Microstructure and Tensile Strength of an Al-Si-Fe-V Alloy: Vanadium and Solidification Thermal Parameters as Recycling Strategies
Abstract
:1. Introduction
- A new knowledge of the combined effects of V and cooling conditions on the formation of V-containing IMCs during solidification;
- Application of a concept of average solidification thermal parameters to investigate the microstructure coarsening in Al-Si-Fe(-V) alloys;
- Determination of quantitative relationships for the prediction of mechanical properties from microstructural parameters.
2. Materials and Methods
2.1. Alloys Preparation
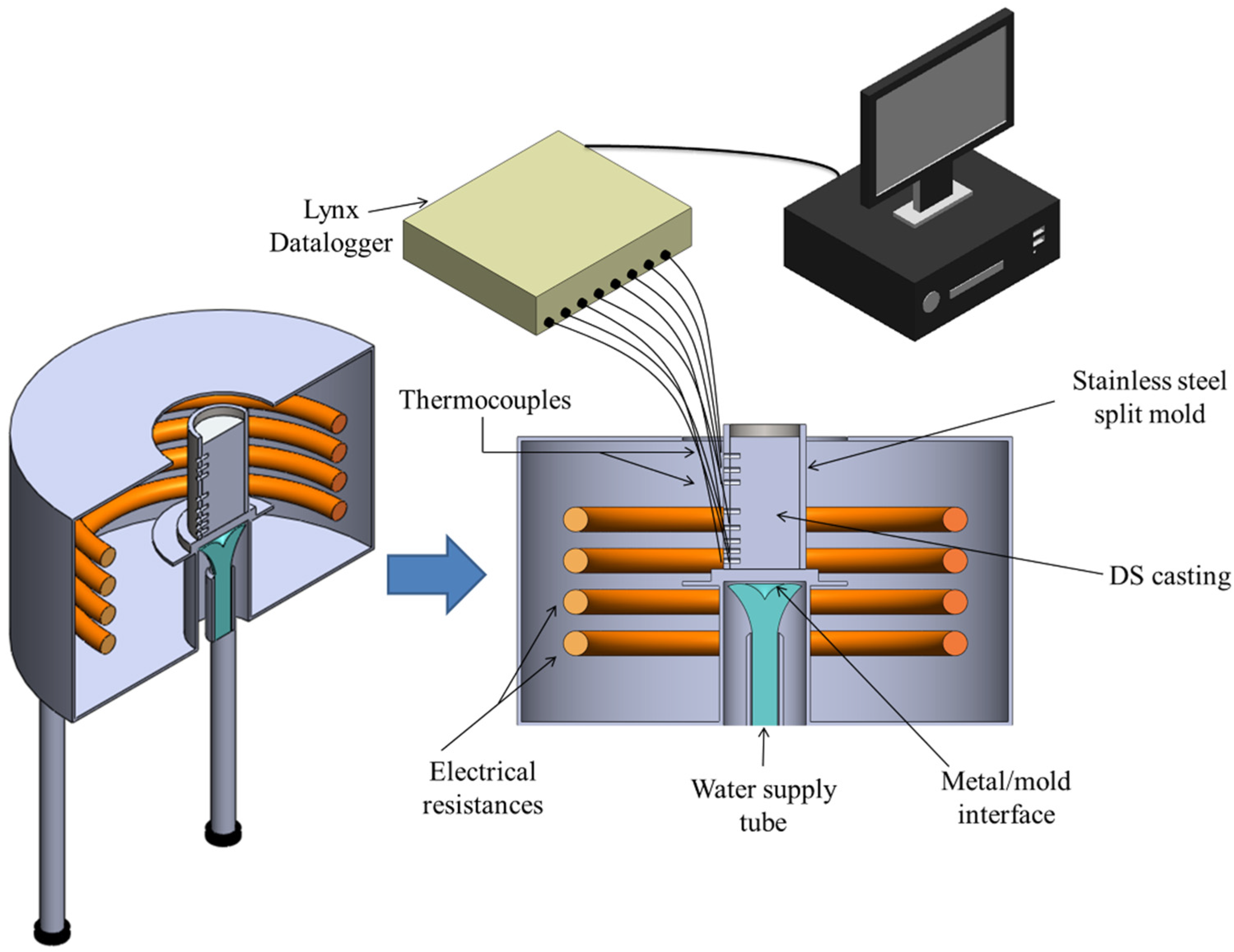
2.2. Directional Solidification
2.3. Calculation of Solidification Thermal Parameters
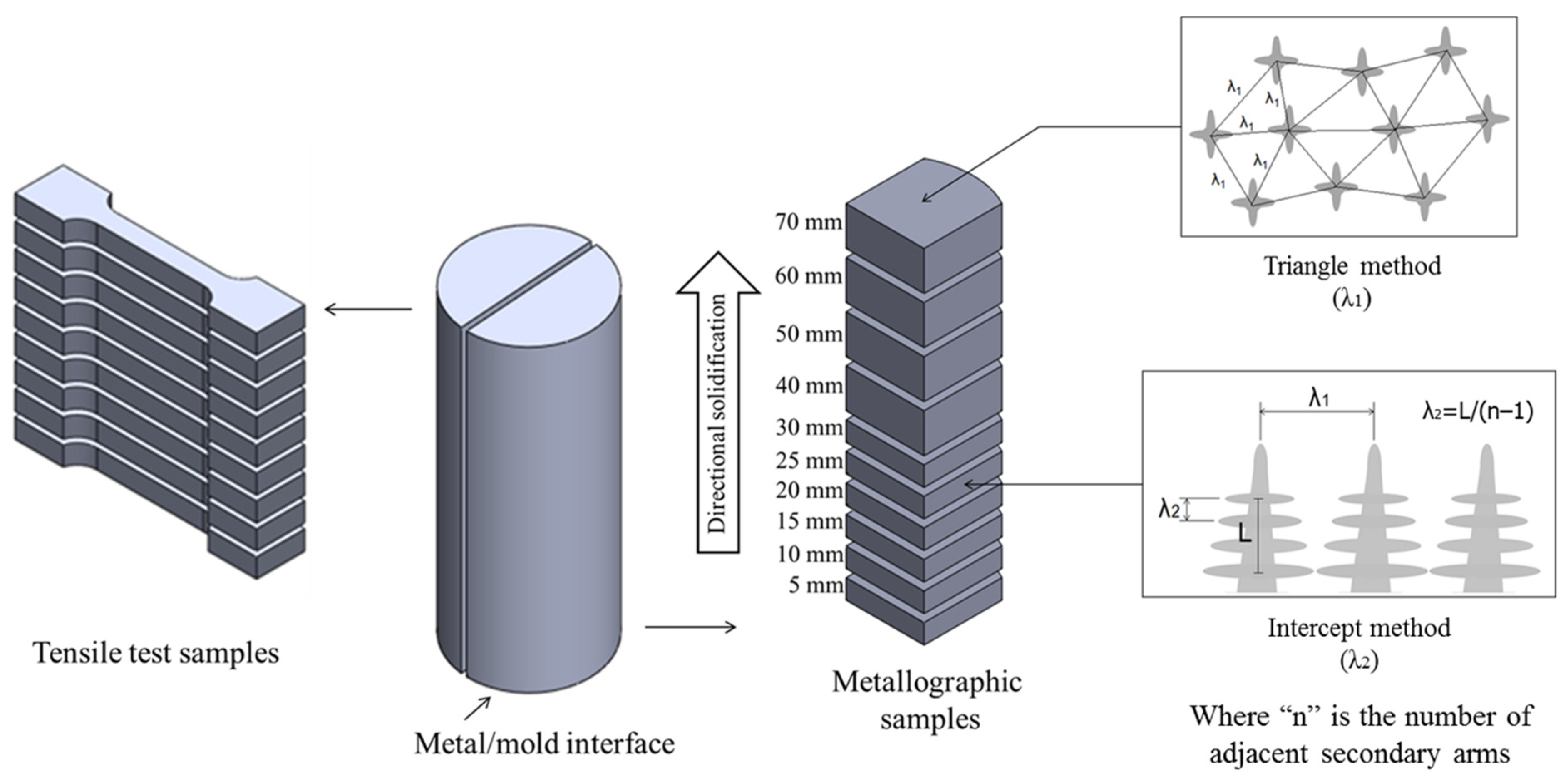
2.4. Macro and Microstructural Characterization
2.5. Mechanical Testing
2.6. Multiple Linear Regression (MLR) Analysis
3. Results
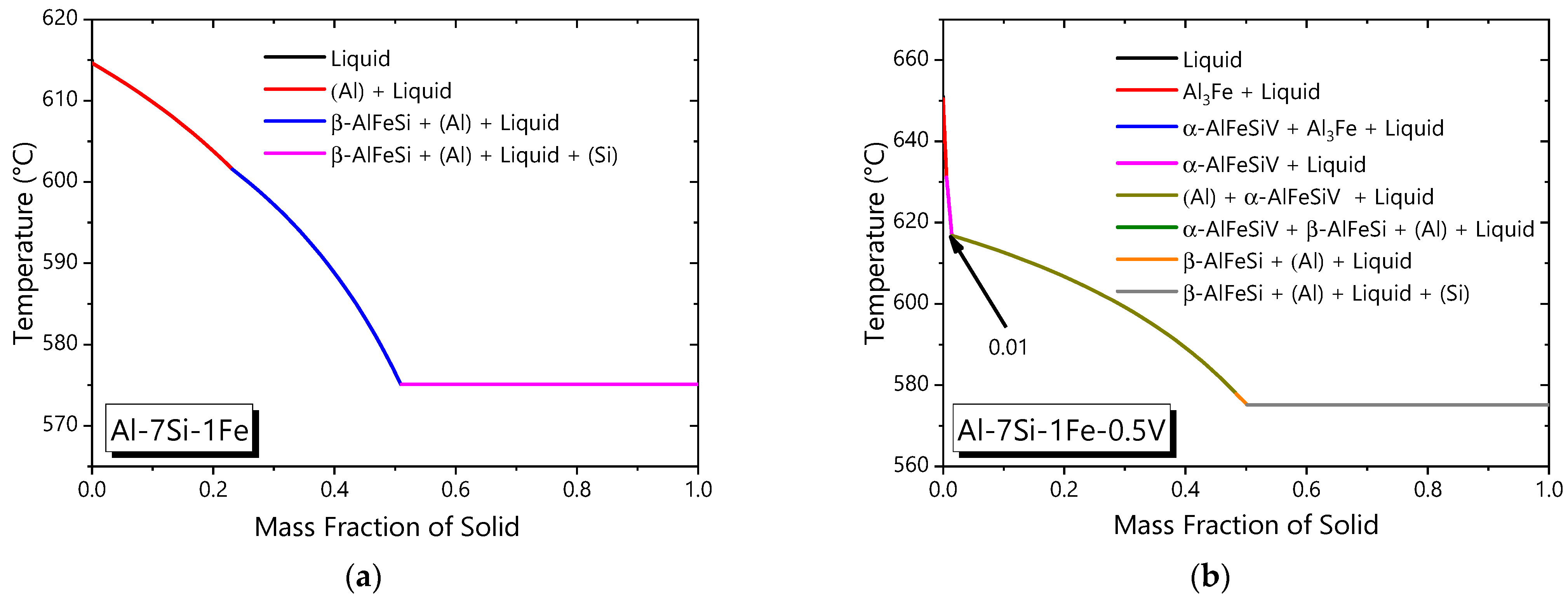
3.1. Thermo-Calc Simulations
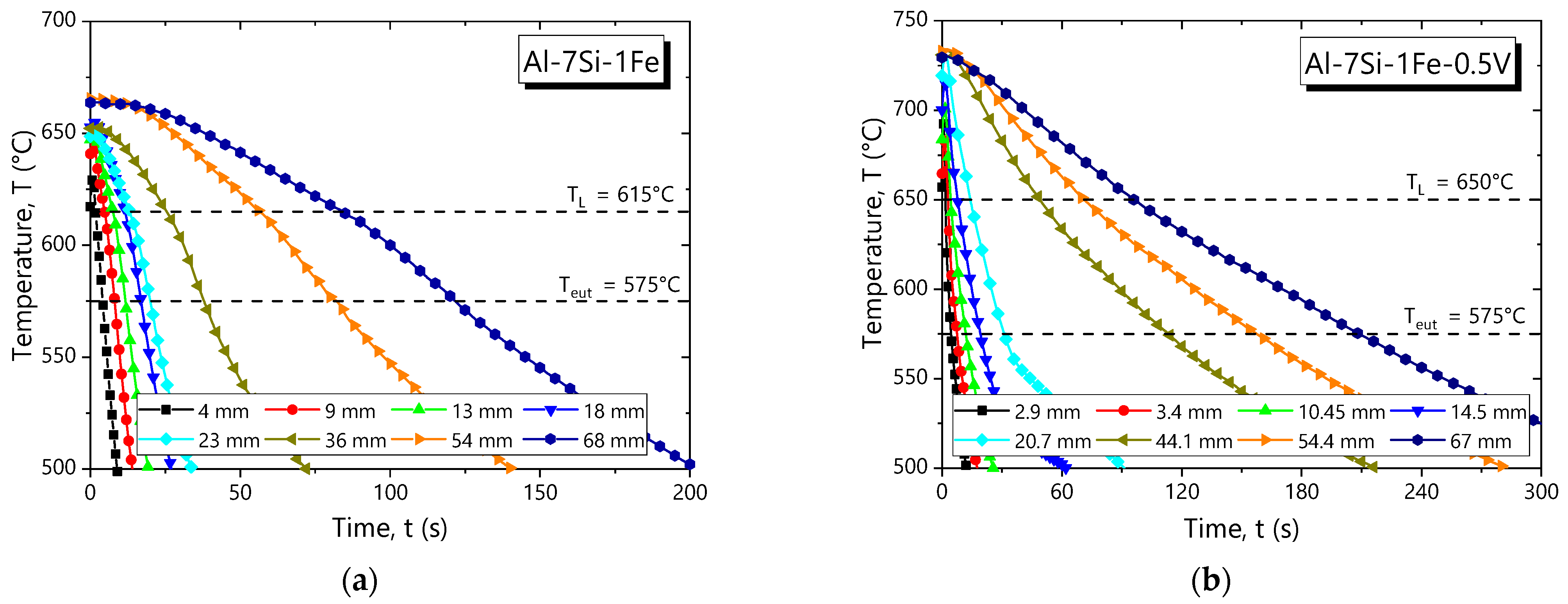
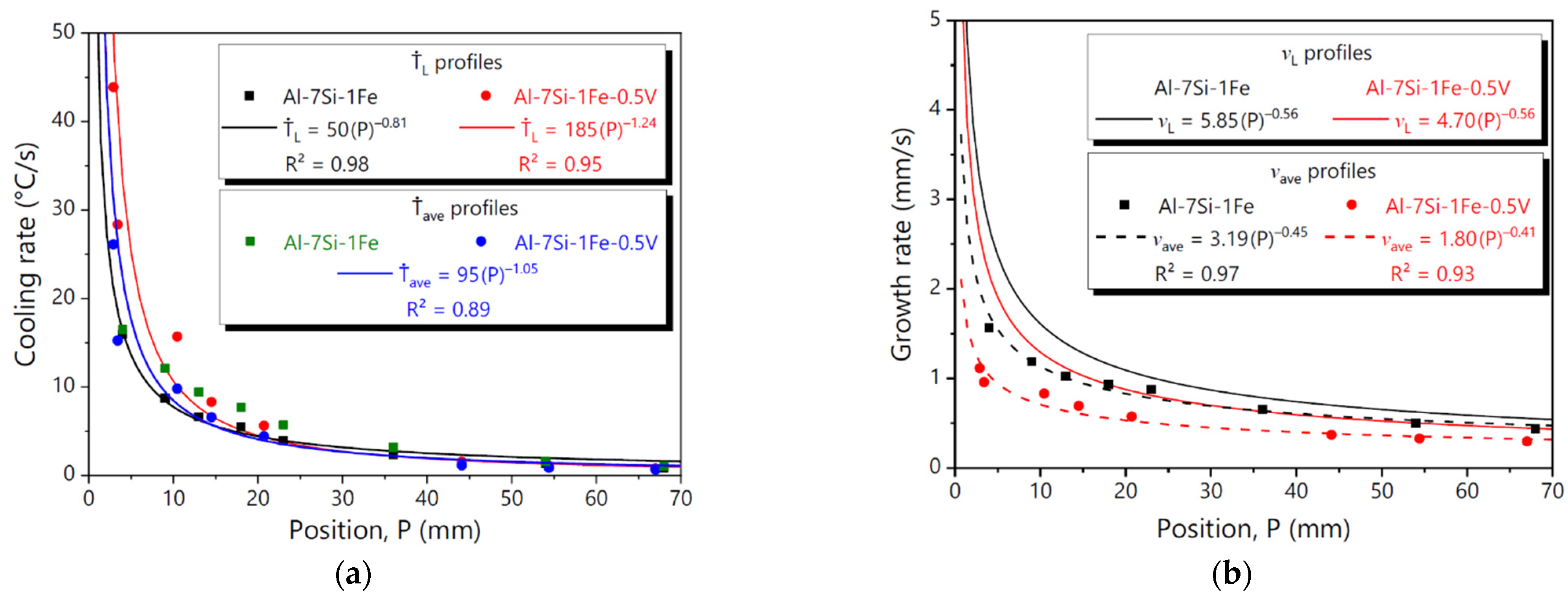
3.2. Solidification Thermal Parameters
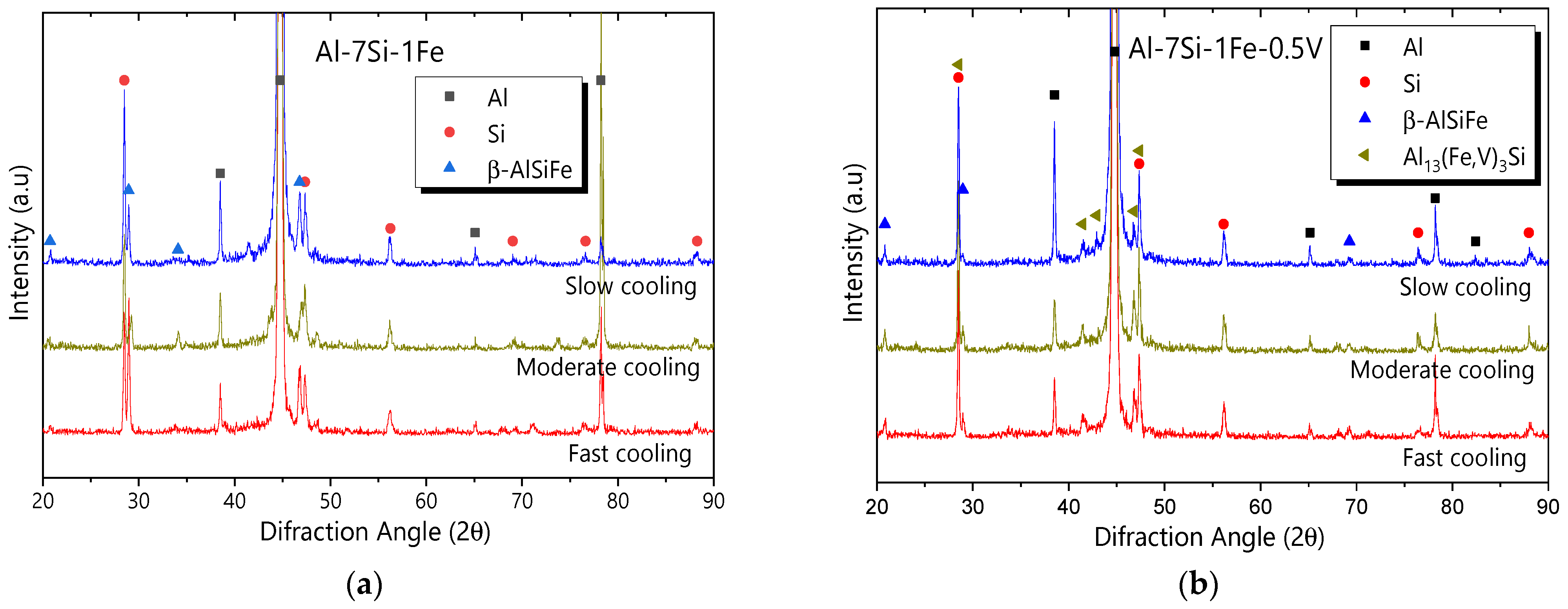
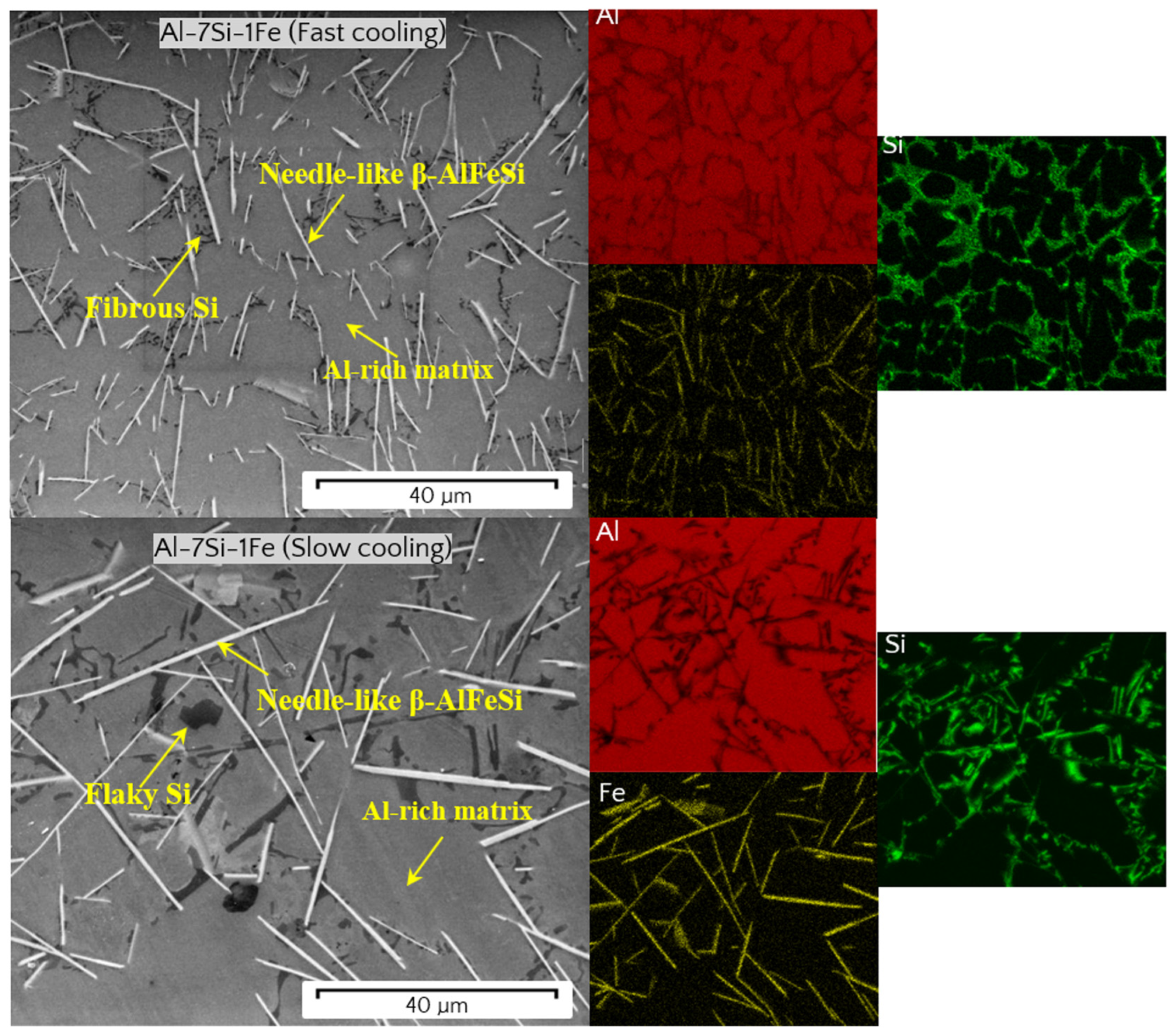
3.3. Microstructural Phases
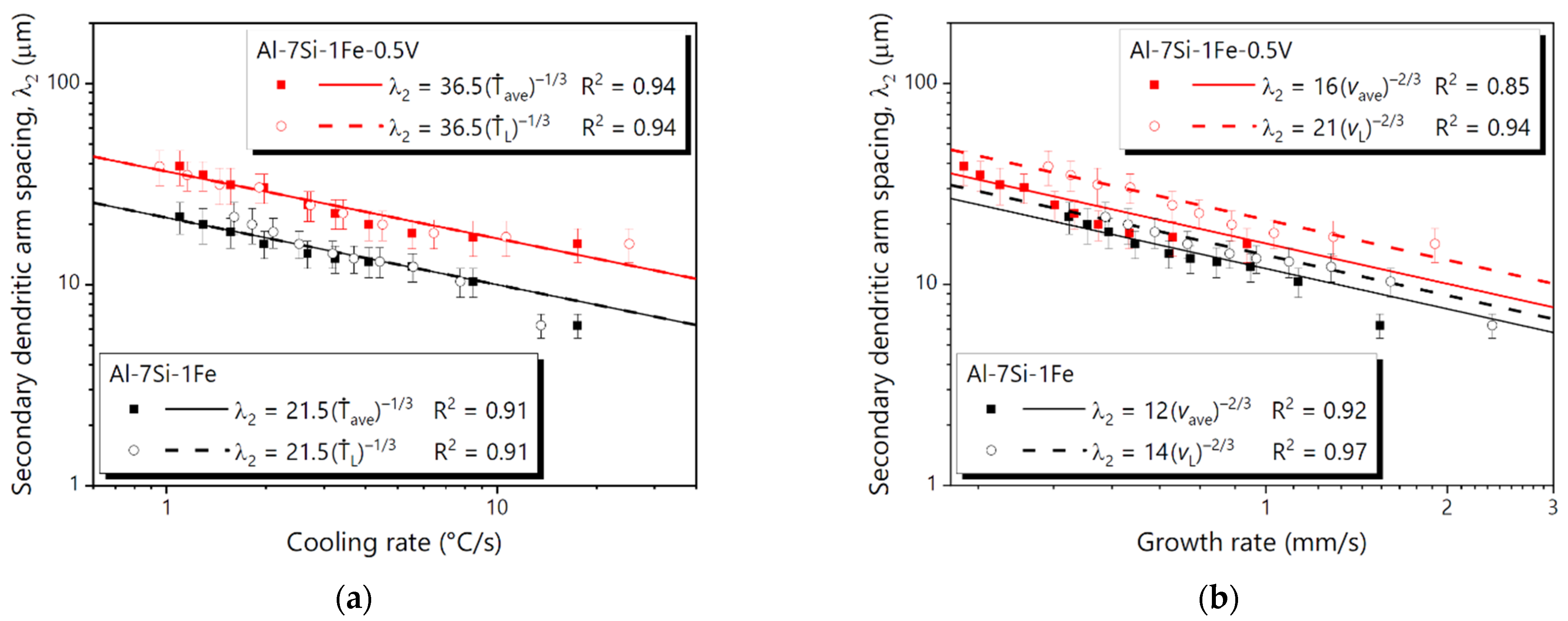
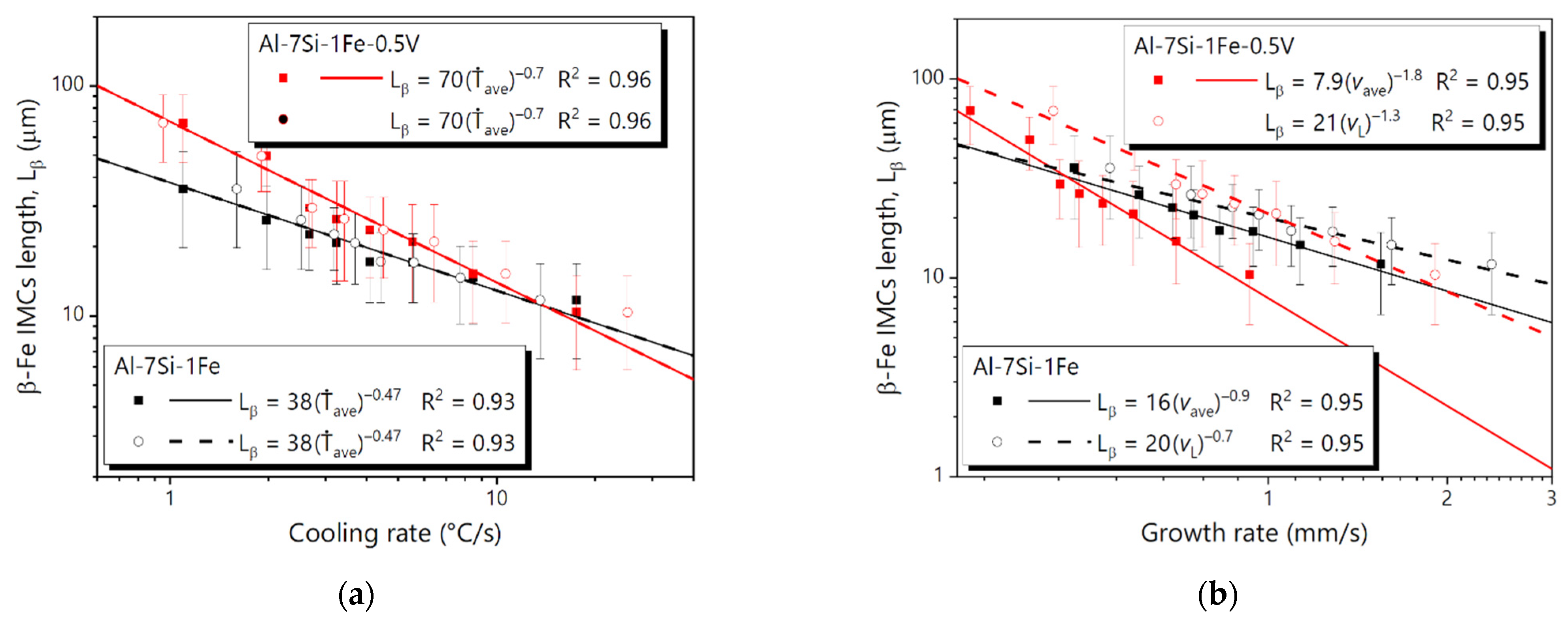
3.4. Microstructural Growth Laws
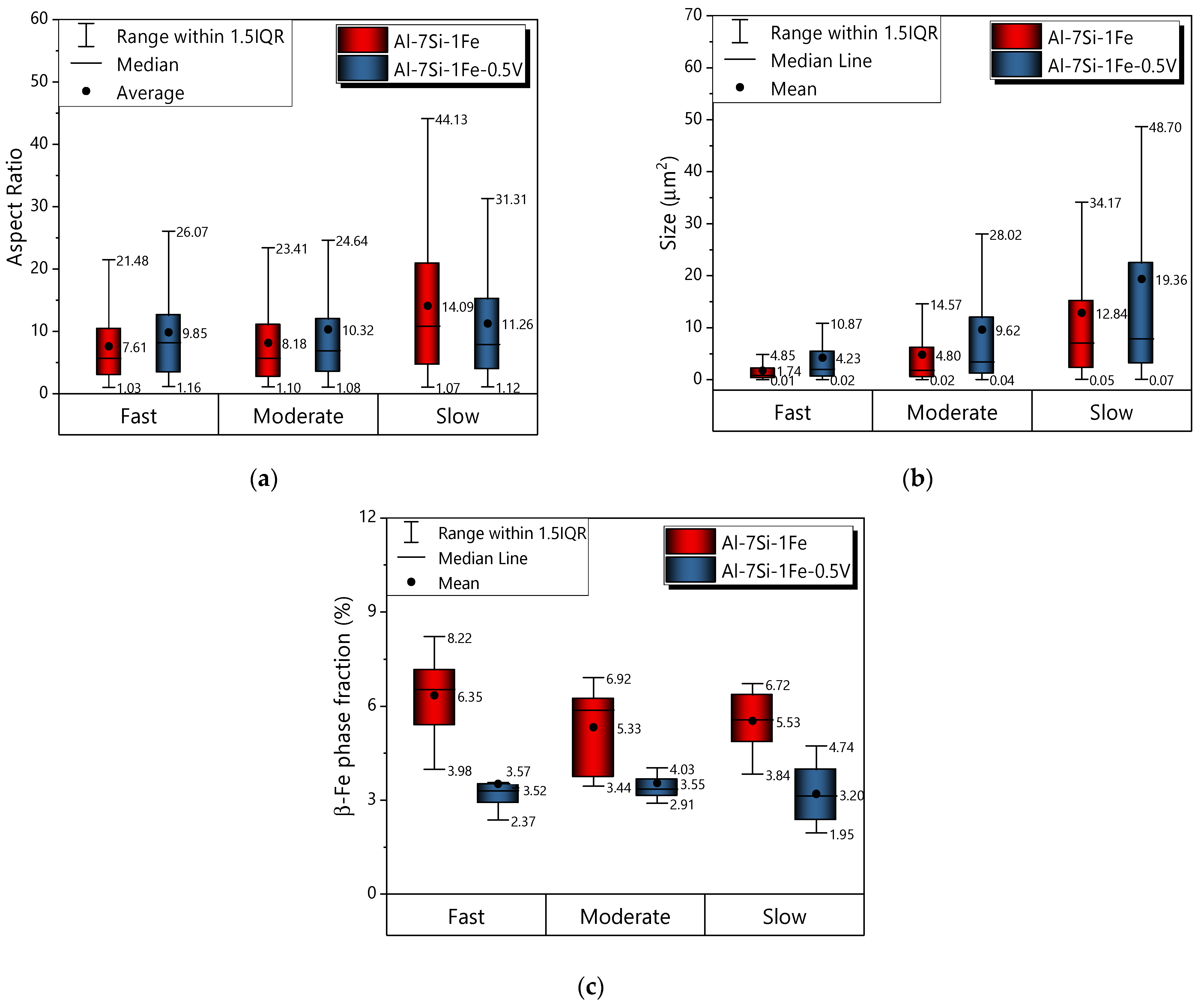
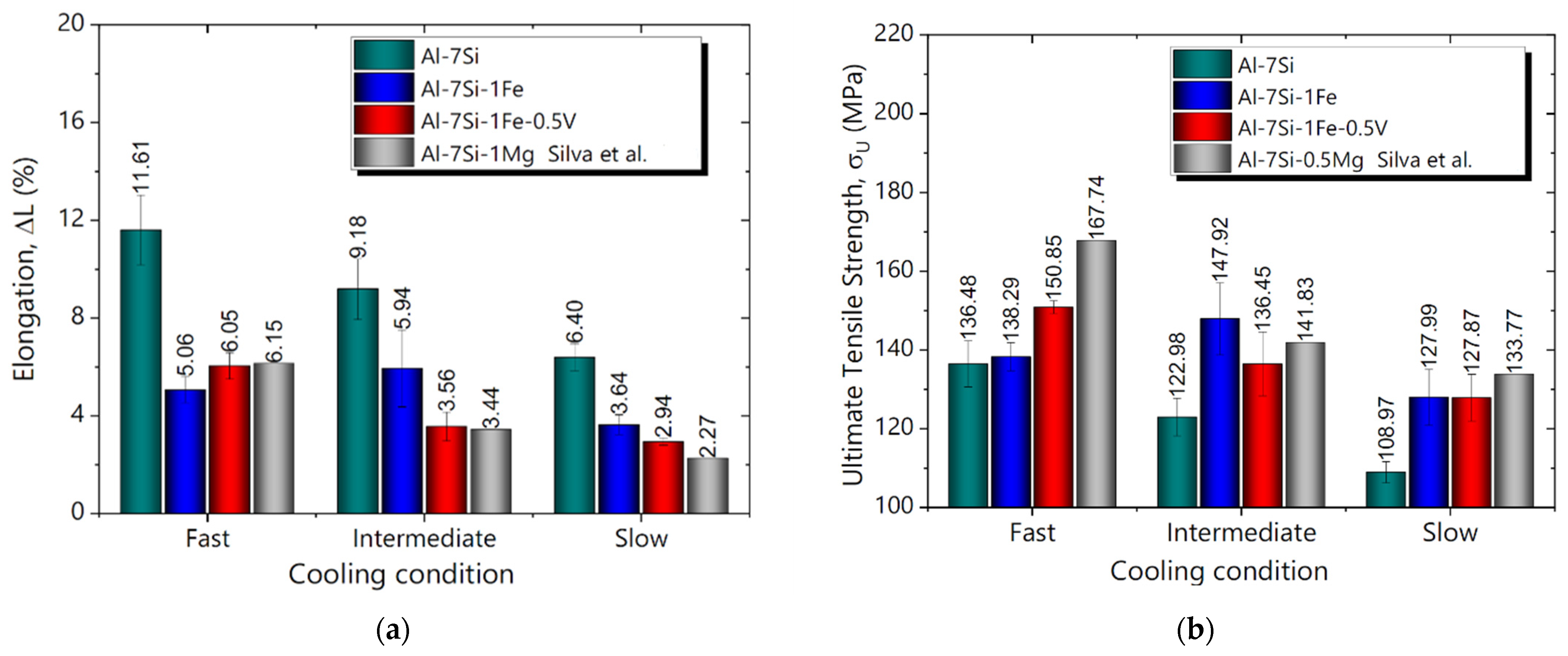
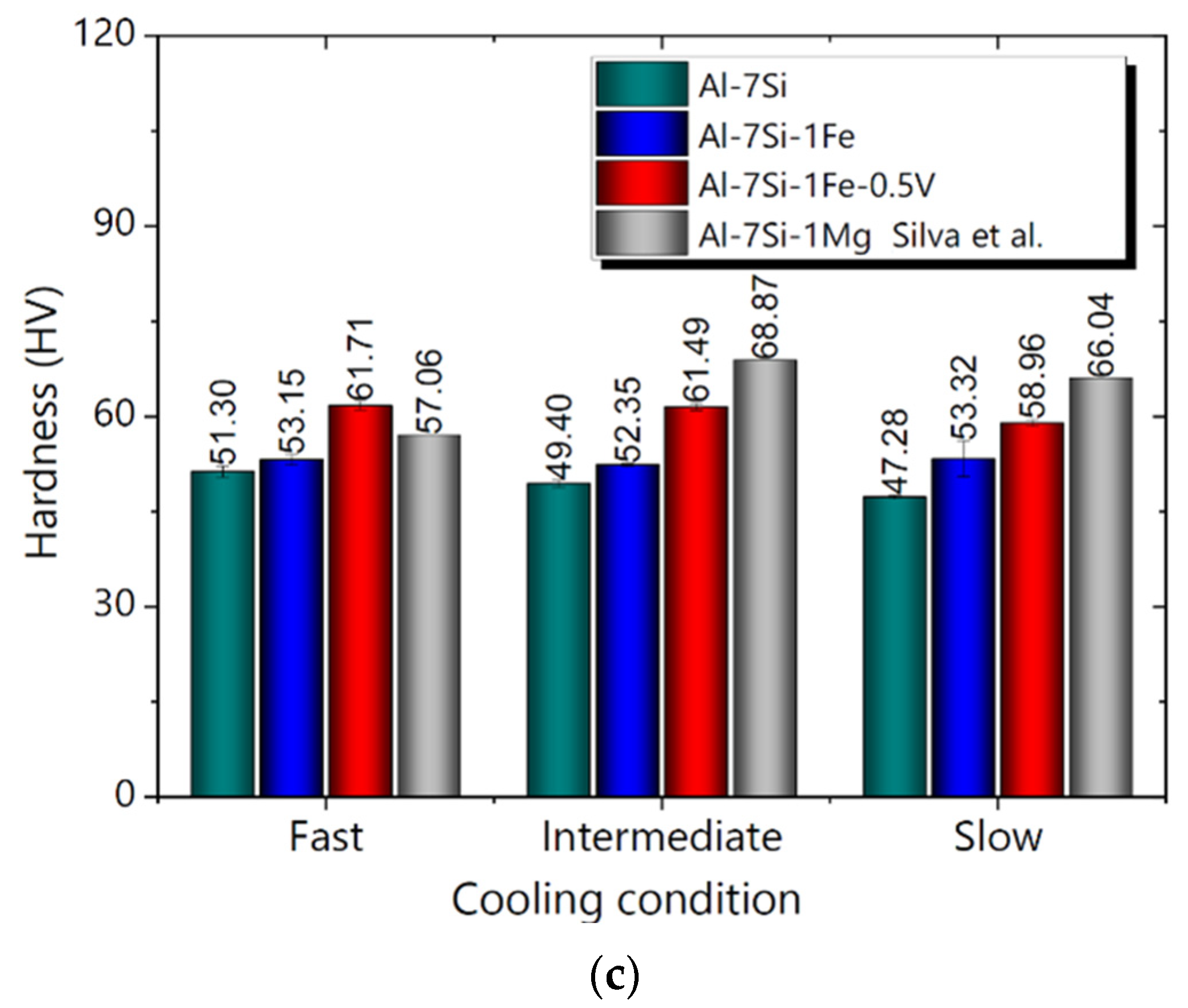
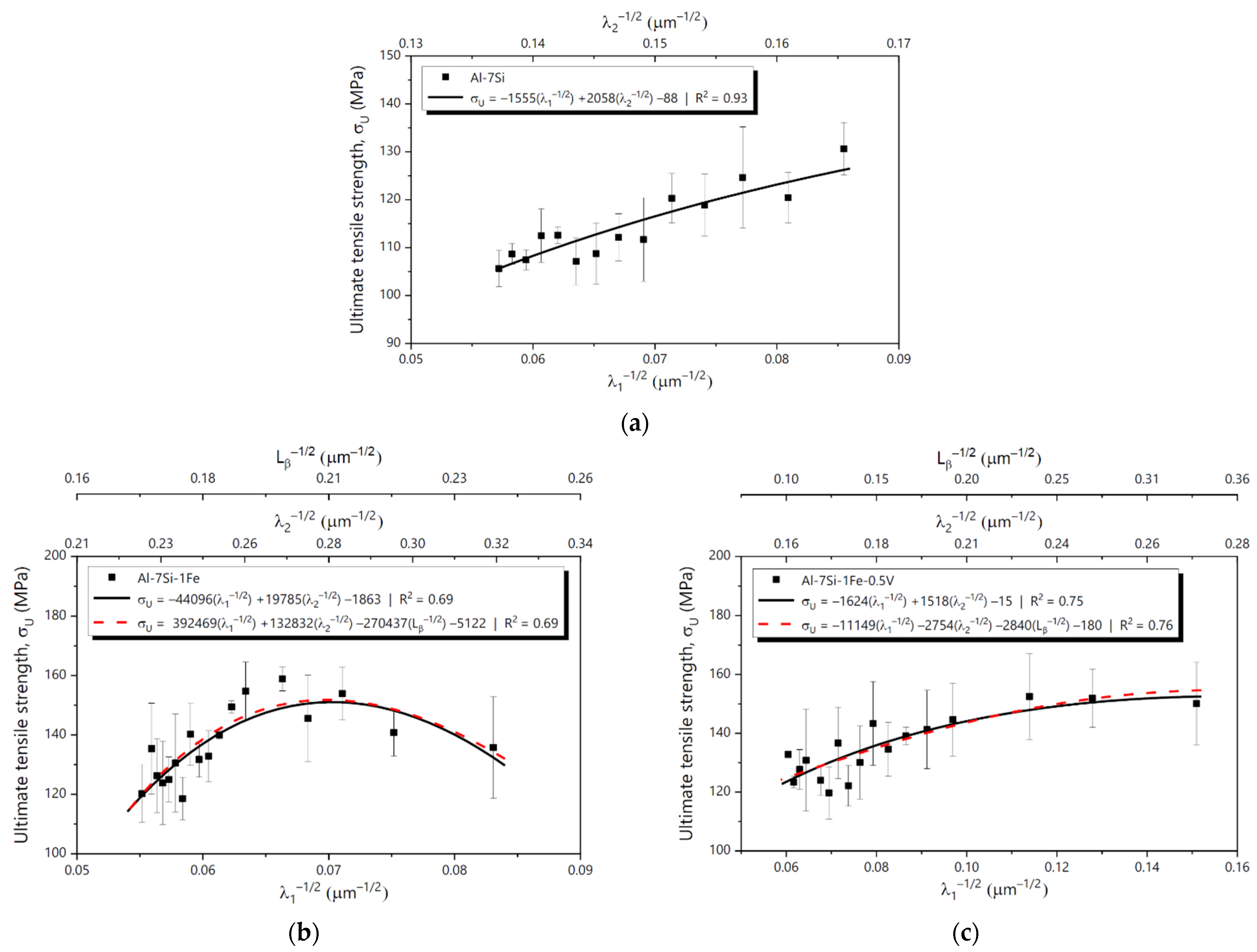
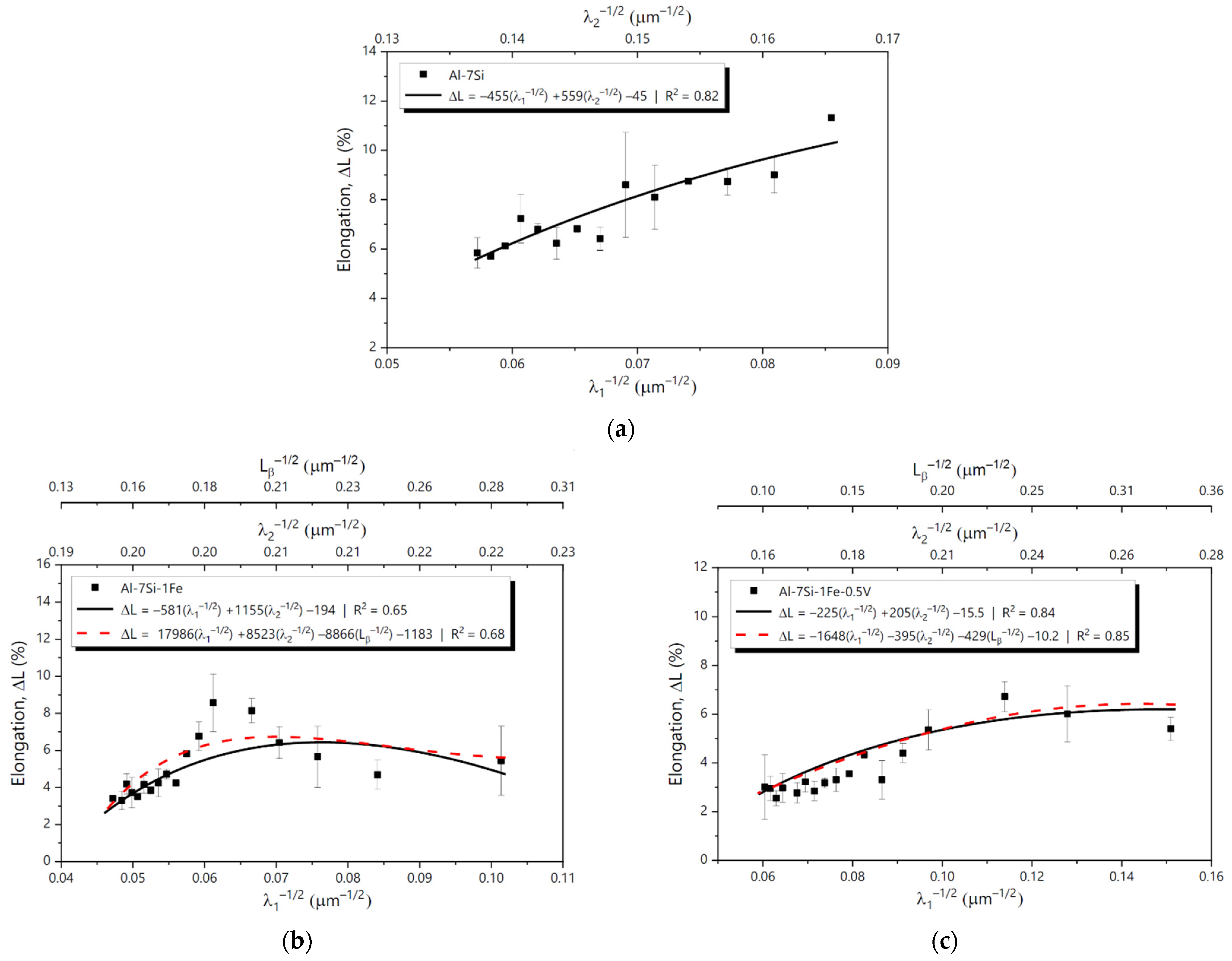
3.5. Mechanical Properties and Cooling Regime
3.6. Tensile Behavior and MLR Analysis
4. Conclusions
- The microstructure of the Al-7Si-1Fe alloy was shown to be composed of Al-rich dendrites surrounded by fibrous/flaky Si and needle-like β-AlFeSi particles. With 0.5V addition, the microstructure consisted of Al-rich dendrites surrounded with V-containing particles (α-AlSiFeV and Al3V), fibrous/flaky Si, and needle-like β-AlFeSi.
- Experimental growth laws relating the dendritic arm spacings (λ1 and λ2) to solidification thermal parameters were determined using characteristic exponents previously reported for several binary and ternary Al-based alloys. The addition of 0.5 wt.%V to the Al-7Si-1Fe alloy was shown to increase λ1 and reduce λ2.
- The addition of 0.5 wt.%V to the Al-7Si-1Fe alloy was shown to be only advantageous when associated with fast cooling conditions, with both elongation (L) and ultimate tensile strength () being improved. Therefore, the addition of 0.5 wt.%V was shown to be a potential alternative for the recycling of the Al-7Si-1Fe alloy for casting technologies associated with faster cooling conditions. The hardness of all studied alloys was shown to be practically unaffected by the cooling regime.
- Hall-Petch-type equations relating and ΔL of the studied alloys to the inverse of the square root of λ1, λ2 and the length of β-AlFeSi particles (Lβ) were proposed in the form of MLR plots. Overall, of the Al-7Si and Al-7Si-1Fe-0.5V alloys were shown to increase with the reduction in the microstructural spacings, whereas σU of the Al-7Si-1Fe alloy achieved a maximum value with the microstructure refinement and then started decreasing. For the Al-7Si and Al-7Si-1Fe-0.5V alloys, ΔL improved with the microstructure refinement. For the Al-7Si-1Fe alloy, however, ΔL reached a maximum and then started decreasing.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Løvik, A.N.; Modaresi, R.; Müller, D.B. Long-Term Strategies for Increased Recycling of Automotive Aluminum and Its Alloying Elements. Environ. Sci. Technol. 2014, 48, 4257–4265. [Google Scholar] [CrossRef] [PubMed]
- Jarfors, A.E.; Du, A.; Yu, G.; Zheng, J.; Wang, K. On the Sustainable Choice of Alloying Elements for Strength of Aluminum-Based Alloys. Sustainability 2020, 12, 1059. [Google Scholar] [CrossRef] [Green Version]
- Yılmaz, T.G.; Tüfekçi, M.; Karpat, F. A Study of Lightweight Door Hinges of Commercial Vehicles Using Aluminum Instead of Steel for Sustainable Transportation. Sustainability 2017, 9, 1661. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, K.T.; Murali, S.; Raman, K.S.; Murthy, K.S.S. Casting and Heat Treatment Variables of Al-7Si-Mg alloy. Mater. Sci. Technol. 1993, 9, 189–203. [Google Scholar]
- Tiryakioǧlu, M.; Staley, J.T.; Campbell, J. Evaluating Structural Integrity of Cast Al-7%Si-Mg Alloys via Work Wardening Char-acteristics II. A New Quality Index. Mater. Sci. Eng. A 2004, 368, 231–238. [Google Scholar] [CrossRef]
- Kaiser, M.S.; Sabbir, S.H.; Kabir, M.S.; Soummo, M.R.; Al Nur, M. Study of Mechanical and Wear Behaviour of Hyper-Eutectic Al-Si Automotive Alloy Through Fe, Ni and Cr Addition. Mater. Res. 2018, 21. [Google Scholar] [CrossRef] [Green Version]
- Wan, B.; Chen, W.; Liu, L.; Cao, X.; Zhou, L.; Fu, Z. Effect of Trace Yttrium Addition on the Microstructure and Tensile Properties of Recycled Al–7Si–0.3Mg–1.0Fe Casting Alloys. Mater. Sci. Eng. A 2016, 666, 165–175. [Google Scholar] [CrossRef]
- Tillová, E.; Chalupová, M.; Borko, K.; Kuchariková, L. Changes of Fracture Surface in Recycled A356 Cast Alloy. Mater. Today Proc. 2016, 3, 1183–1188. [Google Scholar] [CrossRef]
- Baldan, R.; Malavazi, J.; Couto, A.A. Microstructure and Mechanical Behavior of Al9Si0.8Fe Alloy with Different Mn Contents. Mater. Sci. Technol. 2017, 33, 1192–1199. [Google Scholar] [CrossRef]
- Canté, M.V.; Lima, T.S.; Brito, C.; Garcia, A.; Cheung, N.; Spinelli, J.E. An Alternative to the Recycling of Fe-Contaminated Al. J. Sustain. Met. 2018, 4, 412–426. [Google Scholar] [CrossRef]
- Qin, R.; Yan, R.F.; Guan, Z.P.; Zhang, G.Q.; Song, J.W.; Ren, M.W.; Wang, J.G. Effect of Vanadium on Fe-rich Phase, Mechanical Properties and Thermal Conductivity of Hypoeutectic Al–Si Alloy. Mater. Res. Express 2021, 8, 026518. [Google Scholar] [CrossRef]
- Lin, B.; Li, H.; Xu, R.; Xiao, H.; Zhang, W.; Li, S. Effects of Vanadium on Modification of Iron-Rich Intermetallics and Mechanical Properties in A356 Cast Alloys with 1.5 wt.% Fe. J. Mater. Eng. Perform. 2018, 28, 475–484. [Google Scholar] [CrossRef]
- Sahoo, K.L.; Pathakb, B.N. Solidification Behavior, Microstructure and Mechanical Properties of High Fe-Containing Al–Si–V Alloys. J. Mater. Process. Technol. 2009, 209, 798–804. [Google Scholar] [CrossRef]
- Szymczak, T.; Gumienny, G.; Klimek, L.; Goły, M.; Pacyniak, T. Microstructural Characteristics of AlSi9Cu3(Fe) Alloy with High Melting Point Elements. Metals 2020, 10, 1278. [Google Scholar] [CrossRef]
- Essari, A.M.; Ghidhan, S.B.; Kawan, F.A. The Influence of Heat Treatment, Mg Modification and SiCp Reinforcement on Hardness of Al-8Si-8Fe-1.4V Alloy. J. Sci. Technol. (Eng. Appl. Sci.) 2018, 2, 106–112. [Google Scholar]
- Rui, C.H.E.N.; Shi, Y.F.; Xu, Q.Y.; Liu, B.C. Effect of Cooling Rate on Solidification Parameters and Microstructure of Al-7Si-0.3Mg-0.15Fe Alloy. Trans. Nonferrous Met. Soc. China 2014, 24, 1645–1652. [Google Scholar]
- Lima, J.; Barbosa, C.; Magno, I.; Nascimento, J.; Barros, A.; Oliveira, M.; Souza, F.; Rocha, O. Microstructural evolution during unsteady-state horizontal solidification of Al-Si-Mg (356) alloy. Trans. Nonferrous Met. Soc. China 2018, 28, 1073–1083. [Google Scholar] [CrossRef]
- Septimio, R.S.; Costa, T.A.; Silva, C.A.; Vida, T.A.; de Damborenea, J.; Garcia, A.; Cheung, N. Correlation Between Unsteady-State Solidification Thermal Parameters and Micro-structural Growth of Zn–8 mass% Al and Zn–8 mass% Al–XBi Tribological Alloys. J. Therm. Anal. Calorim. 2020, 139, 1741–1761. [Google Scholar] [CrossRef]
- Peres, M.; Siqueira, C.; Garcia, A. Macrostructural and microstructural development in Al–Si alloys directionally solidified under unsteady-state conditions. J. Alloy. Compd. 2004, 381, 168–181. [Google Scholar] [CrossRef]
- Oliveira, R.; Kakitani, R.; Ramos, L.R.; Gonçalves, D.L.; Garcia, A.; Cheung, N. The Roles of Mn and Ni Additions to Fe-Contaminated Al in Neutralizing Fe and Stabilizing the Cellular α-Al Microstructure. J. Sustain. Met. 2019, 5, 561–580. [Google Scholar] [CrossRef]
- Kakitani, R.; Oliveira, R.; Reyes, R.V.; Rodrigues, A.V.; Bertelli, F.; Garcia, A.; Spinelli, J.E.; Cheung, N. Metal/Mold Thermal Conductance Affecting Ultrafine Scale Microstructures in Alu-minum Eutectic Alloys. Case Stud. Therm. Eng. 2021, 26, 101144. [Google Scholar] [CrossRef]
- Gündüz, M.; Çadirli, E. Directional Solidification of Aluminium–Copper Alloys. Mater. Sci. Eng. A 2002, 327, 167–185. [Google Scholar] [CrossRef]
- ASTM E8; ASTM E8/E8M Standard Test Methods for Tension Testing of Metallic Materials 1. Annual Book of ASTM Standards 4 2010; ASTM International: West Conshohocken, PA, USA, 2021; pp. 1–27.
- ASTM E384-17; ASTM E384-17 Standard Test Method for Microindentation Hardness of Materials. ASTM International: West Conshohocken, PA, USA, 2017; pp. 1–40.
- Kistler, N.A.; Corbin, D.J.; Nassar, A.R.; Reutzel, E.W.; Beese, A.M. Effect of processing conditions on the microstructure, porosity, and mechanical properties of Ti-6Al-4V repair fabricated by directed energy deposition. J. Mater. Process. Technol. 2018, 264, 172–181. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, J.; Damoah, L.N.W.; Robertson, D.G. Removal of Iron from Aluminum: A review. Miner. Process. Extr. Metall. Rev. 2012, 33, 99–157. [Google Scholar] [CrossRef]
- Dinnis, C.M.; Taylor, J.A.; Dahle, A.K. As-cast morphology of iron-intermetallics in Al–Si foundry alloys. Scr. Mater. 2005, 53, 955–958. [Google Scholar] [CrossRef]
- Hearn, W.; Bogno, A.-A.; Spinelli, J.; Valloton, J.; Henein, H. Microstructure Solidification Maps for Al-10 Wt Pct Si Alloys. Met. Mater. Trans. A 2019, 50, 1333–1345. [Google Scholar] [CrossRef]
- Mahta, M.; Emamy, M.; Daman, A.; Keyvani, A.; Campbell, J. Precipitation of Fe rich intermetallics in Cr- and Co-modified A413 alloy. Int. J. Cast Met. Res. 2005, 18, 73–79. [Google Scholar] [CrossRef]
- Becker, H.; Bergh, T.; Vullum, P.E.; Leineweber, A.; Li, Y. Effect of Mn and Cooling Rates on α-, β-and δ-Al–Fe–Si Intermetallic Phase Formation in a Secondary Al–Si Alloy. Materialia 2019, 5, 100198. [Google Scholar] [CrossRef]
- Silva, C.; Barros, A.; Vida, T.; Garcia, A.; Cheung, N.; Reis, D.A.P.; Brito, C. Assessing Microstructure Tensile Properties Relationships in Al-7Si-Mg Alloys via Multiple Regression. Metals 2022, 12, 1040. [Google Scholar] [CrossRef]
- Brito, C.; Costa, T.A.; Vida, T.A.; Bertelli, F.; Cheung, N.; Spinelli, J.E.; Garcia, A. Characterization of Dendritic Microstructure, Intermetallic Phases, and Hardness of Direc-tionally Solidified Al-Mg and Al-Mg-Si Alloys. Metall. Mater. Trans. A 2015, 46, 3342–3355. [Google Scholar] [CrossRef]
- Duarte, R.N.; Faria, J.D.; Brito, C.; Verissimo, N.; Cheung, N.; Garcia, A. Length scale of the dendritic microstructure affecting tensile properties of Al–(Ag)–(Cu) alloys. Int. J. Mod. Phys. B 2016, 30, 1550261. [Google Scholar] [CrossRef]




| Metals | Al | Si | Fe | V | Mg | Cu | Pb | Zn | C | P | S |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Balance | 0.006 | 0.073 | - | 0.001 | 0.01 | 0.006 | 0.005 | - | - | - |
| Si | 0.109 | Balance | 0.316 | - | - | - | - | - | - | - | - |
| Fe | - | - | Balance | - | - | - | - | <0.02 | - | - | - |
| V-Fe | 0.04 | 0.96 | 16.76 | 81.9 | - | - | - | - | 0.28 | 0.04 | 0.02 |
| Alloy and Mechanical Property | Statistical Variable | Statistical Values | ||||||
|---|---|---|---|---|---|---|---|---|
| Al-7Si σu | R2 | 0.93 | - | |||||
| F | 1.79 × 10−9 | - | ||||||
| Intercept | λ1−1/2 | λ2−1/2 | Intercept | λ1−1/2 | λ2−1/2 | Lβ−1/2 | ||
| Coefficient | −88.35 | −1555.46 | 2058.07 | - | - | - | - | |
| p-value | 0.00711 | 0.0000898 | 0.000012 | - | - | - | - | |
| Al-7Si ΔL | R2 | 0.82 | - | |||||
| F | 2.47 × 10−6 | - | ||||||
| Intercept | λ1−1/2 | λ2−1/2 | Intercept | λ1−1/2 | λ2−1/2 | Lβ−1/2 | ||
| Coefficient | −45.22 | −454.76 | 558.90 | - | - | - | - | |
| p-value | 0.00027 | 0.00035 | 0.00011 | - | - | - | - | |
| Al-7Si-1Fe σu | R2 | 0.69 | 0.69 | |||||
| F | 0.000164 | 0.000767 | ||||||
| Intercept | λ1−1/2 | λ2−1/2 | Intercept | λ1−1/2 | λ2−1/2 | Lβ−1/2 | ||
| Coefficient | −1863.1 | −44,096.3 | 19,785.01 | −5122.54 | 392,468.74 | 132,831.98 | −270,436.78 | |
| p-value | 0.00051 | 0.00040 | 0.00034 | 0.7258 | 0.8407 | 0.7929 | 0.8231 | |
| Al-7Si-1Fe ΔL | R2 | 0.65 | 0.68 | |||||
| F | 0.000385 | 0.00091 | ||||||
| Intercept | λ1−1/2 | λ2−1/2 | Intercept | λ1−1/2 | λ2−1/2 | Lβ−1/2 | ||
| Coefficient | −194.25 | −581.59 | 1155.09 | −1182.70 | 17,986.26 | 8522.66 | −8865.95 | |
| p-value | 0.00119 | 0.00218 | 0.00111 | 0.1875 | 0.2801 | 0.2014 | 0.2655 | |
| Al-7Si-1Fe-0.5V σu | R2 | 0.75 | 0.76 | |||||
| F | 0.0000291 | 0.000129 | ||||||
| Intercept | λ1−1/2 | λ2−1/2 | Intercept | λ1−1/2 | λ2−1/2 | Lβ−1/2 | ||
| Coefficient | −14.88 | −1624.33 | 1518.88 | 180.71 | 11,163.26 | −2759.05 | −2843.66 | |
| p-value | 0.6964 | 0.00704 | 0.00298 | 0.5406 | 0.5597 | 0.6662 | 0.5047 | |
| Al-7Si-1Fe-0.5V ΔL | R2 | 0.84 | 0.85 | |||||
| F | 1.02 × 10−6 | 5.56 × 10−6 | ||||||
| Intercept | λ1−1/2 | λ2−1/2 | Intercept | λ1−1/2 | λ2−1/2 | Lβ−1/2 | ||
| Coefficient | −15.51 | −224.59 | 204.75 | 10.23 | 1647.77 | −395.27 | −429.34 | |
| p-value | 0.00189 | 0.00316 | 0.00103 | 0.7623 | 0.5028 | 0.6153 | 0.4473 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, C.; Barros, A.; Rodrigues, A.V.; Kakitani, R.; Soares, T.; Garcia, A.; Cheung, N. Microstructure and Tensile Strength of an Al-Si-Fe-V Alloy: Vanadium and Solidification Thermal Parameters as Recycling Strategies. Sustainability 2022, 14, 13859. https://doi.org/10.3390/su142113859
Silva C, Barros A, Rodrigues AV, Kakitani R, Soares T, Garcia A, Cheung N. Microstructure and Tensile Strength of an Al-Si-Fe-V Alloy: Vanadium and Solidification Thermal Parameters as Recycling Strategies. Sustainability. 2022; 14(21):13859. https://doi.org/10.3390/su142113859
Chicago/Turabian StyleSilva, Cássio, André Barros, Adilson Vitor Rodrigues, Rafael Kakitani, Thiago Soares, Amauri Garcia, and Noé Cheung. 2022. "Microstructure and Tensile Strength of an Al-Si-Fe-V Alloy: Vanadium and Solidification Thermal Parameters as Recycling Strategies" Sustainability 14, no. 21: 13859. https://doi.org/10.3390/su142113859






