Flexible Multifunctional Self-Expanding Electrospun Polyacrylic Acid Covalently Cross-Linked Polyamide 66 Nanocomposite Fiber Membrane with Excellent Oil/Water Separation and High pH Stability Performances
Abstract
1. Introduction
2. Experimental
2.1. Materials
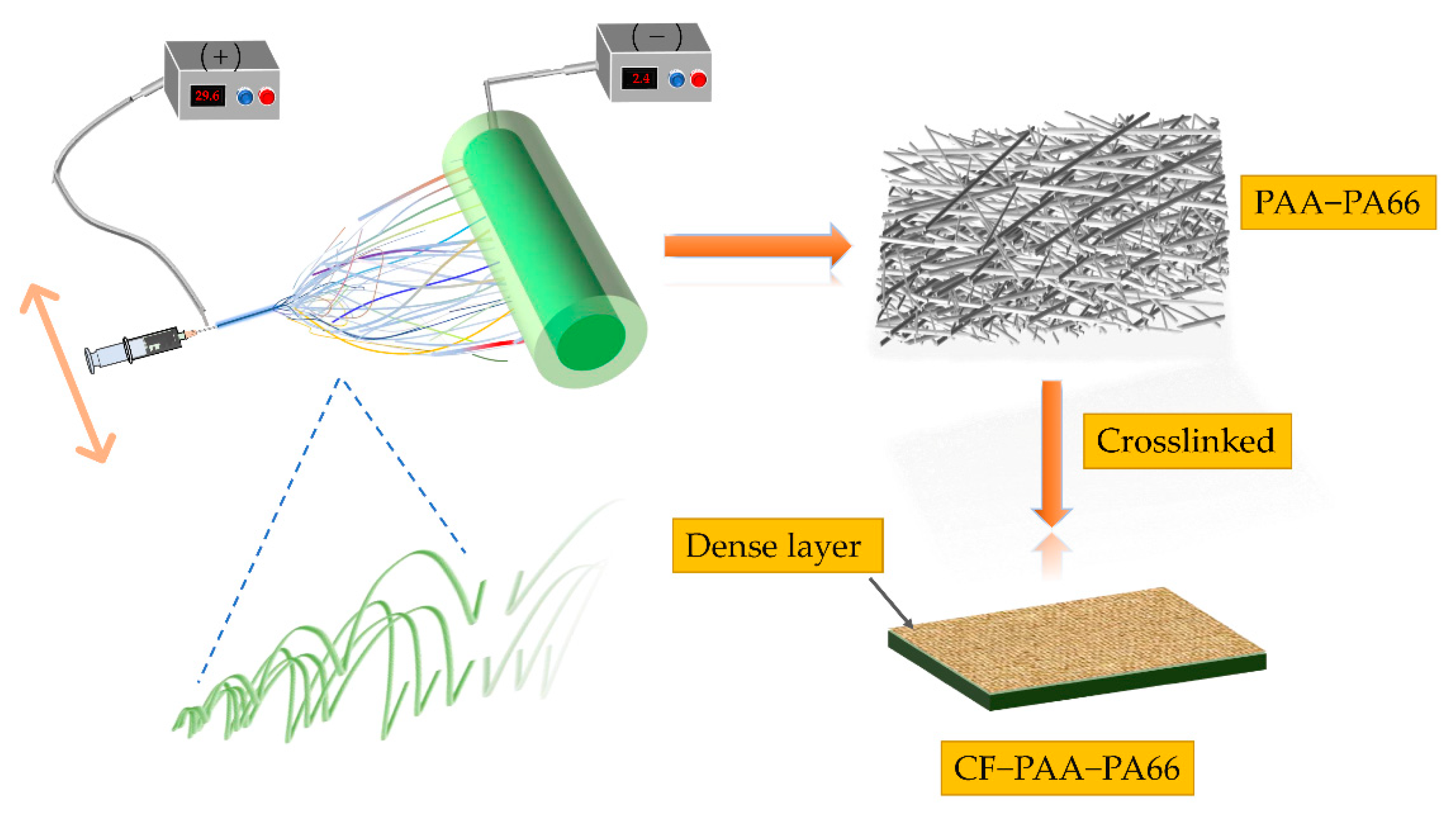
2.2. Preparation of Electrospun PAA-PA66 Nanofiber Membranes
2.3. Polymer Crosslinking (CF-PAA-PA66)
2.4. Membrane Characterizations
2.5. Water Content
2.6. The Overall Pore Volume of Fibrous Membranes
2.7. The Hydrophilicity of Nanofiber Membranes
2.8. Swelling Experiments
2.9. Nanofibers Performance Tests
2.10. Mechanical Property of Nanofiber Membranes
3. Results
3.1. Morphology and Structure of Nanofiber Membranes
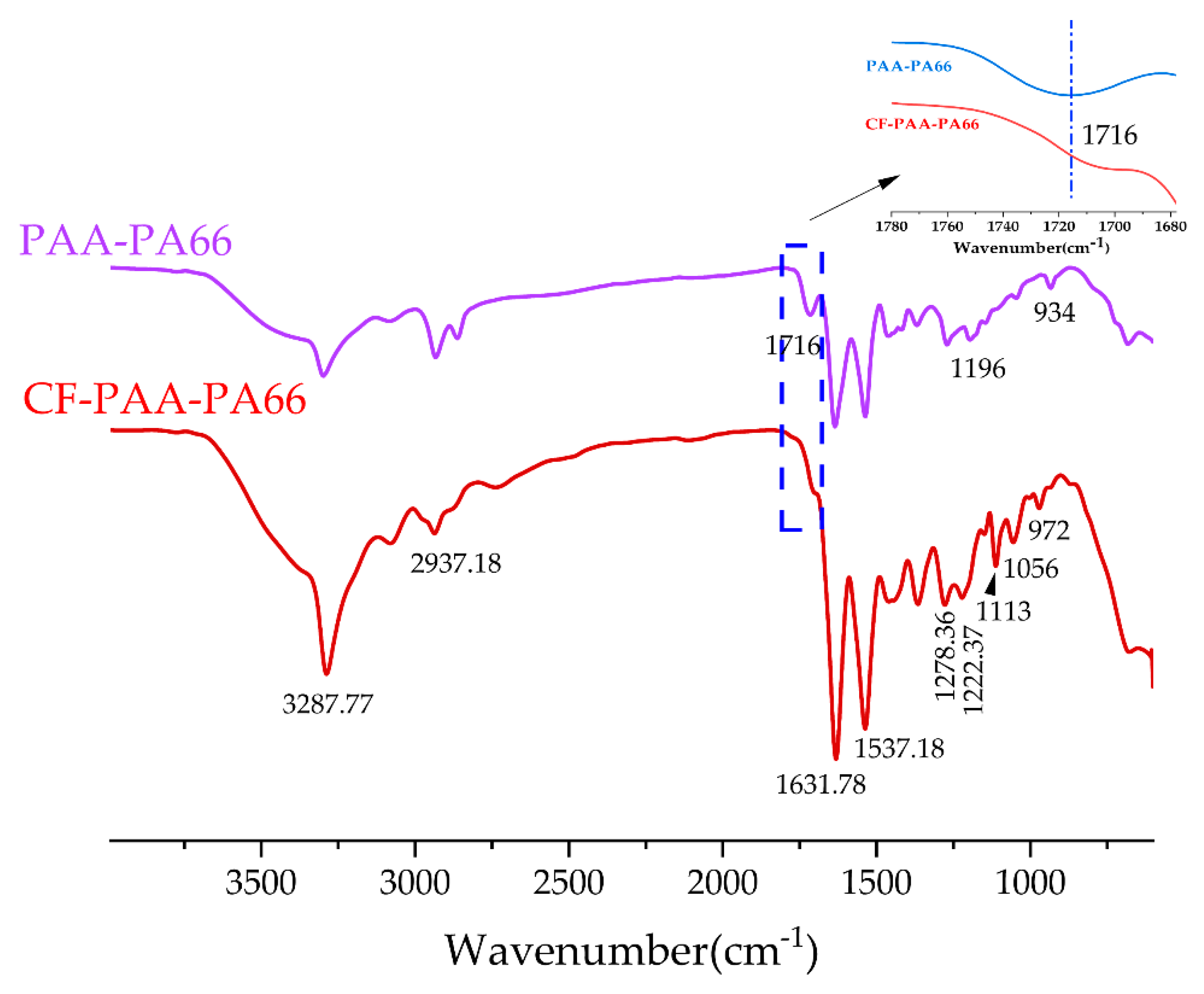
3.2. Chemical Characterizations
3.3. Crystalline Structure
3.4. Thermal Stability
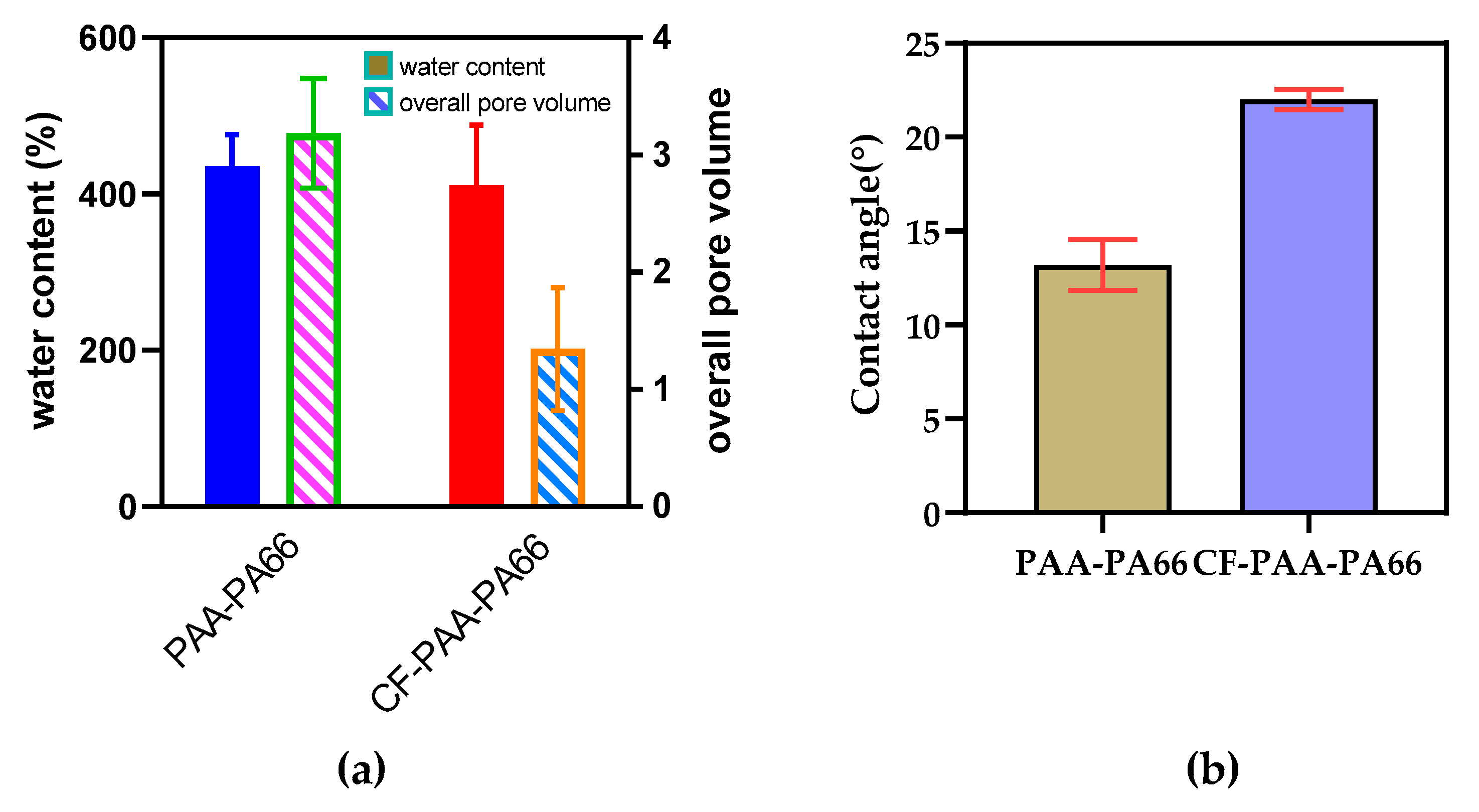
3.5. Water Content, Overall Pore Volume, and Contact Angle
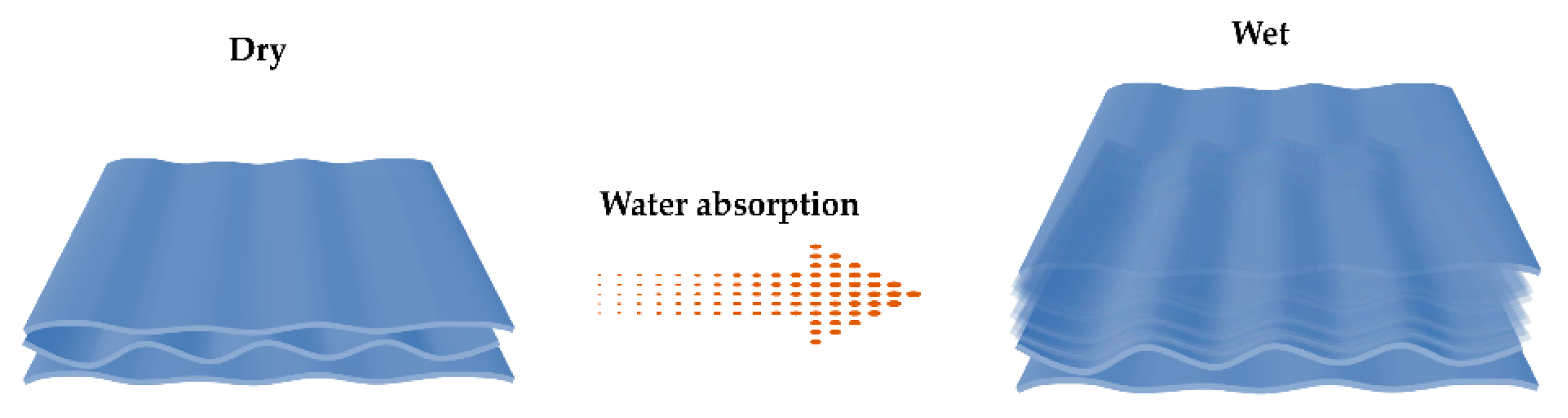
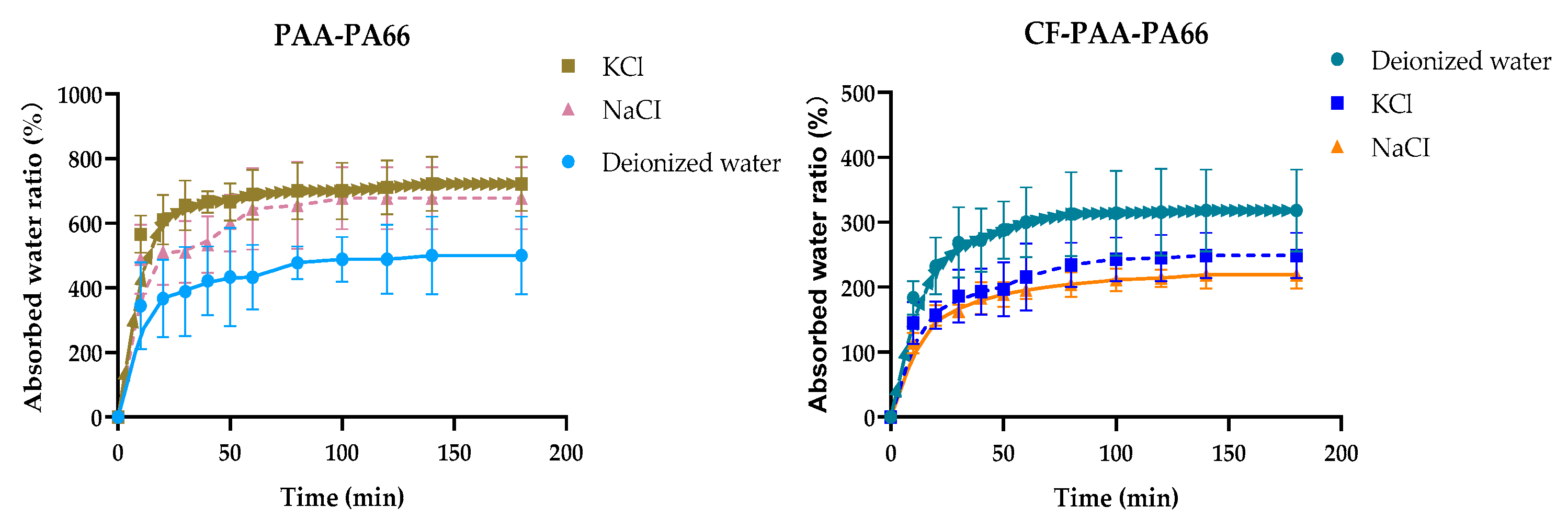
3.6. Equilibrium Swelling Performance and pH-Responsive Swelling Behavior
3.7. Permeability and Rejection Performance
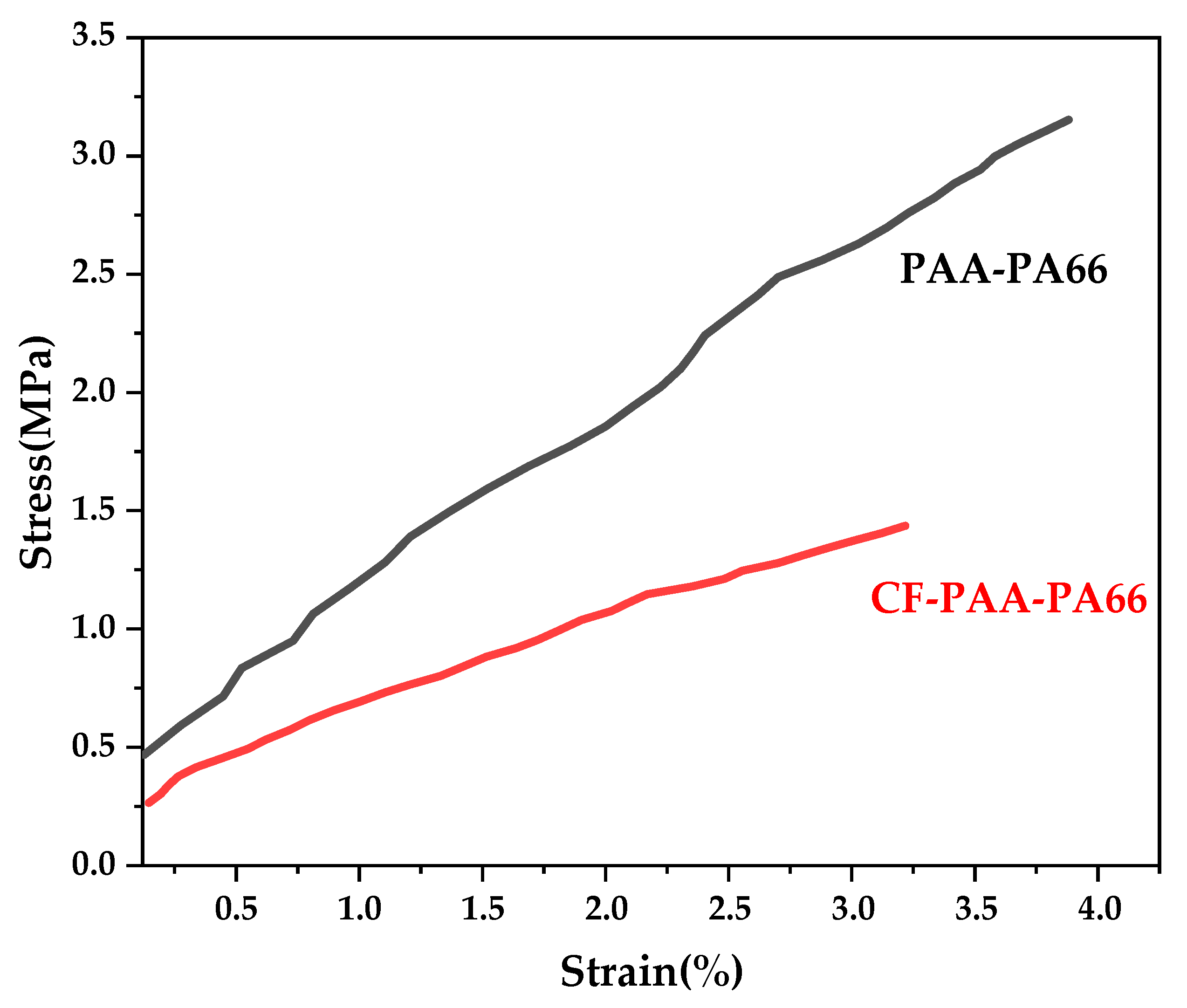
3.8. Mechanical Strength
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Tan, N.B. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Sas, I.; Gorga, R.E.; Joines, J.A.; Thoney, K.A. Literature review on superhydrophobic self-cleaning surfaces produced by electrospinning. J. Polym. Sci. B Polym. Phys. 2012, 50, 824–845. [Google Scholar] [CrossRef]
- Tan, S.-H.; Inai, R.; Kotaki, M.; Ramakrishna, S. Systematic parameter study for ultra-fine fiber fabrication via electrospinning process. Polymer 2005, 46, 6128–6134. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. Drug Delivery Applications of Core-Sheath Nanofibers Prepared by Coaxial Electrospinning: A Review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Lin, J.; Ding, B.; Yang, J.; Yu, J.; Sun, G. Subtle regulation of the micro- and nanostructures of electrospun polystyrene fibers and their application in oil absorption. Nanoscale 2012, 4, 176–182. [Google Scholar] [CrossRef]
- Tobiesen, F.A.; Michielsen, S. Method for grafting poly (acrylic acid) onto nylon 6,6 using amine end groups on nylon surface. J. Polym. Sci. A Polym. Chem. 2002, 40, 719–728. [Google Scholar] [CrossRef]
- Lu, L.; Yang, B.; Liu, J. Flexible multifunctional graphite nanosheet/electrospun-polyamide 66 nanocomposite sensor for ECG, strain, temperature and gas measurements. Chem. Eng. J. 2020, 400, 125928. [Google Scholar] [CrossRef]
- Huang, Y.J.; Huang, C.L.; Lai, R.Y.; Zhuang, C.H.; Chiu, W.H.; Lee, K.M. Microstructure and Biological Properties of Electrospun In Situ Polymerization of Polycaprolactone-Graft-Polyacrylic Acid Nanofibers and Its Composite Nanofiber Dressings. Polymers 2021, 13, 4246. [Google Scholar] [CrossRef]
- Yan, J.; Nie, L.; Li, G.; Zhu, Y.; Gao, M.; Wu, R.; Wang, B. Graphene Oxide Modified Polyamide 66 Ultrafiltration Membranes with Enhanced Anti-Fouling Performance. Membranes 2022, 12, 458. [Google Scholar] [CrossRef]
- Zeng, J.; Hou, H.; Wendorff, J.H.; Greiner, A. Electrospun poly (vinyl alcohol)/poly (acrylic acid) fibres with excellent water-stability. E-Polymers 2004, 4, 899. [Google Scholar] [CrossRef]
- Park, Y.; Lee, S.; Ha, S.S.; Alunda, B.; Noh, D.Y.; Lee, Y.J.; Kim, S.; Seol, J.H. Crosslinking Effect on Thermal Conductivity of Electrospun Poly (acrylic acid) Nanofibers. Polymers 2019, 11, 858. [Google Scholar] [CrossRef] [PubMed]
- Ranjbari, E.; Bazgir, S.; Shirazi, M.M.A. Needleless electrospinning of poly (acrylic acid) superabsorbent: Fabrication, characterization and swelling behavior. Polym. Test. 2020, 84, 106403. [Google Scholar] [CrossRef]
- Wu, S.; Wang, B.; Zheng, G.; Liu, S.; Dai, K.; Liu, C.; Shen, C. Preparation and characterization of macroscopically electrospun polyamide 66 nanofiber bundles. Mater. Lett. 2014, 124, 77–80. [Google Scholar] [CrossRef]
- Li, L.; Hsieh, Y.-L. Ultra-fine polyelectrolyte fibers from electrospinning of poly (acrylic acid). Polymer 2005, 46, 5133–5139. [Google Scholar] [CrossRef]
- Gao, Y.; Sang, X.; Chen, Y.; Li, Y.; Liu, B.; Sheng, J.; Feng, Y.; Li, L.; Liu, H.; Wang, X.; et al. Polydopamine modification electrospun polyacrylonitrile fibrous membrane with decreased pore size and dendrite mitigation for lithium ion battery. J. Mater. Sci. 2019, 55, 3549–3560. [Google Scholar] [CrossRef]
- Yan, J.; Huang, Y.; Miao, Y.E.; Tjiu, W.W.; Liu, T. Polydopamine-coated electrospun poly (vinyl alcohol)/poly (acrylic acid) membranes as efficient dye adsorbent with good recyclability. J. Hazard. Mater. 2015, 283, 730–739. [Google Scholar] [CrossRef]
- Huang, Y.L.; Baji, A.; Tien, H.W.; Yang, Y.K.; Yang, S.Y.; Ma, C.C.; Liu, H.Y.; Mai, Y.W.; Wang, N.H. Self-assembly of graphene onto electrospun polyamide 66 nanofibers as transparent conductive thin films. Nanotechnology 2011, 22, 475603. [Google Scholar] [CrossRef]
- Kim, B.; Park, H.; Lee, S.-H.; Sigmund, W.M. Poly (acrylic acid) nanofibers by electrospinning. Mater. Lett. 2005, 59, 829–832. [Google Scholar] [CrossRef]
- Han, W.; Zheng, G.; Liang, Y.; Dai, K.; Liu, C.; Chen, J.; Shen, C.; Peng, X.; Fu, P.; Cao, W.; et al. HDPE solution crystallization induced by electrospun PA66 nanofiber. Colloid Polym. Sci. 2011, 289, 843–848. [Google Scholar] [CrossRef]
- Poletto, P.; Duarte, J.; Thürmer, M.B.; Santos, V.d.; Zeni, M. Characterization of Polyamide 66 membranes prepared by phase inversion using formic acid and hydrochloric acid such as solvents. Mater. Res. 2011, 14, 547–551. [Google Scholar] [CrossRef]
- Chang, X.; Wang, Z.; Quan, S.; Xu, Y.; Jiang, Z.; Shao, L. Exploring the synergetic effects of graphene oxide (GO) and polyvinylpyrrodione (PVP) on poly (vinylylidenefluoride) (PVDF) ultrafiltration membrane performance. Appl. Surf. Sci. 2014, 316, 537–548. [Google Scholar] [CrossRef]
- Xiang, S.; Guo, Z.; Wang, Y.; Liu, H.; Zhang, J.; Cui, Z.; Wang, H.; Li, J. The microstructure regulation, strengthening, toughening and hydrophilicity of polyamide6 in fabricating poly (vinylidene fluoride)-based flat membrane via the thermally induced phase separation technique. Eur. Polym. J. 2020, 126, 109568. [Google Scholar] [CrossRef]
- Cui, Z.; Hassankiadeh, N.T.; Lee, S.Y.; Lee, J.M.; Woo, K.T.; Sanguineti, A.; Arcella, V.; Lee, Y.M.; Drioli, E. Poly (vinylidene fluoride) membrane preparation with an environmental diluent via thermally induced phase separation. J. Membr. Sci. 2013, 444, 223–236. [Google Scholar] [CrossRef]
- Chao, S.; Li, X.; Li, Y.; Wang, Y.; Wang, C. Preparation of polydopamine-modified zeolitic imidazolate framework-8 functionalized electrospun fibers for efficient removal of tetracycline. J. Colloid Interface Sci. 2019, 552, 506–516. [Google Scholar] [CrossRef]
- Kang, H.-K.; Shin, H.-K.; Jeun, J.-P.; Kim, H.-B.; Kang, P.-H. Fabrication and characterization of electrospun polyamide 66 fibers crosslinked by gamma irradiation. Macromol. Res. 2011, 19, 364–369. [Google Scholar] [CrossRef]
- Ekram, B.; Abd El-Hady, B.M.; El-Kady, A.M.; Amr, S.M.; Gabr, H.; Waly, A.I.; Guirguis, O.W. Enhancing the Stability, Hydrophilicity, Mechanical and Biological Properties of Electrospun Polycaprolactone in Formic Acid/Acetic Acid Solvent System. Fibers Polym. 2019, 20, 715–724. [Google Scholar] [CrossRef]
- Zhang, L.; Han, N.; Tan, L.; Qian, Y.; Cui, Z.; Cai, J. Preparation of hydrolysis of poly (acrylonitrile-co-methyl acrylate) membranes via thermally induced phase separation: Effects of hydrolysis conditions and additives. J. Appl. Polym. Sci. 2018, 135, 46380. [Google Scholar] [CrossRef]









| Parameter | Value |
|---|---|
| Positive-voltage | 29.6 KV |
| Negative-voltage | 2.4 KV |
| Spinneret to rotary collector distance | 8 cm |
| Sliding table speed | 20 mm/min |
| Collector rotation speed | 50 rpm |
| NanofiberPolymer | Modified Additive | Functionalization Method | Advantages | Application | Ref |
|---|---|---|---|---|---|
| PAA | 1,4-butanediol di-glycidyl-ether | Esterification reaction | High swelling rate, high water absorption | The diaper and napkin | [13] |
| PA66 | ________ | ________ | PA66 bundles arranged with different nanofibers | ________ | [14] |
| PAA | β-cyclodextrin | Heat-induced crosslinking | Forming ester bond to improve water resistance of PAA fiber, strongly pH-responsive swelling behaviors | ________ | [15] |
| PA66 | Graphite nanosheet (GN) | Immersed in the GN suspension | A multifunctional sensor, the excellent multifunctional response for strain, temperature and gas sensing | The detection of human motion and physiological ECG signal | [8] |
| PAN | PDA | The spin coating | Increase the stress strength, high electrolyte uptake, high ionic conductivity, wide electrochemical window | Lithium ion battery for energy storage systems | [16] |
| PVA/PAA | PDA | Thermal crosslinking + Dopamine self-polymerizing coating | Efficient adsorption performance toward methyl blue, highly flexible, easy to operate and retrieve, easy to elute and regenerate | Wastewater treatment | [17] |
| PA66 | PAA | Amide crosslinking | Contains dense layer, high water absorption, high rejection rate, high heat stability | Oily sewage treatment, cleaning alternative items | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Nie, L.; Li, G.; Wu, W.; Gao, M.; Zhu, Y.; Wu, W.; Wang, B. Flexible Multifunctional Self-Expanding Electrospun Polyacrylic Acid Covalently Cross-Linked Polyamide 66 Nanocomposite Fiber Membrane with Excellent Oil/Water Separation and High pH Stability Performances. Sustainability 2022, 14, 14097. https://doi.org/10.3390/su142114097
Yan J, Nie L, Li G, Wu W, Gao M, Zhu Y, Wu W, Wang B. Flexible Multifunctional Self-Expanding Electrospun Polyacrylic Acid Covalently Cross-Linked Polyamide 66 Nanocomposite Fiber Membrane with Excellent Oil/Water Separation and High pH Stability Performances. Sustainability. 2022; 14(21):14097. https://doi.org/10.3390/su142114097
Chicago/Turabian StyleYan, Jiangyi, Lihong Nie, Guiliang Li, Wenxin Wu, Ming Gao, Yuanlu Zhu, Weixing Wu, and Beifu Wang. 2022. "Flexible Multifunctional Self-Expanding Electrospun Polyacrylic Acid Covalently Cross-Linked Polyamide 66 Nanocomposite Fiber Membrane with Excellent Oil/Water Separation and High pH Stability Performances" Sustainability 14, no. 21: 14097. https://doi.org/10.3390/su142114097
APA StyleYan, J., Nie, L., Li, G., Wu, W., Gao, M., Zhu, Y., Wu, W., & Wang, B. (2022). Flexible Multifunctional Self-Expanding Electrospun Polyacrylic Acid Covalently Cross-Linked Polyamide 66 Nanocomposite Fiber Membrane with Excellent Oil/Water Separation and High pH Stability Performances. Sustainability, 14(21), 14097. https://doi.org/10.3390/su142114097






