The Electro-Fenton Process for Caffeine Removal from Water and Granular Activated Carbon Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Degradation of Caffeine in Water by Electro-Fenton
2.3. Adsorption of Caffeine on Granular Activated Carbon
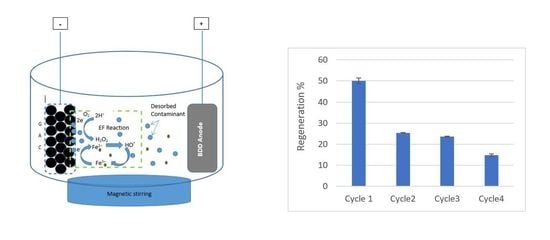
2.4. Electro-Fenton Regeneration of GAC
2.5. Analytical Methods
3. Results
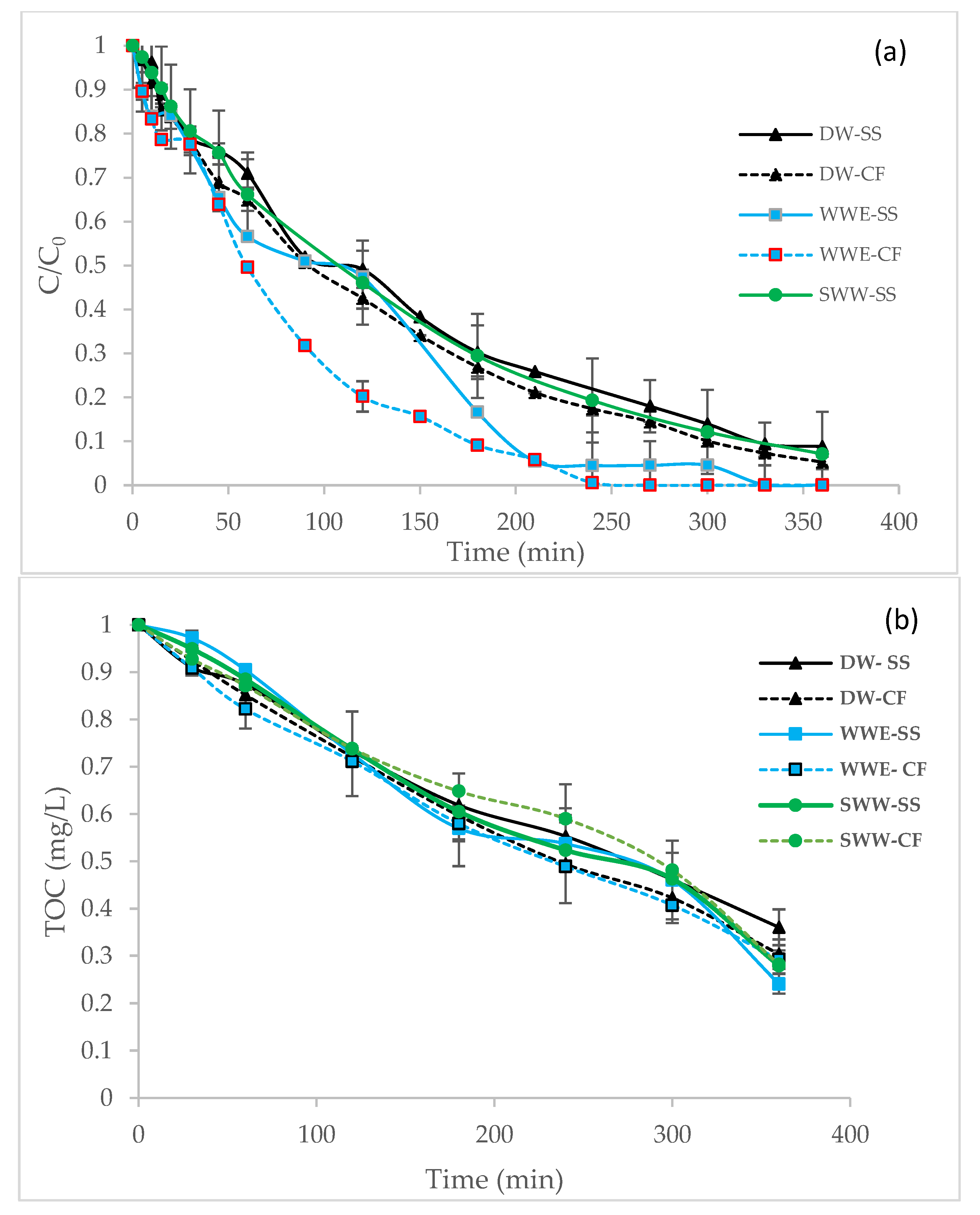
3.1. Effect of the Water Matrix and Cathode Material on Degradation and Mineralization
3.2. Adsorption of Caffeine on Granular Activated Carbon
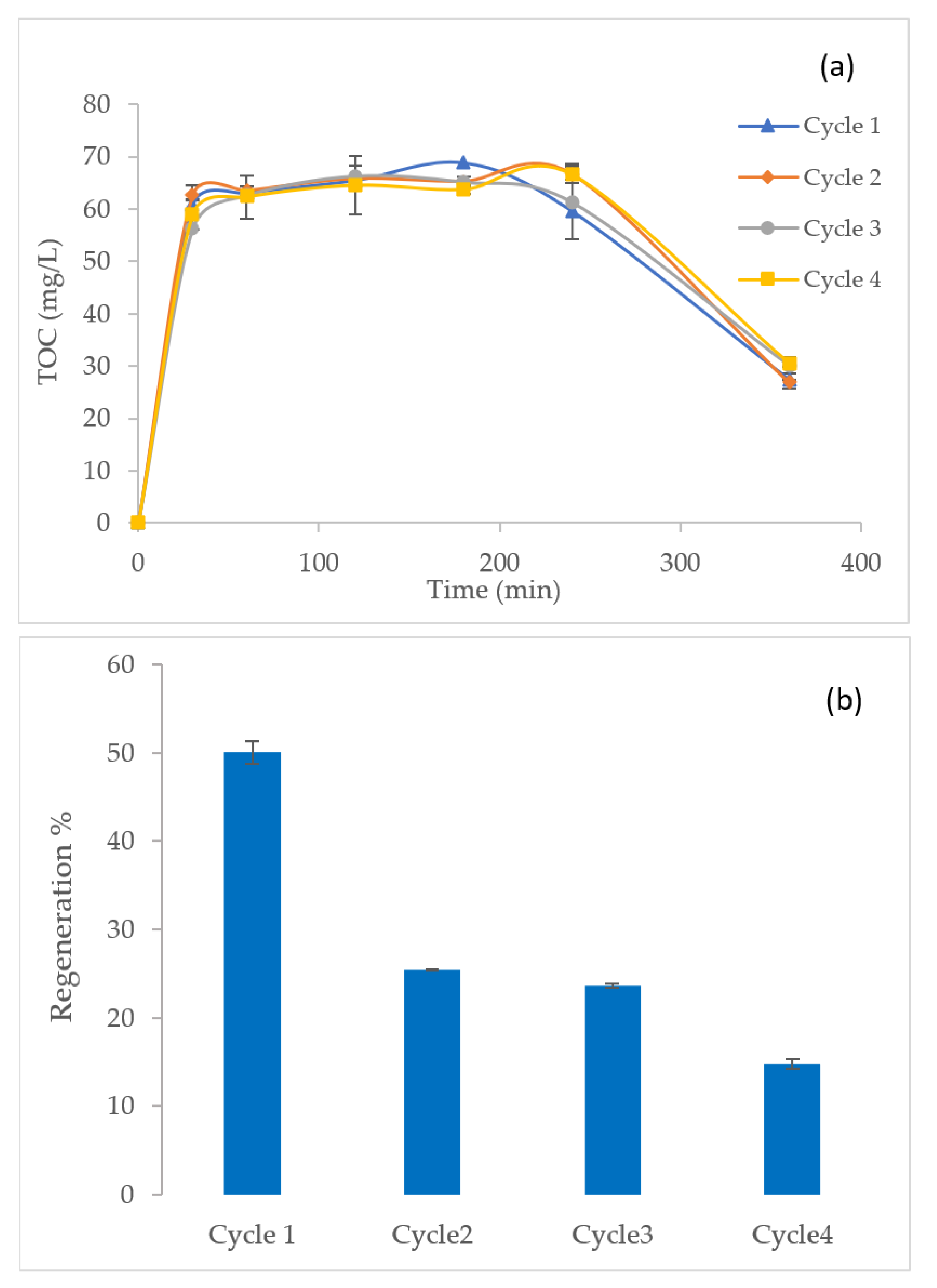
3.3. Caffeine Removal from GAC and Mineralization of Organics during EF Regeneration
3.4. Regeneration Cycles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GAC | Granular activated carbon |
| AC | Activated carbon |
| CF | Carbon felt |
| PAC | Powder activated carbon |
| eAOPs | Electrochemical advanced oxidation processes |
| HO• | Hydroxyl radicals |
| EF | Electro-Fenton |
| BDD | Boron-doped diamond |
| RE | Regeneration efficiency |
| DW | Deionized water |
| SWW | Simulated wastewater |
| WWE | Wastewater effluent |
| SS | Stainless steel |
| RP-HPLC | Reverse-phase high-performance liquid chromatography |
References
- Sotelo, J.L.; Ovejero, G.; Rodríguez, A.; Álvarez, S.; Galán, J.; García, J. Competitive adsorption studies of caffeine and diclofenac aqueous solutions by activated carbon. Chem. Eng. J. 2014, 240, 443–453. [Google Scholar] [CrossRef]
- Xiao, Y.; Hill, J.M. Benefit of Hydrophilicity for Adsorption of Methyl Orange and Electro-Fenton Regeneration of Activated Carbon-Polytetrafluoroethylene Electrodes. Environ. Sci. Technol. 2018, 52, 11760–11768. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Ngo, H.; Kim, S.; Guo, W.; Hagare, P. Adsorption and bioadsorption of granular activated carbon (GAC) for dissolved organic carbon (DOC) removal in wastewater. Bioresour. Technol. 2008, 99, 8674–8678. [Google Scholar] [CrossRef] [Green Version]
- Kosheleva, R.I.; Mitropoulos, A.C.; Kyzas, G.Z. Synthesis of activated carbon from food waste. Environ. Chem. Lett. 2019, 17, 429–438. [Google Scholar] [CrossRef]
- Skoczko, I.; Guminski, R. Research on the Development of Technologies for the Production of Granulated Activated Carbons Using Various Binders. Materials 2020, 13, 5180. [Google Scholar] [CrossRef]
- Bury, N.A.; Mumford, K.A.; Stevens, G.W. The electro-Fenton regeneration of Granular Activated Carbons: Degradation of organic contaminants and the relationship to the carbon surface. J. Hazard. Mater. 2021, 416, 125792. [Google Scholar] [CrossRef]
- Costa, L.R.D.C.; Ribeiro, L.D.M.; Hidalgo, G.E.N.; Féris, L.A. Evaluation of efficiency and capacity of thermal, chemical and ultrasonic regeneration of tetracycline exhausted activated carbon. Environ. Technol. 2020, 43, 907–917. [Google Scholar] [CrossRef]
- Clifford, D.; Chu, P.; Lau, A. Thermal regeneration of powdered activated carbon (pac) and pac-biological sludge mixtures. Water Res. 1983, 17, 1125–1138. [Google Scholar] [CrossRef]
- Sun, B.; Ma, J.; Sedlak, D.L. Chemisorption of Perfluorooctanoic Acid on Powdered Activated Carbon Initiated by Persulfate in Aqueous Solution. Environ. Sci. Technol. 2016, 50, 7618–7624. [Google Scholar] [CrossRef]
- Cho, J.-H.; Kim, Y.-S.; Jeon, S.-B.; Seo, J.-B.; Jung, J.-H.; Oh, K.-J. Improvement of thermal regeneration of spent granular activated carbon using air agent: Application of sintering and deoxygenation. Korean J. Chem. Eng. 2014, 31, 1641–1650. [Google Scholar] [CrossRef]
- Alvarez, P.M. Comparison between Thermal and Ozone Regenerations of Spent Activated Carbon Exhausted with Phenol—ScienceDirect. 2004. Available online: https://www-sciencedirect-com.kuleuven.e-bronnen.be/science/article/pii/S0043135404000533 (accessed on 21 September 2021).
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Mousset, E.; Oturan, N.; Oturan, M.A. An unprecedented route of OH radical reactivity evidenced by an electrocatalytical process: Ipso-substitution with perhalogenocarbon compounds. Appl. Catal. B Environ. 2018, 226, 135–146. [Google Scholar] [CrossRef]
- Coria, G.; Sirés, I.; Brillas, E.; Nava, J.L. Influence of the anode material on the degradation of naproxen by Fenton-based electrochemical processes. Chem. Eng. J. 2016, 304, 817–825. [Google Scholar] [CrossRef]
- Guo, H.; Xu, Z.; Wang, D.; Chen, S.; Qiao, D.; Xu, H.; Yan, W.; Jin, X. Evaluation of diclofenac degradation effect in “active” and “non-active” anodes: A new consideration about mineralization inclination. Chemosphere 2021, 286, 131580. [Google Scholar] [CrossRef] [PubMed]
- Dirany, A.; Aaron, S.E.; Oturan, N.; Sirés, I.; Oturan, M.A.; Aaron, J.J. Study of the toxicity of sulfamethoxazole and its degradation products in water by a bioluminescence method during application of the electro-Fenton treatment. Anal. Bioanal. Chem. 2011, 400, 353–360. [Google Scholar] [CrossRef]
- Nidheesh, P.; Gandhimathi, R. Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination 2012, 299, 989–993. [Google Scholar] [CrossRef]
- Bañuelos, J.A.; Garcia-Rodriguez, O.; Valadez, F.J.R.; Manriquez, J.M.; Bustos, E.; Rodríguez, A.; Godínez, L.A. Cathodic polarization effect on the electro-Fenton regeneration of activated carbon. J. Appl. Electrochem. 2015, 45, 523–531. [Google Scholar] [CrossRef]
- Bañuelos, J.A.; El-Ghenymy, A.; Rodríguez, F.; Manríquez, J.; Bustos, E.; Rodríguez, A.; Brillas, E.; Godínez, L.A. Study of an Air Diffusion Activated Carbon Packed Electrode for an Electro-Fenton Wastewater Treatment. Electrochimica Acta 2014, 140, 412–418. [Google Scholar] [CrossRef]
- Zhou, W.; Meng, X.; Ding, Y.; Rajic, L.; Gao, J.; Qin, Y.; Alshawabkeh, A.N. “Self-cleaning” electrochemical regeneration of dye-loaded activated carbon. Electrochem. Commun. 2019, 100, 85–89. [Google Scholar] [CrossRef]
- Bañuelos, J.A.; Rodríguez, F.J.; Rocha, J.M.; Bustos, E.; Rodríguez, A.; Cruz, J.C.; Arriaga, L.G.; Godínez, L.A. Novel Electro-Fenton Approach for Regeneration of Activated Carbon. Environ. Sci. Technol. 2013, 47, 7927–7933. [Google Scholar] [CrossRef]
- Álvarez-Torrellas, S.; Rodríguez, A.; Ovejero, G.; Gómez, J.M.; García, J. Removal of caffeine from pharmaceutical wastewater by adsorption: Influence of NOM, textural and chemical properties of the adsorbent. Environ. Technol. 2015, 37, 1618–1630. Available online: http://www.tandfonline.com/doi/abs/10.1080/09593330.2015.1122666 (accessed on 13 October 2022). [CrossRef] [PubMed]
- Kim, S.-H.; Tanaka, M.; Lee, M.-H.; Watanabe, T. Enhanced decomposition of caffeine by water plasma combined with mist generator: Effect of operational parameter and decomposition pathway. Chemosphere 2022, 307, 136056. [Google Scholar] [CrossRef] [PubMed]
- Ganzenko, O.; Oturan, N.; Huguenot, D.; van Hullebusch, E.D.; Esposito, G.; Oturan, M.A. Removal of psychoactive pharmaceutical caffeine from water by electro-Fenton process using BDD anode: Effects of operating parameters on removal efficiency. Sep. Purif. Technol. 2015, 156, 987–995. [Google Scholar] [CrossRef]
- Sopaj, F.; Oturan, N.; Pinson, J.; Podvorica, F.I.; Oturan, M.A. Effect of cathode material on electro-Fenton process efficiency for electrocatalytic mineralization of the antibiotic sulfamethazine. Chem. Eng. J. 2020, 384, 123249. [Google Scholar] [CrossRef]
- Zhou, M.; Oturan, M.A.; Sirés, I. (Eds.) Electro-Fenton Process: New Trends and Scale-Up; Springer: Singapore, 2018; Volume 61. [Google Scholar] [CrossRef]
- Feng, L.; Serna-Galvis, E.A.; Oturan, N.; Giannakis, S.; Torres-Palma, R.A.; Oturan, M.A. Evaluation of process influencing factors, degradation products, toxicity evolution and matrix-related effects during electro-Fenton removal of piroxicam from waters. J. Environ. Chem. Eng. 2019, 7, 103400. [Google Scholar] [CrossRef]
- Margot, J.; Kienle, C.; Magnet, A.; Weil, M.; Rossi, L.; de Alencastro, L.F.; Abegglen, C.; Thonney, D.; Chèvre, N.; Schärer, M.; et al. Treatment of micropollutants in municipal wastewater: Ozone or powdered activated carbon? Sci. Total Environ. 2013, 461–462, 480–498. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Rodríguez, M.; Bello-Mendoza, R.; Hernández-Ramírez, A.; Ruiz-Ruiz, E.J. Degradation of anti-inflammatory drugs in municipal wastewater by heterogeneous photocatalysis and electro-Fenton process. Environ. Technol. 2019, 40, 2436–2445. [Google Scholar] [CrossRef]
- Guenfoud, F.; Mokhtari, M.; Akrout, H. Electrochemical degradation of malachite green with BDD electrodes: Effect of electrochemical parameters. Diam. Relat. Mater. 2014, 46, 8–14. [Google Scholar] [CrossRef]
- Do, J.-S.; Chen, C. In Situ Oxidative Degradation of Formaldehyde with Electrogenerated Hydrogen Peroxide. J. Electrochem. Soc. 1993, 140, 1632–1637. [Google Scholar] [CrossRef]
- Scialdone, O.; Galia, A.; Gattuso, C.; Sabatino, S.; Schiavo, B. Effect of air pressure on the electro-generation of H2O2 and the abatement of organic pollutants in water by electro-Fenton process. Electrochimica Acta 2015, 182, 775–780. [Google Scholar] [CrossRef]
- Ganzenko, O.; Trellu, C.; Oturan, N.; Huguenot, D.; Péchaud, Y.; van Hullebusch, E.D.; Oturan, M.A. Electro-Fenton treatment of a complex pharmaceutical mixture: Mineralization efficiency and biodegradability enhancement. Chemosphere 2020, 253, 126659. [Google Scholar] [CrossRef] [PubMed]
- Couto, O.M.; Matos, I.; da Fonseca, I.M.; Arroyo, P.A.; da Silva, E.A.; de Barros, M.A.S.D. Effect of solution pH and influence of water hardness on caffeine adsorption onto activated carbons. Can. J. Chem. Eng. 2015, 93, 68–77. [Google Scholar] [CrossRef]
- Trellu, C.; Gibert-Vilas, M.; Pechaud, Y.; Oturan, N.; Oturan, M.A. Clofibric acid removal at activated carbon fibers by adsorption and electro-Fenton regeneration—Modeling and limiting phenomena. Electrochimica Acta 2021, 382, 138283. [Google Scholar] [CrossRef]
- Galhetas, M.; Mestre, A.S.; Pinto, M.L.; Gulyurtlu, I.; Lopes, H.; Carvalho, A.P. Chars from gasification of coal and pine activated with K2CO3: Acetaminophen and caffeine adsorption from aqueous solutions. J. Colloid Interface Sci. 2014, 433, 94–103. [Google Scholar] [CrossRef] [Green Version]
- McQuillan, R.V.; Stevens, G.W.; Mumford, K.A. The electrochemical regeneration of granular activated carbons: A review. J. Hazard. Mater. 2018, 355, 34–49. [Google Scholar] [CrossRef]

| DW-SS | DW-CF | WWE-SS | WWE-CF | SWW-SS | SWW-CF | ||
|---|---|---|---|---|---|---|---|
| Degradation | K1 (min−1) | 6.7 × 10−3 | 7.8 × 10−3 | 10.1 × 10−3 | 13.6 × 10−3 | 7.1 × 10−3 | - |
| R2 Removal % | 0.996 91 | 0.995 95 | 0.990 100 | 0.990 100 | 0.998 93 | - - | |
| Mineralization | K0 (mg/L.min) | 1.7 × 10−3 | 1.9 × 10−3 | 2.0 × 10−3 | 1.9 × 10−3 | 1.9 × 10−3 | 1.7 × 10−3 |
| R2 | 0.990 | 0.995 | 0.975 | 0.990 | 0.988 | 0.990 | |
| Mineralization % | 64 | 70 | 72 | 76 | 71 | - |
| Electrodes Process Matrix | Experimental Parameters | C0 | Removal % | Removal Rate | Reference |
|---|---|---|---|---|---|
| BDD anode/ CF cathode EF PW | Fe2+ = 0.2 mM; Na2SO4 = 0.05 M V = 0.2 L pH = 3; I = 300 mA | 0.1 mM | 93 | 2.48 × 10−9 M−1 S−1 | [24] |
| BDD anode CF cathode EF Mixture of pharmaceuticals | Fe2+ = 0.2 mM; Na2SO4 = 0.05 M V = 0.2 L pH = 3; I = 400 mA | 0.1 mM | - | 1.47 × 10−10 M−1 S−1 * | [33] |
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| b (L/mg) | qmax (mg/g) | R2 | Kf | R2 | n |
| 14.667 | 227.273 | 0.991 | 0.378 | 0.385 | 1.694 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadi, N.; Boelee, N.C.; Dewil, R. The Electro-Fenton Process for Caffeine Removal from Water and Granular Activated Carbon Regeneration. Sustainability 2022, 14, 14313. https://doi.org/10.3390/su142114313
Gadi N, Boelee NC, Dewil R. The Electro-Fenton Process for Caffeine Removal from Water and Granular Activated Carbon Regeneration. Sustainability. 2022; 14(21):14313. https://doi.org/10.3390/su142114313
Chicago/Turabian StyleGadi, Nadia, Nadine C. Boelee, and Raf Dewil. 2022. "The Electro-Fenton Process for Caffeine Removal from Water and Granular Activated Carbon Regeneration" Sustainability 14, no. 21: 14313. https://doi.org/10.3390/su142114313