Review of Adsorption Studies for Contaminant Removal from Wastewater Using Molecular Simulation
Abstract
:1. Introduction
2. Molecular Simulation and Application in Adsorption Study
2.1. Carbon-Based
2.2. Oxides and Hydroxides
2.3. Zeolites
2.4. Metal–Organic Framework
2.5. Clay
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bayantong, A.R.B.; Shih, Y.J.; Ong, D.C.; Abarca, R.R.M.; Dong, C.D.; de Luna, M.D.G. Adsorptive removal of dye in wastewater by metal ferrite-enabled graphene oxide nanocomposites. Chemosphere 2021, 274, 129518. [Google Scholar] [CrossRef] [PubMed]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Badeenezhad, A.; Azhdarpoor, A.; Bahrami, S.; Yousefinejad, S. Removal of methylene blue dye from aqueous solutions by natural clinoptilolite and clinoptilolite modified by iron oxide nanoparticles. Mol. Simul. 2019, 45, 564–571. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, S.; Tang, M.; Jing, R.; Shao, Y.; Liu, X.; Dong, Y.; Li, M.; Liao, Q.; Lv, G.; et al. Adsorption of sulfamethoxazole and tetracycline on montmorillonite in single and binary systems. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 575, 264–270. [Google Scholar] [CrossRef]
- Ba Mohammed, B.; Yamni, K.; Tijani, N.; Alrashdi, A.A.; Zouihri, H.; Dehmani, Y.; Chung, I.M.; Kim, S.H.; Lgaz, H. Adsorptive removal of phenol using faujasite-type Y zeolite: Adsorption isotherms, kinetics and grand canonical Monte Carlo simulation studies. J. Mol. Liq. 2019, 296, 111997. [Google Scholar] [CrossRef]
- Hemavathy, R.V.; Saravanan, A.; Kumar, P.S.; Vo, D.V.N.; Karishma, S.; Jeevanantham, S. Adsorptive removal of Pb(II) ions onto surface modified adsorbents derived from Cassia fistula seeds: Optimization and modelling study. Chemosphere 2021, 283, 131276. [Google Scholar] [CrossRef]
- Kuhlman, T.; Farrington, J. What is sustainability? Sustainability 2010, 2, 3436–3448. [Google Scholar] [CrossRef] [Green Version]
- Kumari, P.; Alam, M.; Siddiqi, W.A. Usage of nanoparticles as adsorbents for waste water treatment: An emerging trend. Sustain. Mater. Technol. 2019, 22, e00128. [Google Scholar] [CrossRef]
- Walker, G.M.; Weatherley, L.R. Textile wastewater treatment using granular activated carbon adsorption in fixed beds. Sep. Sci. Technol. 2000, 35, 1329–1341. [Google Scholar] [CrossRef]
- Paliulis, D. Removal of formaldehyde from synthetic wastewater using natural and modified zeolites. Pol. J. Environ. Stud. 2016, 25, 251–257. [Google Scholar] [CrossRef]
- Lin, S.H.; Juang, R.S. Adsorption of phenol and its derivatives from water using synthetic resins and low-cost natural adsorbents: A review. J. Environ. Manage. 2009, 90, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- McKay, G.; Ramprasad, G.; Pratapa Mowli, P. Equilibrium studies for the adsorption of dyestuffs from aqueous solutions by low-cost materials. Water Air Soil Pollut. 1986, 29, 273–283. [Google Scholar] [CrossRef]
- Pérez Jiménez, V.A.; Hernández-Montoya, V.; Ramírez-Montoya, L.A.; Castillo-Borja, F.; Tovar-Gómez, R.; Montes-Morán, M.A. Adsorption of impurities from nickel-plating baths using commercial sorbents to reduce wastewater discharges. J. Environ. Manag. 2021, 284, 112024. [Google Scholar] [CrossRef] [PubMed]
- Wanyonyi, F.S.; Fidelis, T.T.; Mutua, G.K.; Orata, F.; Pembere, A.M.S. Role of pore chemistry and topology in the heavy metal sorption by zeolites: From molecular simulation to machine learning. Comput. Mater. Sci. 2021, 195, 110519. [Google Scholar] [CrossRef]
- Asif, K.; Lock, S.S.M.; Taqvi, S.A.A.; Jusoh, N.; Yiin, C.L.; Chin, B.L.F. A molecular simulation study on amine-functionalized silica/polysulfone mixed matrix membrane for mixed gas separation. Chemosphere 2023, 311, 136936. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, X.; Shi, C.; Yan, B.; Gong, L.; Chen, J.; Xiang, L.; Xu, H.; Liu, Q.; Zeng, H. Unraveling the molecular interaction mechanism between graphene oxide and aromatic organic compounds with implications on wastewater treatment. Chem. Eng. J. 2019, 358, 842–849. [Google Scholar] [CrossRef]
- Delgadillo-Velasco, L.; Hernández-Montoya, V.; Rangel-Vázquez, N.A.; Cervantes, F.J.; Montes-Morán, M.A.; del Rosario Moreno-Virgen, M. Screening of commercial sorbents for the removal of phosphates from water and modeling by molecular simulation. J. Mol. Liq. 2018, 262, 443–450. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Taha, M.; Abdel-Gawad, H.; Mahdy, F.; Hegazi, B. Zeolitic imidazolate frameworks: Experimental and molecular simulation studies for efficient capture of pesticides from wastewater. J. Environ. Chem. Eng. 2019, 7, 103499. [Google Scholar] [CrossRef]
- Chang, S.; Zhang, Q.; Lu, Y.; Wu, S.; Wang, W. High-efficiency and selective adsorption of organic pollutants by magnetic CoFe2O4/graphene oxide adsorbents: Experimental and molecular dynamics simulation study. Sep. Purif. Technol. 2020, 238, 116400. [Google Scholar] [CrossRef]
- Meng, Z.; Wu, M.; Yu, Y.; Meng, F.; Liu, A.; Komarneni, S.; Zhang, Q. Selective removal of methyl orange and Cr anionic contaminants from mixed wastewater by in-situ formation of Zn-Al layered double hydroxides. Appl. Clay Sci. 2018, 161, 1–5. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Zhang, Z.; Wang, S. Investigation of the interaction between xanthate and kaolinite based on experiments, molecular dynamics simulation, and density functional theory. J. Mol. Liq. 2021, 336, 116298. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, Y.; Wu, L. Influence of electrostatic field on the adsorption of phenol on single-walled carbon nanotubes: A study by molecular dynamics simulation. Chem. Eng. J. 2019, 363, 278–284. [Google Scholar] [CrossRef]
- Pelalak, R.; Soltani, R.; Heidari, Z.; Malekshah, R.E.; Aallaei, M.; Marjani, A.; Rezakazemi, M.; Kurniawan, T.A.; Shirazian, S. Molecular dynamics simulation of novel diamino-functionalized hollow mesosilica spheres for adsorption of dyes from synthetic wastewater. J. Mol. Liq. 2021, 322, 114812. [Google Scholar] [CrossRef]
- Khanmohammadi, H.; Bayati, B.; Rahbar Shahrouzi, J.; Babaluo, A.A.; Ghorbani, A. Molecular simulation of the ion exchange behavior of Cu2+, Cd2+ and Pb2+ ions on different zeolites exchanged with sodium. J. Environ. Chem. Eng. 2019, 7, 103040. [Google Scholar] [CrossRef]
- Pelalak, R.; Soltani, R.; Heidari, Z.; Malekshah, R.E.; Aallaei, M.; Marjani, A.; Rezakazemi, M.; Shirazian, S. Synthesis, molecular dynamics simulation and adsorption study of different pollutants on functionalized mesosilica. Sci. Rep. 2021, 11, 1967. [Google Scholar] [CrossRef]
- Liu, X.; Tu, Y.; Liu, S.; Liu, K.; Zhang, L.; Li, G.; Xu, Z. Adsorption of ammonia nitrogen and phenol onto the lignite surface: An experimental and molecular dynamics simulation study. J. Hazard. Mater. 2021, 416, 125966. [Google Scholar] [CrossRef]
- Tuzen, M.; Sarı, A.; Afshar Mogaddam, M.R.; Kaya, S.; Katin, K.P.; Altunay, N. Synthesis of carbon modified with polymer of diethylenetriamine and trimesoyl chloride for the dual removal of Hg (II) and methyl mercury ([CH3Hg]+) from wastewater: Theoretical and experimental analyses. Mater. Chem. Phys. 2022, 277, 125501. [Google Scholar] [CrossRef]
- Hossain, S.; Rahman, M.S.; Dhar, L.; Quraishi, S.B.; Abser, M.N.; Rahman, F.; Rahman, M.T. Increasing the potentiality of graphene oxide by chloroacetic acid for the adsorption of lead with molecular dynamic interpretation. Curr. Res. Green Sustain. Chem. 2021, 4, 100095. [Google Scholar] [CrossRef]
- Dryaz, A.R.; Shaban, M.; AlMohamadi, H.; Al-Ola, K.A.A.; Hamd, A.; Soliman, N.K.; Ahmed, S.A. Design, characterization, and adsorption properties of Padina gymnospora/zeolite nanocomposite for Congo red dye removal from wastewater. Sci. Rep. 2021, 11, 21058. [Google Scholar] [CrossRef]
- Taylor, P.; Siepmann, J.I. Configurational bias Monte Carlo: A new sampling scheme for flexible chains. Mol. Phys. 2013, 75, 37–41. [Google Scholar]
- Metropolis, N.; Rosenbluth, A.W.; Rosenbluth, M.N.; Teller, A.H.; Teller, E. Equation of state calculations by fast computing machines. J. Chem. Physics 1953, 21, 1087–1092. [Google Scholar] [CrossRef]
- Tao, Y.R.; Zhang, G.H.; Xu, H.J. Grand canonical Monte Carlo (GCMC) study on adsorption performance of metal organic frameworks (MOFs) for carbon capture. Sustain. Mater. Technol. 2022, 32, e00383. [Google Scholar] [CrossRef]
- Levy, M. Universal variational functionals of electron densities, first-order density matrices, and natural spin-orbitals and solution of the v-representability problem. Proc. Natl. Acad. Sci. USA 1979, 76, 6062–6065. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Zhou, J.; Li, D.; Ao, Z.; An, T.; Wang, G. Density functional theory study on the enhanced adsorption mechanism of gaseous pollutants on Al-doped Ti2CO2 monolayer. Sustain. Mater. Technol. 2021, 29, e00294. [Google Scholar] [CrossRef]
- Yee, C.Y.; Lim, L.G.; Lock, S.S.M.; Jusoh, N.; Yiin, C.L.; Chin, B.L.F.; Chan, Y.H.; Loy, A.C.M.; Mubashir, M. A systematic review of the molecular simulation of hybrid membranes for performance enhancements and contaminant removals. Chemosphere 2022, 307, 135844. [Google Scholar] [CrossRef]
- Huang, B.; Zhao, R.; Xu, H.; Deng, J.; Li, W.; Wang, J.; Yang, H.; Zhang, L. Adsorption of Methylene Blue on Bituminous Coal: Adsorption Mechanism and Molecular Simulation. ACS Omega 2019, 4, 14032–14039. [Google Scholar] [CrossRef] [Green Version]
- Galdino, A.L.; Oliveira, J.C.A.; Magalhaes, M.L.; Lucena, S.M.P. Prediction of the phenol removal capacity from water by adsorption on activated carbon. Water Sci. Technol. 2021, 84, 135–143. [Google Scholar] [CrossRef]
- Han, Y.; Wang, N.; Guo, X.; Jiao, T.; Ding, H. Influence of ultrasound on the adsorption of single-walled carbon nanotubes to phenol: A study by molecular dynamics simulation and experiment. Chem. Eng. J. 2022, 427, 131819. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, Y. Molecular simulation of adsorption and its implications to protein chromatography: A review. Biochem. Eng. J. 2010, 48, 408–415. [Google Scholar] [CrossRef]
- Li, L.; He, M.; Feng, Y.; Wei, H.; You, X.; Yu, H.; Wang, Q.; Wang, J.X. Adsorption of xanthate from aqueous solution by multilayer graphene oxide: An experimental and molecular dynamics simulation study. Adv. Compos. Hybrid. Mater. 2021, 4, 725–732. [Google Scholar] [CrossRef]
- Theiss, F.L.; Ayoko, G.A.; Frost, R.L. Removal of boron species by layered double hydroxides: A review. J. Colloid Interface Sci. 2013, 402, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Dhar, L.; Hossain, S.; Rahman, M.S.; Quraishi, S.B.; Saha, K.; Rahman, F.; Rahman, M.T. Adsorption Mechanism of Methylene Blue by Graphene Oxide-Shielded Mg-Al-Layered Double Hydroxide from Synthetic Wastewater. J. Phys. Chem. A 2021, 125, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Malekshah, R.E.; Heidari, Z.; Pelalak, R.; Marjani, A.; Shirazian, S. Molecular dynamic simulations and quantum chemical calculations of adsorption process using amino-functionalized silica. J. Mol. Liq. 2021, 330, 115544. [Google Scholar] [CrossRef]
- Ma, Y.; Chew, J.W. Investigation of membrane fouling phenomenon using molecular dynamics simulations: A review. J. Membr. Sci. 2022, 661, 120874. [Google Scholar] [CrossRef]
- Jiang, N.; Erdős, M.; Moultos, O.A.; Shang, R.; Vlugt, T.J.H.; Heijman, S.G.J.; Rietveld, L.C. The adsorption mechanisms of organic micropollutants on high-silica zeolites causing S-shaped adsorption isotherms: An experimental and Monte Carlo simulation study. Chem. Eng. J. 2020, 389, 123968. [Google Scholar] [CrossRef]
- Capa-Cobos, L.F.; Jaramillo-Fierro, X.; González, S. Computational study of the adsorption of phosphates as wastewater pollutant molecules on faujasites. Processes 2021, 9, 1821. [Google Scholar] [CrossRef]
- Damjanović, L.; Rakić, V.; Rac, V.; Stošić, D.; Auroux, A. The investigation of phenol removal from aqueous solutions by zeolites as solid adsorbents. J. Hazard. Mater. 2010, 184, 477–484. [Google Scholar] [CrossRef]
- Gao, G.; Xing, Y.; Liu, T.; Wang, J.; Hou, X. UiO-66 (Zr) as sorbent for porous membrane protected micro-solid-phase extraction androgens and progestogens in environmental water samples coupled with LC-MS/MS analysis: The application of experimental and molecular simulation method. Microchem. J. 2019, 146, 126–133. [Google Scholar] [CrossRef]
- Bigdeli, A.; Khorasheh, F.; Tourani, S.; Khoshgard, A.; Bidaroni, H.H. Molecular Simulation Study of the Adsorption and Diffusion Properties of Terephthalic Acid in Various Metal Organic Frameworks. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1643–1652. [Google Scholar] [CrossRef]
- Dadashi Firouzjaei, M.; Akbari Afkhami, F.; Rabbani Esfahani, M.; Turner, C.H.; Nejati, S. Experimental and molecular dynamics study on dye removal from water by a graphene oxide-copper-metal organic framework nanocomposite. J. Water Process Eng. 2020, 34, 101180. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Taha, M.; Abdel-Gawad, H.; Hegazi, B. Amino-functionalized Al-MIL-53 for dimethoate pesticide removal from wastewater and their intermolecular interactions. J. Mol. Liq. 2021, 327, 114852. [Google Scholar] [CrossRef]
- Hong, T.Z.X.; Dahanayaka, M.; Liu, B.; Law, A.W.K.; Zhou, K. Zeolitic imidazolate frameworks as capacitive deionization electrodes for water desalination and Cr(VI) adsorption: A molecular simulation study. Appl. Surf. Sci. 2021, 546, 149080. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, S.; Hao, G.; Hu, Y.; Wu, F.; Jiang, W. Fabrication of thermoresponsive metal–organic nanotube sponge and its application on the adsorption of endocrine-disrupting compounds and pharmaceuticals/personal care products: Experiment and molecular simulation study. Environ. Pollut. 2021, 273, 116466. [Google Scholar] [CrossRef]
- Li, X.; Wan, J.; Wang, Y.; Ding, S.; Sun, J. Improvement of selective catalytic oxidation capacity of phthalates from surface molecular-imprinted catalysis materials: Design, mechanism, and application. Chem. Eng. J. 2021, 413, 127406. [Google Scholar] [CrossRef]
- Bergaoui, M.; Nakhli, A.; Benguerba, Y.; Khalfaoui, M.; Erto, A.; Soetaredjo, F.E.; Ismadji, S.; Ernst, B. Novel insights into the adsorption mechanism of methylene blue onto organo-bentonite: Adsorption isotherms modeling and molecular simulation. J. Mol. Liq. 2018, 272, 697–707. [Google Scholar] [CrossRef]
- Li, P.; Khan, M.A.; Xia, M.; Lei, W.; Zhu, S.; Wang, F. Efficient preparation and molecular dynamic (MD) simulations of Gemini surfactant modified layered montmorillonite to potentially remove emerging organic contaminants from wastewater. Ceram. Int. 2019, 45, 10782–10791. [Google Scholar] [CrossRef]
- Chang, P.H.; Liu, P.; Sarkar, B.; Mukhopadhyay, R.; Yang, Q.Y.; Tzou, Y.M.; Zhong, B.; Li, X.; Owens, G. Unravelling the mechanism of amitriptyline removal from water by natural montmorillonite through batch adsorption, molecular simulation and adsorbent characterization studies. J. Colloid Interface Sci. 2021, 598, 379–387. [Google Scholar] [CrossRef]
- Hounfodji, J.W.; Kanhounnon, W.G.; Kpotin, G.; Atohoun, G.S.; Lainé, J.; Foucaud, Y.; Badawi, M. Molecular insights on the adsorption of some pharmaceutical residues from wastewater on kaolinite surfaces. Chem. Eng. J. 2021, 407, 127176. [Google Scholar] [CrossRef]
- Ouachtak, H.; El, R.; El, A.; Haounati, R.; Amaterz, E.; Ait, A.; Akbal, F.; Labd, M. Experimental and molecular dynamics simulation study on the adsorption of Rhodamine B dye on magnetic montmorillonite composite γ-Fe2O3@Mt. J. Mol. Liq. 2020, 309, 113142. [Google Scholar] [CrossRef]
- Ouachtak, H.; El Guerdaoui, A.; Haounati, R.; Akhouairi, S.; El Haouti, R.; Hafid, N.; Ait Addi, A.; Šljukić, B.; Santos, D.M.F.; Taha, M.L. Highly efficient and fast batch adsorption of orange G dye from polluted water using superb organo-montmorillonite: Experimental study and molecular dynamics investigation. J. Mol. Liq. 2021, 335, 116560. [Google Scholar] [CrossRef]



| Adsorbent | Study Domain | Simulation Environment Operating Conditions | Surface Area (m2/g) | Simulation Result | Adsorption Capacity (mg/g) | References |
|---|---|---|---|---|---|---|
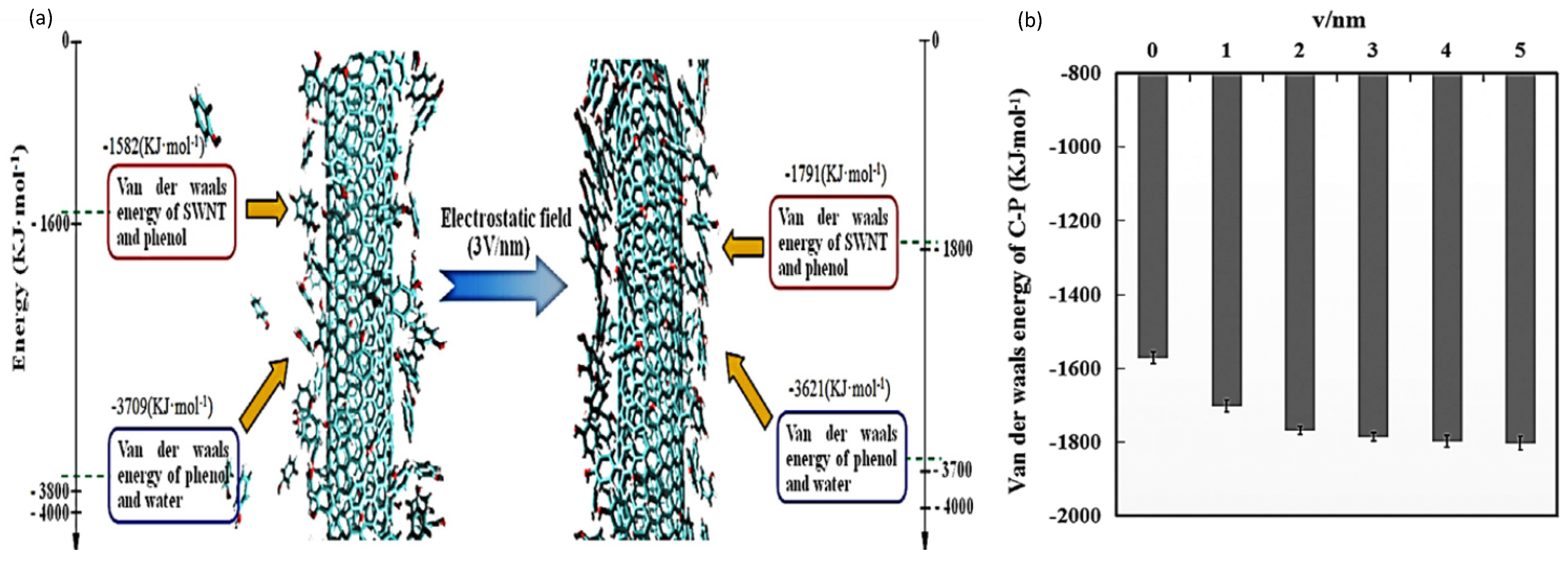
| Single-walled carbon nanotubes | A molecular dynamics simulation study for adsorption of phenol on single-walled carbon nanotubes under the influence of electrostatic field. |
| NA | The electrostatic field strengths and the value of Van der Waal energies in kJ/mol were:
| NA | Zhang et al. (2019) [22] |
| Bituminous Coal | Adsorption mechanism and molecular simulation for methylene blue (MB) adsorption on Bituminous Coal. | 298 K. | NA |
| NA | Huang et al. (2019) [36] |
| Activated carbon | Estimation of the phenol adsorption on activated carbon by pore methodology. | 301 K | NA |
| Max. adsorption capacity;
| Galdino et al. (2021) [37] |
| Bituminous activated carbon (BAC), bone char (BC), iron modified activated carbon (FeC), coconut shell activated carbon (CC), manganese modified zeolite (KL), natural zeolite (NZ) and silica (S) | Removal of impurities from nickel-plating baths by adsorption on commercial sorbents to reduce wastewater discharges. |
| NA |
| The adsorption capacity of Zn2+:
| Jiménez et al. (2021) [13]. |
| Lignite (Coal) | To understand adsorption phenomenon of ammonia nitrogen and phenol onto lignite surface using experimental and molecular dynamics simulation study. | 298 K | 7.102 | Adsorption energies in kcal/mol were:
| NA | Liu et al. (2021) [26]. |
| Single-walled carbon | A study by molecular dynamics simulation and experiment for influence of ultrasound on the adsorption of single-walled carbon nanotubes to phenol | 300 K | NA | For ultrasound with frequencies, the values of Van der Waals energies of SWNTs-phenol in kJ/mol were:
| NA | Han et al. (2022) [38]. |
| Activated carbon (AC) with diethylenetriamine (DETA)-trimesoyl chloride (TMC) copolymer | Theoretical and experimental analyses for synthesis of carbon modified with polymer of diethylenetriamine and trimesoyl chloride for the dual removal of Hg (II) and methyl mercury ([CH3Hg]+) from wastewater. | NA | NA | Calculated adsorption energies (Ea) in kcal/mol were:
| The adsorption capacity of developed composite:
| Tuzen et al. (2022) [27] |
| Adsorbent | Study Domain | Simulation Environment Operating Conditions | Surface Area (m2/g) | Simulation Result | Adsorption Capacity (mg/g) | References |
|---|---|---|---|---|---|---|
| Activated carbon, bone-char, catalytic carbon, natural-silica, natural-zeolite, manganese(II) oxide-composite, iron(III)hydroxide | A molecular simulation modeling for screening of different commercial sorbents for the removal of phosphates from water. |
|
| Gibbs free energy in kcal/mol was:
| Fe(OH)3 had the higher adsorption capacity of:
| Delgadillo-Velasco et al. (2018) [17]. |
| 0Zn-Al layered double hydroxides (ZnAl-LDH) | Adsorption of methyl orange and Cr anionic contaminants (present in wastewater) by in situ formation of Zn-Al layered double hydroxides. |
| NA | The binding energies in eV were:
| NA | Meng et al. (2018) [20]. |
| Graphene oxide (GO) | Understanding the molecular interlinkage between graphene oxide and aromatic organic compounds with implications on wastewater treatment. |
| NA | Adsorption energies Ead in kcal mol−1 were:
| NA | Zhang et al. (2019) [16]. |
| Magnetic CoFe2O4/graphene oxide | Experimental and molecular dynamics simulation study for high-efficiency and selective adsorption of organic pollutants on magnetic CoFe2O4/graphene oxides. | (298 K). | NA | Adsorption energies in kcal/mol were:
| The maximum adsorption capacity from Langmuir equation were:
| Chang et al. (2020) [19]. |
| MFe2O4@GO | Removal of dye by metal ferrite-enabled graphene oxide nanocomposites using adsorption. |
| NA | (1) The enthalpy energies of the adsorption reaction in kcal/mol were:
| The adsorption capacity of varying GO nanocomposites were:
| Bayantong et al. (2021) [1]. |
| Amino-functionalized silica | Understanding adsorption process using molecular dynamic simulations and quantum chemical calculations for wastewater treatment using amino-functionalized silica. | NA | NA | Adsorption energies in kcal/mol were:
| NA | Cao et al. (2021) [43] |
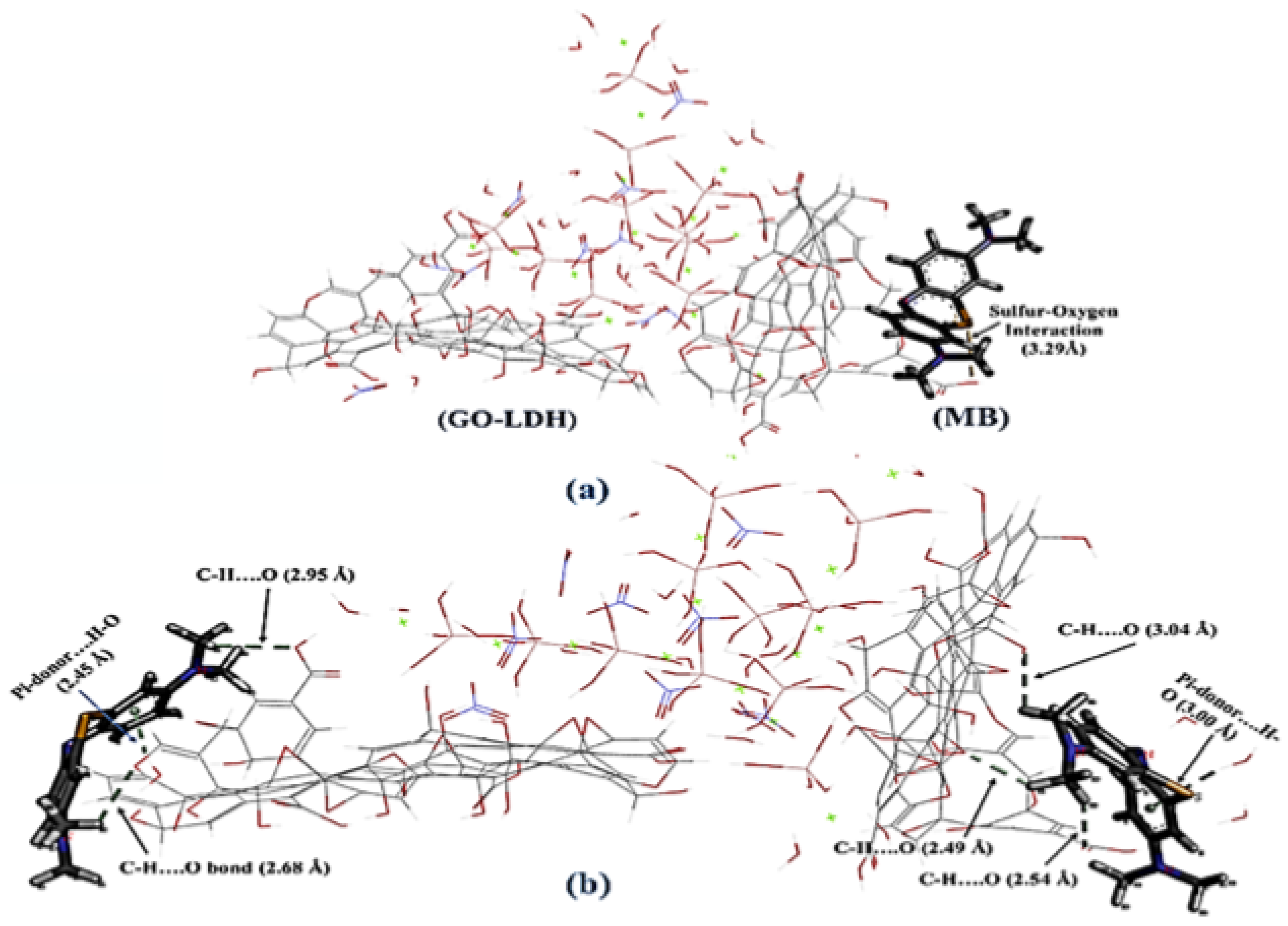
| Graphene Oxide Shielded Mg−Al-Layered Double Hydroxide | Methylene blue removal by Graphene Oxide Shielded Mg−Al-Layered Double Hydroxide from synthetic wastewater using adsorption. | 298 K | NA | Strong nonbonding interactions were observed as follows:
| The maximum adsorption capacity was:
| Dhar et al. (2021) [42]. |
| Graphene oxide-chloroacetic acid | Molecular dynamics simulation study for lead removal using adsorption of graphene oxide with chloroacetic acid (to increase the potentiality of GO) | 298 K | NA | Total energies of GO layers in kJ/mol were: GO: Pb+2 vs. energy values
| According to the Langmuir isotherm, maximum adsorption capacities (qe) were:
| Hossain et al. (2021) [28] |
| Multilayer graphene oxide | An experimental and molecular dynamics simulation study for xanthate removal from aqueous solution by multilayer graphene oxides adsorbent. | Simulation at two stages.
| NA | As the simulation progressed from time 250 ps to time 750 ps, the xanthate molecules gradually deformed, which showed a tendency to be gradually adsorbed on the MGO surface. | NA | Li et al. (2021) [40]. |
| Diamino-functionalized hollow mesosilica spheres | Molecular dynamics simulation for dye removal from synthetic wastewater using novel diamino-functionalized hollow mesosilica spheres. | NA | NA | The adsorption energy in kcal/mol after optimization were:
| NA | Pelalak et al. (2021) [23]. |
| Functionalized Meso-silica | A molecular dynamics simulation for removal of different pollutants using functionalized mesosilica adsorbent. | NA | NA | Adsorption energy in kcal/mol were:
| NA | Pelalak et al. (2021) [25]. |
| Adsorbent | Study Domain | Simulation Environment Operating Conditions | Surface Area (m2/g) | Simulation Result | Adsorption Capacity | References |
|---|---|---|---|---|---|---|
| Clinoptilolite | Adsorption of methylene blue dye from wastewater using natural clinoptilolite and clinoptilolite modified by iron oxide nanoparticles. | pH 2–10 | NA | Effective adsorption because of high surface-to-volume ratio of the modified adsorbent as well as the density of the functional groups at the surface of the adsorbent. | NA | Badeenezhad et al. (2019) [3] |
| FLTA, FAU, EDI, THO, NAT and LTN zeolites | Molecular simulation study for adsorption mechanism of Cu2+, Cd2+ and Pb2+ ions on different zeolites exchanged with sodium. | Temp 298 K | NA | The cadmium adsorptions in % were:
| NA | Khanmohammadi et al. (2019) [24]. |
| Faujasite-type Y zeolite (NaY) | Adsorption isotherms, kinetics and grand canonical Monte Carlo simulation studies for adsorption of phenol on faujasite-type Y zeolite |
| 558.75 | The phenol uptakes of zeolite in mg/g were:
| The phenol uptakes of zeolite in mg/g were:
| Mohammed et al. (2019) [5]. |
| High-silica Zeolite | An experimental and Monte Carlo simulation study for adsorption mechanisms of organic micropollutants on high-silica-zeolites causing S-shaped adsorption isotherms | NA | NA | The adsorption loadings of TCP were:
| NA | Jiang et al. (2020) [45]. |
| X- and Y-type faujasite zeolites | A computational study for phosphate removal from wastewater using faujasites. | NA | NA |
| NA | Capa-Cabos et al. (2021) [46]. |
| Padina gymnospora/zeolite nanocomposite | Design, characterization and adsorption properties of Padina gymnospora/zeolite nanocomposite for removal of Congo red dye from wastewater | NA |
| Adsorption energies (kcal/mol) of Congo red adsorbed on zeolite clinoptilolite with 3, 5 and 7 nm simple box systems:
| The adsorption capacities were:
| Dryaz et al. (2021) [29]. |
| Zeolite framework | Role of pore chemistry and topology for heavy metal removal using zeolite (a molecular simulation to machine learning). | NA | NA | Loadings of Cr (VI) ions were:
| NA | Wanyonyi et al.(2021) [14] |
| Adsorbent | Study Domain | Simulation Environment Operating Conditions | Surface Area (m2/g) | Simulation Result | Adsorption Capacity (mg/g) | References |
|---|---|---|---|---|---|---|
| Zeolitic imidazolate frameworks | Experimental and molecular simulation studies for efficient removal of pesticides from wastewater using Zeolitic imidazolate frameworks | 298 K | NA | The maximum loadings of pesticides were:
| Adsorption capacity of pesticides were:
| Abdelhameed et al. (2019) [18] |
| Various metal organic frameworks; MIL-101(Cr), MIL-100(Cr), Cu-BTC, DUT-23(Cu), UIO-66 and UMCM-2 | The adsorption and diffusion properties of Terephthalic Acid for different metal organic frameworks was studied using molecular simulation. | 298 K |
| TPA adsorption in mg/g were:
| The TPA adsorption capacities were:
| Bigdeli et al. (2019) [49]. |
| U iO-66(Zr) | The application of experimental and molecular simulation method for extraction of androgens and progestogens in environmental water samples using UiO-66(Zr) as sorbent. | NA | NA |
| NA | Gao et al. (2019) [48]. |
| Graphene oxide-copper-metal organic framework nanocomposite | Experimental and molecular dynamics study for adsorption of dye from water using a graphene oxide-copper-metal organic framework nanocomposite. |
| NA | Adsorption energies in kcal/mol were:
| Adsorption capacity of GO-Cu-MOF at different temperatures was:
| Firouzjaei et al. (2020) [50]. |
| Amino-functionalized Al-MIL-53 | Molecular dynamic study for intermolecular interactions of dimethoate pesticide on amino-functionalized Al-MIL-53 | NA |
| The adsorption energies of dimethoate onto Al-MOFs in kcal/mol were:
| Pesticide adsorption capacities were:
| Abdelhameed et al. (2021) [51]. |
| Zeolitic imidazolate frameworks | A molecular simulation study for Zeolitic imidazolate frameworks as capacitive deionization electrodes for water desalination and Cr(VI) adsorption | 300 K | NA | CdIF-1 (96.5%) has the second highest average R-value despite having the highest average water flux R-value (0.0308 Å/ns) | NA | Hong et al. (2021) [52]. |
| Metalorganic nanotube sponge | Experiment and molecular simulation study for fabrication of thermoresponsive metalorganic nanotube sponge and its adsorption of endocrine-disrupting compounds and pharmaceuticals/personal care products | 303 K | 137 | Adsorption energy () of DBP and PCMX on different crystal surfaces in kcal/mol were:
| At an initial concentration of 50 mg/L, maximum adsorption capacities were:
| Li et al. (2021) [53]. |
| Molecular imprinting material (C-MIL-100-MIP) | Design, mechanism and application for improvement of selective catalytic oxidation capacity of phthalates from surface molecular-imprinted catalysis materials | 300 K–600 K | 144 |
| Diethyl phthalate adsorption on molecular imprinting material (C-MIL-100-MIP) was 19.1 | Li 2021 02 [54]. |
| Adsorbent | Study Domain | Simulation Environment Operating Conditions | Surface Area (m2/g) | Simulation Result | Adsorption Capacity (mg/g) | References |
|---|---|---|---|---|---|---|
| Organo-bentonite | Adsorption isotherms modeling and molecular simulation for adsorption mechanism of methylene blue onto organo-bentonite | 60 °C | 84.6 | Van der Waals forces (VWF) in kcal/mol were:
| 321 | Bergaoui et al. (2018) [55]. |
| Gemini surfactant modified layered montmorillonite | Efficient preparation and molecular dynamic (MD) simulations of Gemini surfactant modified layered montmorillonite to potentially adsorb organic contaminants from wastewater | NA |
| The loading of 16-3-16 increased from 1.0 to 1.5 CEC (Cation Exchange Capacity). | Maximum adsorption capacities on Ca-Mt-1.0 at equilibrium were:
| Li et al. (2019) [56]. |
| Natural montmorillonite | Molecular simulation and adsorbent characterization studies for the mechanism of amitriptyline adsorption on natural montmorillonite through batch adsorption | 303 K | NA | The calculated occupied areas of AMI molecules and CEC (Cation Exchange Capacity in (meq/g) were:
| Maximum AMI adsorption at pH 7–8 was 276 mg/g | Chang et al. (2021) [57]. |
| Kaolinite | Molecular insights to understand removal of pharmaceutical residues from wastewater on kaolinite surfaces | NA | NA | Adsorption energy in kJ/mol were:
| NA | Hounfodji et al. (2021) [58]. |
| Magnetic montmorillonite composite γ-Fe2O3@Mt | Experimental and molecular dynamics simulation study for removal of Rhodamine B dye on magnetic montmorillonite composite γ-Fe2O3@Mt | NA | NA | The adsorption energy of a single RhB molecule adsorbed on the maghemite (311) nanosurface was −1259.9 kcal/mol | The maximum adsorption amount of RhB was 209.20 | Ouachtak et al., 2020 [59]. |
| Organo-montmorillonite | Experimental study and molecular dynamics to understand adsorption of orange G dye from polluted water using superb organo-montmorillonite | 298 K | CTAB@Mt: 52 | The adsorption energies in kcal/mol were:
| Using Langmuir isotherm, the maximum adsorption capacity calculated was 167 | Ouachtak et al., 2021 [60]. |
| Montmorillonite | Study of removal of sulfamethoxazole and tetracycline using montmorillonite in single and binary systems | 298 K | NA | As the TC concentration increased, the d001-spacing changes were:
| NA | Wu et al. (2019) [4]. |
| Kaolinite | Molecular dynamics simulation and density functional theory to investigate the interaction between xanthate and kaolinite based on experiments | 298 K | NA | The adsorption energies in kJ/mol were:
| NA | Zhang et al. (2021) [21]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hira, N.e.; Lock, S.S.M.; Shoparwe, N.F.; Lock, I.S.M.; Lim, L.G.; Yiin, C.L.; Chan, Y.H.; Hassam, M. Review of Adsorption Studies for Contaminant Removal from Wastewater Using Molecular Simulation. Sustainability 2023, 15, 1510. https://doi.org/10.3390/su15021510
Hira Ne, Lock SSM, Shoparwe NF, Lock ISM, Lim LG, Yiin CL, Chan YH, Hassam M. Review of Adsorption Studies for Contaminant Removal from Wastewater Using Molecular Simulation. Sustainability. 2023; 15(2):1510. https://doi.org/10.3390/su15021510
Chicago/Turabian StyleHira, Noor e, Serene Sow Mun Lock, Noor Fazliani Shoparwe, Irene Sow Mei Lock, Lam Ghai Lim, Chung Loong Yiin, Yi Herng Chan, and Muhammad Hassam. 2023. "Review of Adsorption Studies for Contaminant Removal from Wastewater Using Molecular Simulation" Sustainability 15, no. 2: 1510. https://doi.org/10.3390/su15021510






