Optimized Alternating Current Electric Field and Light Irradiance for Caulerpa lentillifera Biomass Sustainability—An Innovative Approach for Potential Postharvest Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Seagrape Packaging
2.3. Taguchi Orthogonal Array Design (OAD)
2.4. Postharvest Handling
2.5. Chlorophyll and Carotenoid Content Determination
2.6. Electrolyte Leakage Determination
2.7. Color Change Determination
2.8. Firmness Determination
2.9. Statistical Analysis
3. Results and Discussions
3.1. Effect of ACEF Intensity on Postharvest Storage of Seagrape
3.2. Effect of ACEF Treatment Time on Postharvest Storage of Seagrape
| Quality | Factor | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Total Chlorophyll Content | A | 2 | 0.108666 | 0.054333 | 147.97 | 0.007 |
| B | 2 | 0.002348 | 0.001174 | 3.2 | 0.238 | |
| C | 2 | 0.015229 | 0.007614 | 20.74 | 0.046 | |
| Error | 2 | 0.000734 | 0.000367 | |||
| Total | Total | 8 | 0.126977 | |||
| Electrolyte Leakage | A | 2 | 9.3122 | 4.65609 | 123.28 | 0.008 |
| B | 2 | 1.0533 | 0.52666 | 13.95 | 0.067 | |
| C | 2 | 3.4775 | 1.73874 | 46.04 | 0.021 | |
| Error | 2 | 0.0755 | 0.03777 | |||
| Total | Total | 8 | 13.9185 | |||
| Color Change | A | 2 | 32.1844 | 16.0922 | 44.31 | 0.022 |
| B | 2 | 2.0254 | 1.0127 | 2.79 | 0.264 | |
| C | 2 | 14.6101 | 7.3051 | 20.11 | 0.047 | |
| Error | 2 | 0.7263 | 0.3632 | |||
| Total | Total | 8 | 49.5463 | |||
| Firmness | A | 2 | 0.000441 | 0.000221 | 165.27 | 0.006 |
| B | 2 | 0.000092 | 0.000046 | 34.41 | 0.028 | |
| C | 2 | 0.000134 | 0.000067 | 50.37 | 0.019 | |
| Error | 2 | 0.000003 | 0.000001 | |||
| Total | Total | 8 | 0.000670 |
3.3. Effect of Storage Irradiance Level on Postharvest Storage of Seagrape
3.4. Effect of Postharvest Storage Treatments on Physicochemical Properties of Seagrape
3.4.1. Chlorophyll and Carotenoid Content
3.4.2. Electrolyte Leakage
3.4.3. Color Change
3.4.4. Firmness
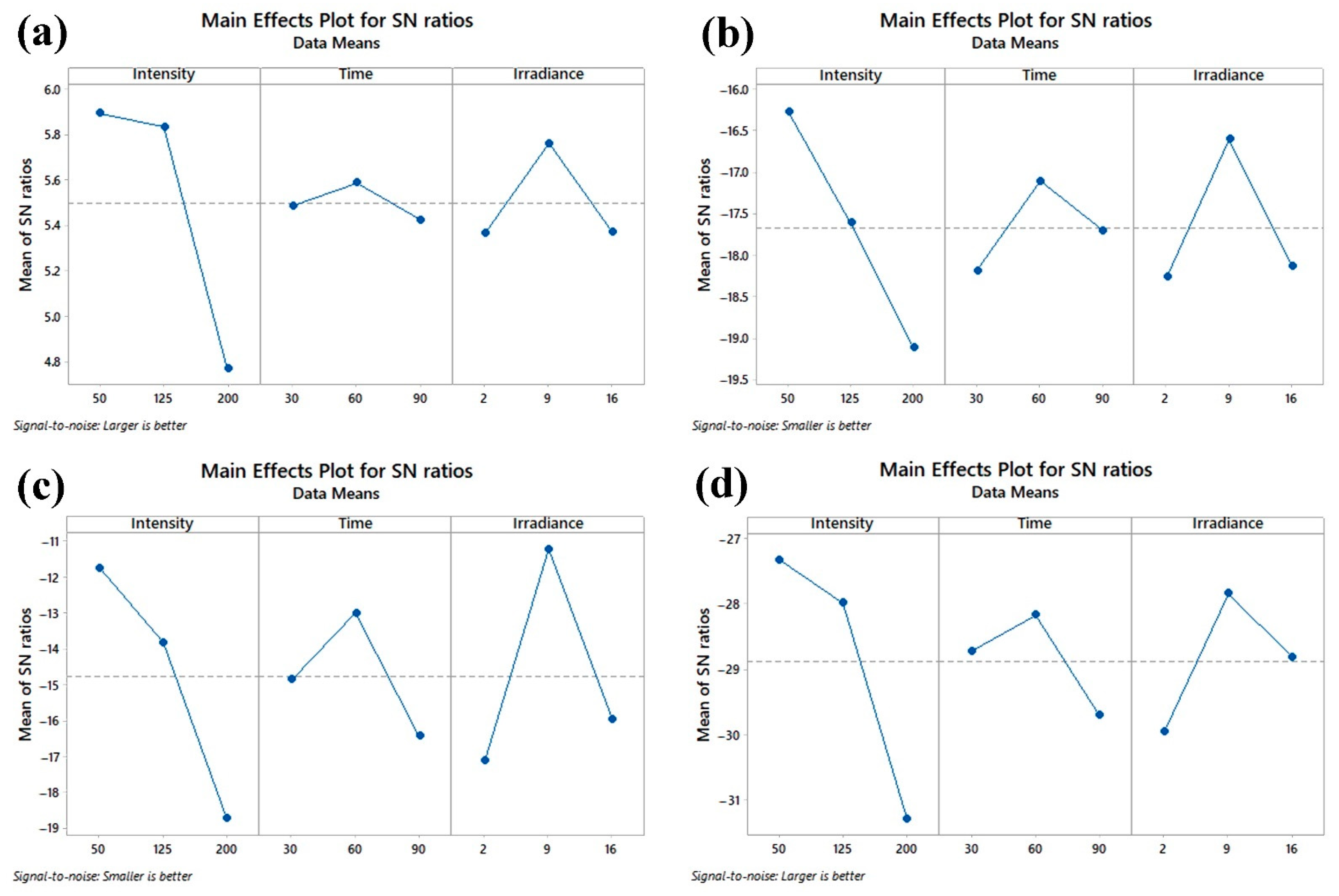
3.5. Determination of Optimum Performance Characteristics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sulaimana, A.S.; Chang, C.K.; Hou, C.Y.; Yudhistira, B.; Punthi, F.; Lung, C.T.; Cheng, K.C.; Santoso, S.P.; Hsieh, C.W. Effect of oxidative stress on physicochemical quality of taiwanese seagrape (Caulerpa lentillifera) with the application of alternating current electric field (ACEF) during post-harvest storage. Processes 2021, 9, 1011. [Google Scholar] [CrossRef]
- Tiitii, U.; Paul, N.; Burkhart, S.; Larson, S.; Swanepoel, L. Traditional Knowledge and Modern Motivations for Consuming Seaweed (Limu) in Samoa. Sustainability 2022, 14, 6212. [Google Scholar] [CrossRef]
- Yamauchi, N. Postharvest chlorophyll degradation and oxidative stress. In Abiotic Stress Biology in Horticultural Plants; Springer: Tokyo, Japan, 2015; pp. 101–113. [Google Scholar]
- Terada, R.; Nakazaki, Y.; Borlongan, I.A.; Endo, H.; Nishihara, G.N. Desiccation effect on the PSII photochemical efficiency of cultivated Japanese Caulerpa lentillifera under the shipping package environment. J Appl. Phycol. 2018, 30, 2533–2538. [Google Scholar] [CrossRef]
- Guo, H.; Yao, J.; Sun, Z.; Duan, D. Effect of temperature, irradiance on the growth of the green alga Caulerpa lentillifera (Bryopsidophyceae, Chlorophyta). J. Appl. Phycol. 2015, 27, 879–885. [Google Scholar] [CrossRef]
- Nassarawa, S.S.; Abdelshafy, A.M.; Xu, Y.; Li, L.; Luo, Z. Effect of Light-Emitting Diodes (LEDs) on the Quality of Fruits and Vegetables During Postharvest Period: A Review. Food Bioprocess Technol. 2021, 14, 388–414. [Google Scholar] [CrossRef]
- Zhao, R.; Hao, J.; Xue, J.; Liu, H.; Li, L. Effect of high-voltage electrostatic field pretreatment on the antioxidant system in stored green mature tomatoes. J. Sci. Food Agric. 2011, 91, 1680–1686. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Chang, C.K.; Wong, L.W.; Hu, C.C.; Lin, J.A.; Hsieh, C.W. Alternating current electric field inhibits browning of Pleurotus ostreatus via inactivation of oxidative enzymes during postharvest storage. LWT-Food Sci. Technol. 2020, 134, 110212. [Google Scholar] [CrossRef]
- Wang, L.X.; Dao, L.P.; Guo, Q.Y.; Chen, T.L.; Fu, L.J.; Zhou, F.C.; Yuan, Y. Investigation on the influence of AC electric filed and KCl on the structure and gel properties of Konjac glucomannan. Food Chem. 2022, 386, 132755. [Google Scholar] [CrossRef]
- Mak, T.M.W.; Xiong, X.; Tsang, D.C.W.; Yu, I.K.M.; Poon, C.S. Sustainable food waste management towards circular bioeconomy: Policy review, limitations and opportunities. Bioresour Technol. 2020, 297, 122497. [Google Scholar] [CrossRef]
- Cornwall, C.E.; Revill, A.T.; Hall-Spencer, J.M.; Milazzo, M.; Raven, J.A.; Hurd, C.L. Inorganic carbon physiology underpins macroalgal responses to elevated CO2. Sci. Rep. 2017, 7, 46297. [Google Scholar] [CrossRef]
- Punthi, F.; Yudhistira, B.; Gavahian, M.; Chang, C.K.; Cheng, K.C.; Hou, C.Y.; Hsieh, C.W. Pulsed electric field-assisted drying: A review of its underlying mechanisms, applications, and role in fresh produce plant-based food preservation. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lung, C.T.; Chang, C.K.; Cheng, F.C.; Hou, C.Y.; Chen, M.H.; Santoso, S.P.; Yudhistira, B.; Hsieh, C.W. Effects of pulsed electric field-assisted thawing on the characteristics and quality of Pekin duck meat. Food Chem. 2022, 390, 133137. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Gonzales, M.; Zúñiga, P.; Petzold, G.; Mella, K.; Muñoz, O. Ohmic heating and pulsed vacuum effect on dehydration processes and polyphenol component retention of osmodehydrated blueberries (cv. Tifblue). Innov. Food Sci. Emerg. Technol. 2016, 36, 112–119. [Google Scholar] [CrossRef]
- Srinorasing, T.; Chirasuwan, N.; Bunnag, B.; Chaiklahan, R. Lipid extracts from Caulerpa lentillifera waste: An alternative product in a circular economy. Sustainability 2021, 13, 4491. [Google Scholar] [CrossRef]
- Butcher, H.; Burkhart, S.; Paul, N.; Tiitii, U.; Tamuera, K.; Eria, T.; Swanepoel, L. Role of seaweed in diets of Samoa and Kiribati: Exploring key motivators for consumption. Sustainability 2020, 12, 7356. [Google Scholar] [CrossRef]
- Arnal, J.; Royo, P.; Pataro, G.; Ferrari, G.; Ferreira, V.J.; López-Sabirón, A.M.; Ferreira, G.A. Implementation of PEF treatment at real-scale tomatoes processing considering LCA methodology as an innovation strategy in the agri-food sector. Sustainability 2018, 10, 979. [Google Scholar] [CrossRef]
- Sun, X.; Shokri, S.; Wang, Z.; Li, B.; Meng, X. Optimization of explosion puffing drying for browning control in Muskmelon (Cucumis melo L.) using Taguchi orthogonal arrays. LWT-Food Sci. Technol. 2021, 142, 111021. [Google Scholar] [CrossRef]
- Ghosh, S.; Gillis, A.; Levkov, K.; Vitkin, E.; Golberg, A. Saving energy on meat air convection drying with pulsed electric field coupled to mechanical press water removal. Innov. Food Sci. Emerg. Technol. 2020, 66, 102509. [Google Scholar] [CrossRef]
- El-Moslamy, S.H.; Elkady, M.F.; Rezk, A.H.; Abdel-Fattah, Y.R. Applying Taguchi design and large-scale strategy for mycosynthesis of nano-silver from endophytic Trichoderma harzianum SYA.F4 and its application against phytopathogens. Sci. Rep. 2017, 7, 45297. [Google Scholar] [CrossRef]
- Jiang, R.; Linzon, Y.; Vitkin, E.; Yakhini, Z.; Chudnovsky, A.; Golberg, A. Thermochemical hydrolysis of macroalgae Ulva for biorefinery: Taguchi robust design method. Sci. Rep. 2016, 6, 27761. [Google Scholar] [CrossRef]
- Ortiz-Sanchez, M.; Cardona, A.C.A. Analysis of the routes for biomass processing towards sustainable development in the conceptual design step: Strategy based on the compendium of bioprocesses portfolio. Bioresour Technol. 2022, 350, 126852. [Google Scholar] [CrossRef] [PubMed]
- Assadpour, E.; Maghsoudlou, Y.; Jafari, S.M.; Ghorbani, M.; Aalami, M. Evaluation of Folic Acid Nano-encapsulation by Double Emulsions Food. Bioprocess Technol. 2016, 9, 2024–2032. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Ding, W.; Zhang, D.; Reed, K.; Zhang, B. Orthogonal Optimization and Physicochemical Characterization of Water-Soluble Gelatin-Chitosan Nanoparticles with Encapsulated Alcohol-Soluble Eugenol Food. Bioprocess Technol. 2020, 13, 1024–1034. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, G.; Chen, H.; Guan, Y.; Li, H. Optimization of an aluminum profile extrusion process based on Taguchi’s method with S/N analysis. Int. J. Adv. Manuf. Technol. 2012, 60, 589–599. [Google Scholar] [CrossRef]
- D’Souza, A.D.; Rao, S.S.; Herbert, M.A. Taguchi method of optimization of process variables for ultimate tensile strength of friction stir welded joint of Al-Ce-Si-Mg aluminium alloy plates Mater. Today Proc. 2021, 46, 2691–2698. [Google Scholar] [CrossRef]
- Nor, S.I.; Irmawati, R.; Omar, H.; Yahaya, M.; Aina, A.A. Removal of free fatty acid (FFA) in crude palm oil (CPO) using potassium oxide/dolomite as an adsorbent: Optimization by Taguchi method. Food Chem. 2022, 373, 131668. [Google Scholar]
- Arshad, R.N.; Abdul-Malek, Z.; Jusoh, Y.M.M.; Radicetti, E.; Tedeschi, P.; Mancinelli, R.; Lorenzo, J.M.; Aadil, R.M. Sustainable Electroporator for Continuous Pasteurisation: Design and Performance Evaluation with Orange Juice. Sustainability 2022, 14, 1896. [Google Scholar] [CrossRef]
- Paull, R.E.; Chen, N.J. Postharvest handling and storage of the edible red seaweed Gracilaria Postharvest. Biol. Technol. 2008, 48, 302–308. [Google Scholar] [CrossRef]
- Kim, K.Y.; Garbary, D.J. Photosynthesis in Codium fragile (Chlorophyta) from a Nova Scotia estuary: Responses to desiccation and hyposalinity. Mar. Biol. 2007, 151, 99–107. [Google Scholar] [CrossRef]
- Halász, K.; Csóka, L. Black chokeberry (Aronia melanocarpa) pomace extract immobilized in chitosan for colorimetric pH indicator film application. Food Packag. Shelf Life. 2018, 16, 185–193. [Google Scholar] [CrossRef]
- Liu, C.E.; Chen, W.J.; Chang, C.K.; Li, P.H.; Lu, P.L.; Hsieh, C.W. Effect of a high voltage electrostatic field (HVEF) on the shelf life of persimmons (Diospyros kaki). LWT-Food Sci. Technol. 2017, 75, 236–242. [Google Scholar] [CrossRef]
- Bai, Y.X.; Li, J.; Mei, Y.; Kang, D.M. Experiment of drying kelp with high voltage electric fields. In Proceedings of the 2008 International Conference on High Voltage Engineering And Application (ICHVE), Chongqing, China, 9–13 November 2008; pp. 732–734. [Google Scholar]
- Fallah-Joshaqani, S.; Hamdami, N.; Keramat, J. Qualitative attributes of button mushroom (Agaricus bisporus) frozen under high voltage electrostatic field. J. Food Eng. 2021, 293, 110384. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Munir, M.A.; Naderipour, A.; Qureshi, M.I.; Bekhit, A.E.; Liu, Z.W.; Aadil, R.M. Pulsed electric field: A potential alternative towards a sustainable food processing. Trends Food Sci. Technol. 2021, 111, 43–54. [Google Scholar] [CrossRef]
- Traffano-Schiffo, M.V.; Laghi, L.; Castro-Giraldez, M.; Tylewicz, U.; Rocculi, P.; Ragni, L.; Rosa, M.D.; Fito, P.J. Osmotic dehydration of organic kiwifruit pre-treated by pulsed electric fields and monitored by NMR. Food Chem. 2017, 236, 87–93. [Google Scholar] [CrossRef]
- Dalvi-Isfahan, M.; Hamdami, N.; Le-Bail, A.; Xanthakisc, E. The principles of high voltage electric field and its application in food processing: A review. Food Res. Int. 2016, 89, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Tanaka, R. The biochemistry, physiology, and evolution of the chlorophyll cycle. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2019; pp. 183–212. [Google Scholar]
- Sánchez-Vega, R.; Elez-Martínez, P.; Martín-Belloso, O. Effects of high-intensity pulsed electric fields processing parameters on the chlorophyll content and its degradation compounds in broccoli juice. Food Bioprocess Technol. 2014, 7, 1137–1148. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Zhang, Q.; Qu, Y.; Li, G. Preparation and antioxidant property of extract and semipurified fractions of Caulerpa racemosa. J. Appl. Phycol. 2012, 24, 1527–1536. [Google Scholar] [CrossRef]
- Sommer, J.; Kunzmann, A.; Stuthmann, L.E.; Springer, K. The antioxidative potential of sea grapes (Caulerpa lentillifera, Chlorophyta) can be triggered by light to reach comparable values of pomegranate and other highly nutritious fruits. Plant Physiol. Rep. 2022, 27, 186–191. [Google Scholar] [CrossRef]
- Luo, F.; Cheng, S.C.; Cai, J.H.; Wei, B.D.; Zhou, X.; Zhou, Q.; Zhao, Y.B.; Ji, S.J. Chlorophyll degradation and carotenoid biosynthetic pathways: Gene expression and pigment content in broccoli during yellowing. Food Chem. 2019, 297, 124964. [Google Scholar] [CrossRef]
- Abedi, E.; Amiri, M.J.; Sahari, M.A. Kinetic, isotherm and thermodynamic investigations on adsorption of trace elements and pigments from soybean oil using high voltage electric field-assisted bleaching: A comparative study. Process Biochem. 2020, 91, 208–222. [Google Scholar] [CrossRef]
- Yan, M.; Yuan, B.; Xie, Y.; Cheng, S.; Huang, H.; Zhang, W.; Chen, J.; Cao, C. Improvement of postharvest quality, enzymes activity and polyphenoloxidase structure of postharvest Agaricus bisporus in response to high voltage electric field. Postharvest Biol. Technol. 2020, 166, 111230. [Google Scholar] [CrossRef]
- Stuthmann, L.E.; Achuthan, R.; Pribbernow, M.; Du, H.T.; Springer, K.; Kunzmann, A. Improving the nutritional value of edible Caulerpa lentillifera (Chlorophyta) using high light intensities. A realistic tool for sea grape farmers. Algal Res. 2022, 66, 102785. [Google Scholar] [CrossRef]
- Terada, R.; Takaesu, M.; Borlongan, I.A.; Nishihara, G.N. The photosynthetic performance of a cultivated Japanese green alga Caulerpa lentillifera in response to three different stressors, temperature, irradiance, and desiccation. J. Appl. Phycol. 2021, 33, 2547–2559. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, S.; Dai, H.; Kong, X.; Hao, J.; Wang, S.; Zhou, X.; Zhao, Y.; Wei, B.; Cheng, S.; et al. Changes in membrane lipid metabolism accompany pitting in blueberry during refrigeration and subsequent storage at room temperature. Front Plant Sci. 2019, 10, 829. [Google Scholar] [CrossRef]
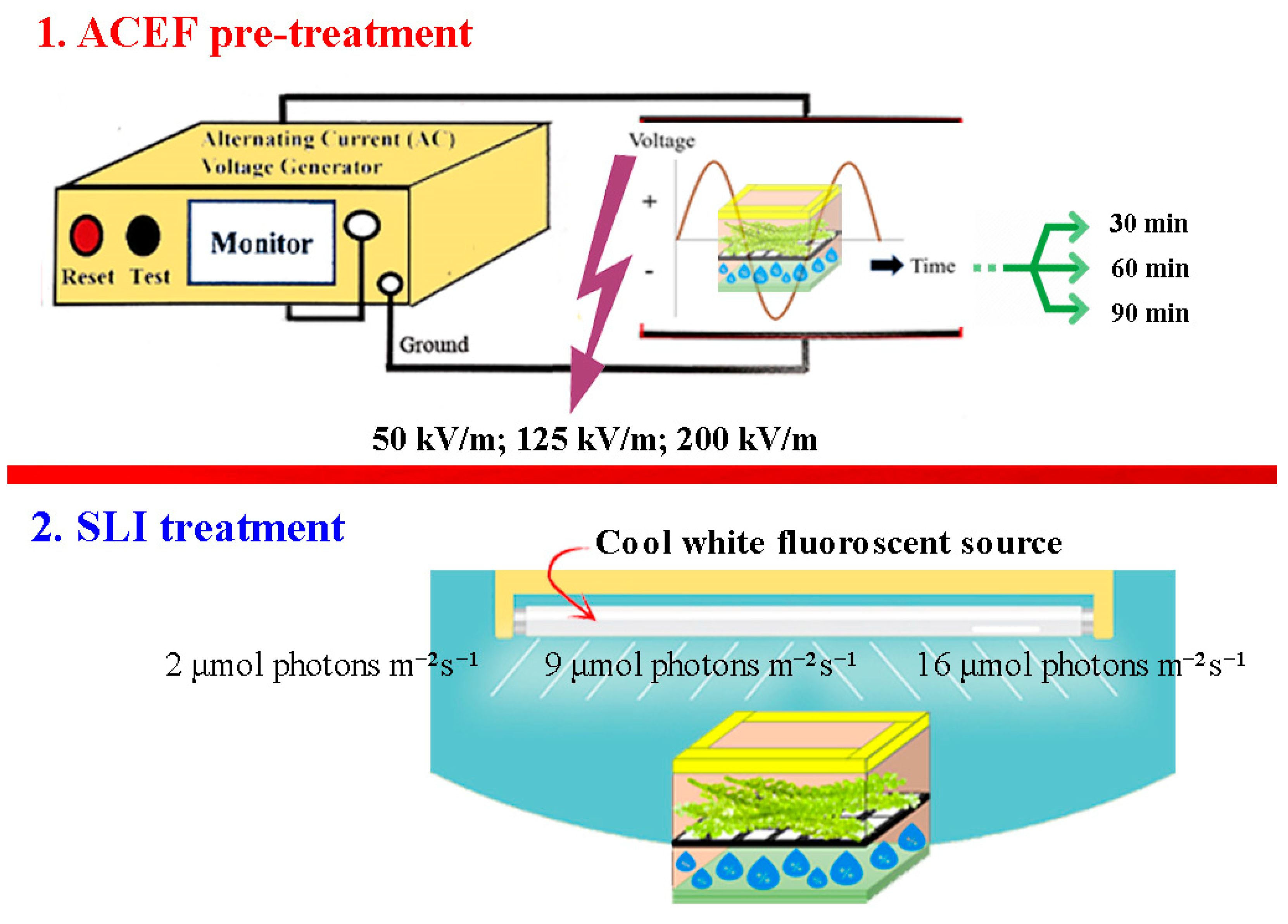

| Sample Runs | Factors | Results of Chlorophyll Content (mg g−1) | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | Chlorophyll a | Chlorophyll b | Carotenoid | Total Chlorophyll | |
| 1 | 1 | 1 | 1 | 1.04 ± 0.03 | 0.69 ± 0.01 | 0.31 ± 0.02 | 1.93 ± 0.07 |
| 2 | 1 | 2 | 2 | 1.13 ± 0.02 | 0.68 ± 0.01 | 0.33 ± 0.01 | 2.06 ± 0.08 |
| 3 | 1 | 3 | 3 | 1.00 ± 0.04 | 0.68 ± 0.02 | 0.32 ± 0.01 | 1.92 ± 0.04 |
| 4 | 2 | 1 | 3 | 1.08 ± 0.05 | 0.69 ± 0.03 | 0.33 ± 0.01 | 2.01 ± 0.05 |
| 5 | 2 | 2 | 1 | 1.05 ± 0.03 | 0.63 ± 0.01 | 0.30 ± 0.02 | 1.94 ± 0.06 |
| 6 | 2 | 3 | 2 | 1.04 ± 0.04 | 0.62 ± 0.03 | 0.30 ± 0.01 | 1.92 ± 0.05 |
| 7 | 3 | 1 | 2 | 0.90 ± 0.07 | 0.58 ± 0.04 | 0.27 ± 0.01 | 1.71 ± 0.02 |
| 8 | 3 | 2 | 3 | 0.91 ± 0.06 | 0.58 ± 0.01 | 0.27 ± 0.02 | 1.72 ± 0.03 |
| 9 | 3 | 3 | 1 | 0.93 ± 0.05 | 0.57 ± 0.02 | 0.28 ± 0.02 | 1.77 ± 0.05 |
| k1 | 1.971 | 1.884 | 1.857 | ||||
| k2 | 1.957 | 1.907 | 1.945 | ||||
| k3 | 1.731 | 1.868 | 1.858 | ||||
| R (delta) | 0.240 | 0.039 | 0.088 | ||||
| Rank | 1 | 3 | 2 | ||||
| Sample Runs | Factors | Results of Physicochemical Properties | ||||
|---|---|---|---|---|---|---|
| A | B | C | Electrolyte Leakage (%) | Color Change (ΔE) | Firmness (N) | |
| 1 | 1 | 1 | 1 | 7.44 ± 0.23 | 5.67 ± 0.42 | 0.0397 ± 0.003 |
| 2 | 1 | 2 | 2 | 5.25 ± 0.34 | 1.78 ± 0.22 | 0.0519 ± 0.002 |
| 3 | 1 | 3 | 3 | 7.05 ± 0.47 | 5.70 ± 0.44 | 0.0386 ± 0.003 |
| 4 | 2 | 1 | 3 | 7.30 ± 0.41 | 3.49 ± 0.53 | 0.0448 ± 0.003 |
| 5 | 2 | 2 | 1 | 7.56 ± 0.52 | 5.08 ± 0.67 | 0.0447 ± 0.005 |
| 6 | 2 | 3 | 2 | 7.95 ± 0.42 | 6.65 ± 0.48 | 0.0316 ± 0.002 |
| 7 | 3 | 1 | 2 | 9.84 ± 0.47 | 8.56 ± 0.59 | 0.0276 ± 0.003 |
| 8 | 3 | 2 | 3 | 9.28 ± 0.59 | 9.81 ± 0.34 | 0.0256 ± 0.004 |
| 9 | 3 | 3 | 1 | 8.06 ± 0.45 | 7.72 ± 0.42 | 0.0286 ± 0.004 |
| k1 | 6.582 | 8.195 | 8.225 | Electrolyte Leakage (%) | ||
| k2 | 7.604 | 7.364 | 6.871 | |||
| k3 | 9.061 | 7.688 | 8.151 | |||
| R (delta) | 2.479 | 0.831 | 1.354 | |||
| Rank | 1 | 3 | 2 | |||
| k1 | 4.384 | 5.906 | 7.374 | Color Change (ΔE) | ||
| k2 | 5.073 | 5.556 | 4.330 | |||
| k3 | 8.695 | 6.691 | 6.448 | |||
| R (delta) | 4.312 | 1.135 | 3.044 | |||
| Rank | 1 | 3 | 2 | |||
| k1 | 0.043 | 0.037 | 0.032 | Firmness (N) | ||
| k2 | 0.040 | 0.041 | 0.042 | |||
| k3 | 0.027 | 0.033 | 0.037 | |||
| R (delta) | 0.016 | 0.008 | 0.009 | |||
| Rank | 1 | 3 | 2 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulaimana, A.S.; Yudhistira, B.; Chang, C.-K.; Gavahian, M.; Yu, C.-C.; Hou, C.-Y.; Hsieh, C.-W. Optimized Alternating Current Electric Field and Light Irradiance for Caulerpa lentillifera Biomass Sustainability—An Innovative Approach for Potential Postharvest Applications. Sustainability 2022, 14, 14361. https://doi.org/10.3390/su142114361
Sulaimana AS, Yudhistira B, Chang C-K, Gavahian M, Yu C-C, Hou C-Y, Hsieh C-W. Optimized Alternating Current Electric Field and Light Irradiance for Caulerpa lentillifera Biomass Sustainability—An Innovative Approach for Potential Postharvest Applications. Sustainability. 2022; 14(21):14361. https://doi.org/10.3390/su142114361
Chicago/Turabian StyleSulaimana, Andi Syahrullah, Bara Yudhistira, Chao-Kai Chang, Mohsen Gavahian, Cheng-Chia Yu, Chih-Yao Hou, and Chang-Wei Hsieh. 2022. "Optimized Alternating Current Electric Field and Light Irradiance for Caulerpa lentillifera Biomass Sustainability—An Innovative Approach for Potential Postharvest Applications" Sustainability 14, no. 21: 14361. https://doi.org/10.3390/su142114361
APA StyleSulaimana, A. S., Yudhistira, B., Chang, C.-K., Gavahian, M., Yu, C.-C., Hou, C.-Y., & Hsieh, C.-W. (2022). Optimized Alternating Current Electric Field and Light Irradiance for Caulerpa lentillifera Biomass Sustainability—An Innovative Approach for Potential Postharvest Applications. Sustainability, 14(21), 14361. https://doi.org/10.3390/su142114361











