Azorean Vascular Plants with Potential Use in Constructed Wetlands with Horizontal Subsurface Flow
Abstract
:1. Introduction
1.1. Constructed Wetlands as Nature-Based Solutions
1.2. Case Study Azores
1.2.1. Domestic effluents
1.2.2. Agricultural effluents
1.2.3. Potential of CWs in the Azores
2. Materials and Methods
2.1. Climate and Geographical Framework
2.2. Flora of the Azores
2.3. Evaluation of the Azorean Vascular Flora for CW Projects
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Potschin, M.; Kretsch, C.; Haines-Young, R.; Furman, E.; Berry, P.; Baró, F. Nature-based solutions. In OpenNESS Ecosystem Service Reference Book. Potschin, M., Jax, K., Eds.; Reference Book. EC FP7 Grant Agreement n. 308428; 2015.. Available online: http://www.openness-project.eu/library/reference-book (accessed on 10 July 2022).
- Cohen-Shacham, E.; Walters, G.; Janzen, C.; Maginnis, S. (Eds.) Nature-based solutions to address global societal challenges; IUCN: Gland, Switzerland, 2016; Volume 97, pp. 2016–2036. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Towards an EU research and innovation policy agenda for nature-based solutions and re-naturing cities. In Final Report of the Horizon 2020 Expert Group on Nature-Based Solutions and re-Naturing Cities; Publications Office: Brussels, Belgium, 2015; Available online: http://data.europa.eu/doi/10.2777/479582 (accessed on 2 August 2022).
- U.S. EPA: United States Environmental Protection Agency. Wastewater Technology Fact Sheet Wetlands: Subsurface Flow; EPA 832-F-00-023; EPA: Washington, DC, USA, 2000.
- Vymazal, J. Constructed wetlands for wastewater treatment. Water 2010, 2, 530–549. [Google Scholar] [CrossRef] [Green Version]
- Haddis, A.; Van der Bruggen, B.; Smets, I. Constructed wetlands as nature-based solutions in removing organic pollutants from wastewater under irregular flow conditions in a tropical climate. Ecohydrol. Hydrobiol. 2020, 20, 38–47. [Google Scholar] [CrossRef]
- Li, X.; Wu, S.; Yang, C.; Zeng, G. Microalgal and duckweed based constructed wetlands for swine wastewater treatment: A review. Bioresour. Technol. 2020, 318, 123858. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.; Gupta, A.K.; Ghosal, P.S.; Majumder, A. A review on performance of constructed wetlands in tropical and cold climate: Insights of mechanism, role of influencing factors, and system modification in low temperature. Sci. Total Environ. 2021, 755, 142540. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. The use constructed wetlands with horizontal sub-surface flow for various types of wastewater. Ecol. Eng. 2009, 35, 1–17. [Google Scholar] [CrossRef]
- Wang, H.; Sheng, L.; Xu, J. Clogging mechanisms of constructed wetlands: A critical review. J. Clean. Prod. 2021, 295, 126455. [Google Scholar] [CrossRef]
- Brix, H. The use of aquatic macrophytes in water-pollution control. Ambio 1989, 28, 100–107. [Google Scholar]
- Vymazal, J.; Zhao, Y.; Mander, Ü. Recent research challenges in constructed wetlands for wastewater treatment: A review. Ecol. Eng. 2021, 169, 106318. [Google Scholar] [CrossRef]
- Vymazal, J.; Kröpfelová, L. Wastewater Treatment in Constructed Wetlands with Horizontal Sub-Surface Flow (Vol. 14); Springer Science & Business Media: Prague, Czech Republic, 2008. [Google Scholar]
- Brix, H. Functions of macrophytes in constructed wetlands. Water Sci. Technol. 1994, 29, 71–78. [Google Scholar] [CrossRef]
- Cui, L.; Ouyang, Y.; Yang, W.; Huang, Z.; Xu, Q.; Yu, G. Removal of nutrients from septic tank effluent with baffle subsurface-flow constructed wetlands. J. Environ. Manag. 2015, 153, 33–39. [Google Scholar] [CrossRef]
- Vymazal, J. The use of sub-surface constructed wetlands for wastewater treatment in the Czech Republic: 10 years experience. Ecol. Eng. 2002, 18, 633–646. [Google Scholar] [CrossRef]
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Maucieri, C.; Barbera, A.; Vymazal, J.; Borin, M. A review of the main affecting factors of the greenhouse gases emission in constructed wetlands. Agric. For. Meteorol. 2017, 236, 175–193. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, H.; Yan, B.; Shutes, B.; Xing, D.; Banuelos, G.; Cheng, R.; Wang, X. Greenhouse gas emissions and wastewater treatment performance by three plant species in subsurface flow constructed wetland mesocosms. Chemosphere 2020, 239, 124795. [Google Scholar] [CrossRef]
- Syranidou, E.; Christofilopoulos, S.; Kalogerakis, N. Juncus spp.—The helophyte for all (phyto) remediation purposes? New Biotechnol. 2017, 38, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. The use of hybrid constructed wetlands for wastewater treatment with special attention to nitrogen removal: A review of a recent development. Water Res. 2013, 47, 4795–4811. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.; Kalra, S.J.; Naraian, R. Environmental perspectives of Phragmites australis (Cav.) Trin. Ex. Steudel. Appl. Water Sci. 2014, 4, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Vymazal, J. Plants used in constructed wetlands with horizontal subsurface flow: A review. Hydrobiologia 2011, 674, 133–156. [Google Scholar] [CrossRef]
- Healy, M.G.; Rodgers, M.; Mulqueen, J. Treatment of dairy wastewater using constructed wetlands and intermittent sand filters. Bioresour. Technol. 2007, 98, 2268–2281. [Google Scholar] [CrossRef]
- Abeysinghe, T.; Simic Milas, A.; Arend, K.; Hohman, B.; Reil, P.; Gregory, A.; Vázquez-Ortega, A. Mapping invasive Phragmites australis in the Old Woman Creek estuary using UAV remote sensing and machine learning classifiers. Remote Sens. 2019, 11, 1380. [Google Scholar] [CrossRef]
- Gonzalez Mateu, M.; Baldwin, A.H.; Maul, J.E.; Yarwood, S.A. Dark septate endophyte improves salt tolerance of native and invasive lineages of Phragmites australis. ISME J. 2020, 14, 1943–1954. [Google Scholar] [CrossRef] [PubMed]
- Pyšek, P.; Skálová, H.; Cuda, J.; Guo, W.; Dolezal, J.; Kauzál, O.; Lambertini, C.; Pyšková, K.; Brix, H.; Meyerson, L.A. Physiology of a plant invasion: Biomass production, growth and tissue chemistry of invasive and native Phragmites australis populations. Preslia 2019, 91, 51–75. [Google Scholar] [CrossRef]
- Quirion, B.; Simek, Z.; Dávalos, A.; Blossey, B. Management of invasive Phragmites australis in the Adirondacks: A cautionary tale about prospects of eradication. Biol. Invasions 2018, 20, 59–73. [Google Scholar] [CrossRef] [Green Version]
- PGRH. Plano de Gestão da Região Hidrográfica dos Açores (RH9) 2016-2021—Síntese da Caracterização e Diagnóstico (Management Plan for the Azores Hydrographic Region (RH9) 2016–2021—Synthesis of Characterization and Diagnosis), Strategic Environmental Assessment, Regional Secretary of Agriculture and Environment. 2015. Available online: https://servicos-sraa.azores.gov.pt/grastore/DRA/PGRHA_2016-2021/PGRH-A_2016-2021_AAE-RA.PDF (accessed on 15 June 2022).
- Del Rosario, K.; Mitra, S.; Humphrey Jr, C.; O’Driscoll, M. Detection of pharmaceuticals and other personal care products in groundwater beneath and adjacent to onsite wastewater treatment systems in a coastal plain shallow aquifer. Sci. Total Environ. 2014, 487, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Hube, S.; Wu, B. Mitigation of emerging pollutants and pathogens in decentralized wastewater treatment processes: A review. Sci. Total Environ. 2021, 779, 146545. [Google Scholar] [CrossRef] [PubMed]
- Withers, P.J.; Jordan, P.; May, L.; Jarvie, H.P.; Deal, N.E. Do septic tank systems pose a hidden threat to water quality? Front. Ecol. Environ. 2014, 12, 123–130. [Google Scholar] [CrossRef]
- INE: National Statistics Institute. The Agricultural Census. Analysis of the Main Results—2019, Lisboa. 2019. Available online: www.ine.pt (accessed on 10 July 2022).
- Morais, T.G.; Teixeira, R.F.; Rodrigues, N.R.; Domingos, T. Carbon footprint of milk from pasture-based dairy farms in Azores, Portugal. Sustainability 2018, 10, 3658. [Google Scholar] [CrossRef] [Green Version]
- Bajouco, R.; Pinheiro, J.; Pereira, B.; Ferreira, R.; Coutinho, J. Risk of phosphorus losses from Andosols under fertilized pasture. Environ. Sci. Pollut. Res. 2020, 27, 19592–19602. [Google Scholar] [CrossRef]
- Cordeiro, R.; Luz, R.; Vilaverde, J.; Vasconcelos, V.; Fonseca, A.; Gonçalves, V. Distribution of Toxic Cyanobacteria in Volcanic lakes of the Azores Islands. Water 2020, 12, 3385. [Google Scholar] [CrossRef]
- Pinheiro, J.; Matos, M.D.L. Characterization and strategies for the control of eutrophication in the Furnas watershed (Azores Portugal). In Novel Methods for Reducingagricultural Nutrient Loadingand Eutrophication, Proceedings of the Meeting of Cost 869, Jokioinen, Finland, 14–16 June 2010; Turtola, E., Ekholm, P., Chardon, W., Eds.; MTT Agrifood Research Finland: Jokioinen, Finland, 2010. [Google Scholar]
- Cruz, J.V.; Silva, M.O.; Dias, M.I.; Prudêncio, M.I. Groundwater composition and pollution due to agricultural practices at Sete Cidades volcano (Azores, Portugal). Appl. Geochem. 2013, 29, 162–173. [Google Scholar] [CrossRef]
- Pereira, A.; Fernandes, R.; Martins, A.; Freire, J. Exploration of Constructed Wetlands WWTPs: Seven Years of Águas do Algarve Experience. In INCREaSE 2017; Springer: Cham, Switzerland, 2018; pp. 612–623. [Google Scholar] [CrossRef]
- Molle, P.; Lombard Latune, R.; Riegel, C.; Lacombe, G.; Esser, D.; Mangeot, L. French vertical-flow constructed wetland design: Adaptations for tropical climates. Water Sci. Technol. 2015, 71, 1516–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quadros, S.; Rio, P.; Mesquita, E.; Rosa, M.J. Exploring the use of Azorean native plants and rock material for sustainable wastewater treatment through constructed wetland systems. In Proceedings of the 2nd European Water Association Spring Conference, Lisbon, Portugal, 10 May–11 May 2017. [Google Scholar]
- Miranda, P.M.A.; Valente, M.A.V.; Tomé, A.R.; Trigo, R.; Coelho, M.F.; Aguiar, A.; Azevedo, B. O clima de Portugal nos séculos XX e XXI. In Alterações Climáticas em Portugal: Cenários, Impactos e Medidas de Adaptação; Santos, F.D., Miranda, P.M.A., Eds.; Gradiva: Lisboa, Portugal, 2006; pp. 47–113. [Google Scholar]
- PROTA: Azores Regional Planning Plan 2021. Available online: http://ot.azores.gov.pt/Instrumentos-de-Gestao-Territorial-Documento.aspx?id=2 (accessed on 10 December 2021).
- Borges, P.A.; Costa, A.; Cunha, R.; Gabriel, R.; Gonçalves, V.; Martins, A.F.; Melo, I.; Parente, M.; Raposeiro, P.; Rodrigues, P.; et al. (Eds.) A List of the Terrestrial and Marine Biota from de Azores; Princípia: Cascais, Portugal, 2010. [Google Scholar]
- Borges Silva, L.; Pavão, D.C.; Elias, R.B.; Moura, M.; Ventura, M.A.; Silva, L. Taxonomic, structural diversity and carbon stocks in a gradient of island forests. Sci. Rep. 2022, 12, 1038. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, R.W.; Silva, D.C.C.; Martins, A.C.G.; Sales, J.C.A.; Roveda, S.R.M.M.; Roveda, J.A.F. Use of fuzzy systems in the elaboration of an anthropic pressure indicator to evaluate the remaining forest fragments. Environ. Earth Sci. 2015, 74, 2481–2488. [Google Scholar] [CrossRef]
- Costa, H.; Bettencourt, M.J.; Silva, C.; Teodósio, J.; Gil, A.; Silva, L. Invasive alien plants in the Azorean protected areas: Invasion status and mitigation actions. In Plant Invasions in Protected Areas; Foxcroft, L., Pyšek, P., Richardson, D., Genovesi, P., Eds.; Invading Nature—Springer Series in Invasion Ecology; Springer: Dordrecht, The Netherlands, 2013; Volume 7, pp. 375–394. [Google Scholar] [CrossRef]
- Silva, L.; Land, E.O.; Luengo, J.L.R. (Eds.) Invasive Terrestrial Flora and Fauna in Macaronesia: TOP 100 in Azores, Madeira and Canaries; ARENA: Ponta Delgada, Portugal, 2008. [Google Scholar]
- Hand, M.L.; Cogan, N.O.; Stewart, A.V.; Forster, J.W. Evolutionary history of tall fescue morphotypes inferred from molecular phylogenetics of the Lolium-Festuca species complex. BMC Evol. Biol. 2010, 10, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura, M.; Silva, L.; Dias, E.F.; Schaefer, H.; Carine, M.A. A revision of the genus Leontodon in the Azores based on morphological and molecular evidence. Phytotaxa 2015, 210, 024–046. [Google Scholar] [CrossRef] [Green Version]
- Dias, E.F.; Moura, M.; Schaefer, H.; Silva, L. Geographical distance and barriers explain population genetic patterns in an endangered island perennial. AoB Plants 2016, 8, plw072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ABP. Azorean Biodiversity Portal. 2022. Available online: https://azoresbioportal.uac.pt (accessed on 20 July 2022).
- CABI. Invasive Species Compendium; CAB International: Wallingford, UK, 2022; Available online: www.cabi.org/isc (accessed on 1 September 2022).
- EOL. Encyclopedia of Life. 2022. Available online: http://eol.org (accessed on 22 July 2022).
- Franco, J.A.; Afonso, M.L.R. Nova Flora de Portugal—Vol. III—Fascículo I; Escolar Editora: Forte da Casa, Portugal, 1994. [Google Scholar]
- Franco, J.A.; Afonso, M.L.R. Nova Flora de Portugal—Vol. III—Fascículo II; Escolar Editora: Forte da Casa, Portugal, 1998. [Google Scholar]
- Franco, J.A.; Afonso, M.L.R. Nova Flora de Portugal—Vol. III—Fascículo III; Escolar Editora: Forte da Casa, Portugal, 2003. [Google Scholar]
- GBIF.Org GBIF Home Page. 2022. Available online: https://www.gbif.org (accessed on 22 July 2022).
- JBUTAD. Portal do Jardim Botânico da Universidade de Trás-os-Montes e Alto Douro, Flora Digital de Portugal. 2022. Available online: https://jb.utad.pt/ (accessed on 22 July 2022).
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet. 2022. Available online: http://www.plantsoftheworldonline.org/ (accessed on 22 July 2022).
- USDA, NRCS. United States Department of Agriculture -Natural Resources Conservation Service. The Plants Database, Greensboro, NC USA. 2022. Available online: https://www.nrcs.usda.gov/ (accessed on 22 July 2022).
- Calheiros, C.S.C.; Rangel, A.O.S.S.; Castro, P.M.L. Constructed wetland systems vegetated with different plants applied to the treatment of tannery wastewater. Water Res. 2007, 41, 1790–1798. [Google Scholar] [CrossRef]
- Korkusuz, E.A. Manual of practice on constructed wetlands for wastewater treatment and reuse in Mediterranean countries. Tech. Rep. AVKR 2005, 5. [Google Scholar] [CrossRef]
- Simpson, D.A.; Yesson, C.; Culham, A.; Couch, C.A.; Muasya, A.M. Climate change and Cyperaceae. In Climate Change, Ecology and Systematics; Hodkinson, T., Jones, M., Waldren, S., Parnell, J., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 439–456. [Google Scholar]
- Murray-Gulde, C.L.; Huddleston, G.M.; Garber, K.V.; Rodgers, J.H. Contributions of Schoenoplectus californicus in a constructed wetland system receiving copper contaminated wastewater. Water Air Soil Pollut. 2005, 163, 355–378. [Google Scholar] [CrossRef]
- Tanner, C.C. Plants for constructed wetland treatment systems: A comparison of the growth and nutrient uptake of eight emergent species. Ecol. Eng. 1996, 7, 59–83. [Google Scholar] [CrossRef]
- Ganjo, D.G.A.; Mirza, H.A. Cyperus longus L. as a biological purifier of wastewater for irrigation purposes: Removal efficiency and Zn, Cd, Cu, Fe and Mn. Environ. Health Risk VII 2013, 16, 249. [Google Scholar]
- Greenway, M.; Woolley, A. Constructed wetlands in Queensland: Performance efficiency and nutrient bioaccumulation. Ecol. Eng. 1999, 12, 39–55. [Google Scholar] [CrossRef]
- Greenway, M. Suitability of macrophytes for nutrient removal from surface flow constructed wetlands receiving secondary treated sewage effluent in Queensland, Australia. Water Sci. Technol. 2003, 48, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Greenway, M. The role of macrophytes in nutrient removal using constructed wetlands. In Environmental Bioremediation Technologies; Singh, S.N., Tripathi, R.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 331–351. [Google Scholar]
- Jampeetong, A.; Brix, H.; Kantawanichkul, S. Effects of inorganic nitrogen forms on growth, morphology, nitrogen uptake capacity and nutrient allocation of four tropical aquatic macrophytes (Salvinia cucullata, Ipomoea aquatica, Cyperus involucratus and Vetiveria zizanioides). Aquat. Bot. 2012, 97, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Sohsalam, P.; Englande, A.J.; Sirianuntapiboon, S. Seafood wastewater treatment in constructed wetland: Tropical case. Bioresour. Technol. 2008, 99, 1218–1224. [Google Scholar] [CrossRef]
- Pincam, T.; Brix, H.; Jampeetong, A. Growth performance of tropical wetland species (Cyperus involucratus Rottb. and Thalia geniculata L.) in anaerobic digester effluent and their water treatment efficiency. Ecol. Eng. 2020, 143, 105667. [Google Scholar] [CrossRef]
- Cui, L.H.; Zhu, X.Z.; Ouyang, Y.; Chen, Y.; Yang, F.L. Total phosphorus removal from domestic wastewater with Cyperus alternifolius in vertical-flow constructed wetlands at the microcosm level. Int. J. Phytoremediation 2011, 13, 692–701. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Taheri, E.; Ehrampoush, M.H.; Nasiri, S.; Jalali, F.; Soltani, R.; Fatehizadeh, A. Efficiency of constructed wetland vegetated with Cyperus alternifolius applied for municipal wastewater treatment. J. Environ. Public Health 2013, 815962. [Google Scholar] [CrossRef] [Green Version]
- Thongtha, S.; Teamkao, P.; Boonapatcharoen, N.; Tripetchkul, S.; Techkarnjararuk, S.; Thiravetyan, P. Phosphorus removal from domestic wastewater by Nelumbo nucifera Gaertn. and Cyperus alternifolius L. J. Environ. Manag. 2014, 137, 54–60. [Google Scholar] [CrossRef]
- Liao, X.; Luo, S.; Wu, Y.; Wang, Z. Comparison of nutrient removal ability between Cyperus alternifolius and Vetiveria zizanioides in constructed wetlands. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2005, 16, 156–160. [Google Scholar]
- Bragato, C.; Brix, H.; Malagoli, M. Accumulation of nutrients and heavy metals in Phragmites australis (Cav.) Trin. ex Steudel and Bolboschoenus maritimus (L.) Palla in a constructed wetland of the Venice lagoon watershed. Environ. Pollut. 2006, 144, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.M.; Inouye, R.S. Vegetative nutrient pools in a constructed wetland in southeaster Idaho. J. Freshw. Ecol. 2006, 21, 593–601. [Google Scholar] [CrossRef]
- de Oliveira, J.P.V.; Pereira, M.P.; Duarte, V.P.; Corrêa, F.F.; de Castro, E.M.; Pereira, F.J. Root anatomy, growth, and development of Typha domingensis Pers. (Typhaceae) and their relationship with cadmium absorption, accumulation, and tolerance. Environ. Sci. Pollut. Res. 2022, 29, 19878–19889. [Google Scholar] [CrossRef]
- Hegazy, A.K.; Abdel-Ghani, N.T.; El-Chaghaby, G.A. Phytoremediation of industrial wastewater potentiality by Typha domingensis. Int. J. Environ. Sci. Technol. 2011, 8, 639–648. [Google Scholar] [CrossRef] [Green Version]
- Lorenzen, B.; Brix, H.; Mendelssohn, I.A.; McKee, K.L.; Miao, S.L. Growth, biomass allocation and nutrient use efficiency in Cladium jamaicense and Typha domingensis as affected by phosphorus and oxygen availability. Aquat. Bot. 2001, 70, 117–133. [Google Scholar] [CrossRef]
- Balachandran, T.; Nanthakumaran, A.; Sukanyah, S.; Sivanesan, K.S. Role of Colocasia esculenta in Constructed Wetlands for Treating Rice mill Wastewater. J. Agric. Sci. 2018, 12, 19–26. [Google Scholar] [CrossRef]
- Bindu, T.; Sylas, V.P.; Mahesh, M.; Rakesh, P.S.; Ramasamy, E.V. Pollutant removal from domestic wastewater with Taro (Colocasia esculenta) planted in a subsurface flow system. Ecol. Eng. 2008, 33, 68–82. [Google Scholar] [CrossRef]
- Chayapan, P.; Kruatrachue, M.; Meetam, M.; Pokethitiyook, P. Phytoremediation potential of Cd and Zn by wetland plants, Colocasia esculenta L. Schott., Cyperus malaccensis Lam., and Typha angustifolia L. grown in hydroponics. J. Environ. Biol. 2015, 36, 1179. [Google Scholar]
- Madera-Parra, C.A.; Peña-Salamanca, E.J.; Peña, M.R.; Rousseau DP, L.; Lens, P.N.L. Phytoremediation of landfill leachate with Colocasia esculenta, Gynerum sagittatum and Heliconia psittacorum in constructed wetlands. Int. J. Phytoremediation 2015, 17, 16–24. [Google Scholar] [CrossRef]
- Rai, U.N.; Tripathi, R.D.; Singh, N.K.; Upadhyay, A.K.; Dwivedi, S.; Shukla, M.K.; Mallick, S.; Singh, S.N.; Nautiyal, C.S. Constructed wetland as an ecotechnological tool for pollution treatment for conservation of Ganga river. Bioresour. Technol. 2013, 148, 535–541. [Google Scholar] [CrossRef]
- Rana, V.; Maiti, S.K. Municipal wastewater treatment potential and metal accumulation strategies of Colocasia esculenta (L.) Schott and Typha latifolia L. in a constructed wetland. Environ. Monit. Assess. 2018, 190, 328. [Google Scholar] [CrossRef] [PubMed]
- Skinner, K.W.N.; Porter-Goff, E. Mercury uptake and accumulation by four species of aquatic plants. Environ. Pollut. 2007, 145, 234–237. [Google Scholar] [CrossRef]
- Mbuligwe, S.E. Comparative effectiveness of engineered wetland systems in the treatment of anaerobically pre-treated domestic wastewater. Ecol. Eng. 2004, 23, 269–284. [Google Scholar] [CrossRef]
- Coleman, J.; Hench, K.; Garbutt, K.; Sexstone, A.; Bissonnette, G.; Skousen, J. Treatment of domestic wastewater by three plant species in constructed wetlands. Water Air Soil Pollut. 2001, 128, 283–295. [Google Scholar] [CrossRef]
- Dias, V.N.; Pacheco, P.M. Constructed wetlands for wastewater treatment in Portugal: A global overview. In Transformations of Nutrients in Natural and Constructed Wetlands; Vymazal, J., Ed.; Backhuys Publishers: Leiden, The Netherlands, 2001; pp. 271–303. [Google Scholar]
- Duarte, A.A.; Seco, T.C.; Peres, J.A.; Bentes, I.; Pinto, J. Performance Evaluation of Portuguese Constructed Wetlands for Municipal Wastewater Treatment. In Advances in Waste Management, Proceedings of the 4th WSEAS International Conference on Waste Management, Water Pollution, Air Pollution, Indoor Climate (WWAI ‘10), Kantaoui, Tunisia, 3–6 May 2010; World Scientific and Engineering Academy and Society (WSEAS), 2010; Available online: https://hdl.handle.net/1822/17617 (accessed on 20 August 2022).
- Maurício, J.F.M. Zonas Húmidas Construídas Para Tratamento Complementar de Efluentes Domésticos: Ensaio Experimental na ETAR da Maia –Ribeira Grande (Constructed Wetlands for the Complementary Treatment of domestic Effluents: Experimental Test at the WWTP of Maia –Ribeira Grande). Master’s Thesis, University of Azores, Ponta Delgada, Portugal, 2019. [Google Scholar]
- Batriu, E.; Ninot, J.M.; Pino, J. Interactions between transplants of Phragmites australis and Juncus acutus in Mediterranean coastal marshes: The modulating role of environmental gradients. Aquat. Bot. 2015, 124, 29–38. [Google Scholar] [CrossRef]
- Liu, F.; Sun, L.; Wan, J.; Shen, L.; Yu, Y.; Hu, L.; Zhou, Y. Performance of different macrophytes in the decontamination of and electricity generation from swine wastewater via an integrated constructed wetland-microbial fuel cell process. J. Environ. Sci. 2020, 89, 252–263. [Google Scholar] [CrossRef]
- Mohammed, N.A.; Ismail, Z.Z. Green sustainable technology for biotreatment of actual dairy wastewater in constructed wetland. J. Chem. Technol. Biotechnol. 2020, 96, 1197–1204. [Google Scholar] [CrossRef]
- Lai, W.L.; Wang, S.Q.; Peng, C.L.; Chen, Z.H. Root features related to plant growth and nutrient removal of 35 wetland plants. Water Res. 2011, 45, 3941–3950. [Google Scholar] [CrossRef]
- Stottmeister, U.; Wießner, A.; Kuschk, P.; Kappelmeyer, U.; Kästner, M.; Bederski, O.; Müller, R.A.; Moormann, H. Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol. Adv. 2003, 22, 93–117. [Google Scholar] [CrossRef]
- Weber, K.P.; Gehder, M.; Legge, R.L. Assessment of changes in the microbial community of constructed wetland mesocosms in response to acid mine drainage exposure. Water Res. 2008, 42, 180–188. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Chen, W.Y.; Gu, B.H.; Liu, X.C.; Chen, F.; Chen, Z.H.; Zhou, X.Y.; Li, Y.X.; Huang, H.; Chen, Y.J. Morphology, ecology and contaminant removal efficiency of eight wetland plants with differing root systems. Hydrobiologia 2009, 623, 77–85. [Google Scholar] [CrossRef]
- Kyambadde, J.; Kansiimea, F.; Gumaeliusb, L.; Dalhammar, G. A comparative study of Cyperus papyrus and Miscanthidium violaceum-based constructed wetlands for wastewater treatment in a tropical climate. Water Res. 2004, 38, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Karathanasis, A.D.; Potter, C.L.; Coyne, M.S. Vegetation effects on fecal bacteria, BOD, and suspended solid removal in constructed wetlands treating domestic wastewater. Ecol. Eng. 2003, 20, 157–169. [Google Scholar] [CrossRef]
- Juárez-Rosete, C.R.; Olivo-Rivas, A.; Aguilar-Castillo, J.A.; Bugarín-Montoya, R.; Arrieta-Ramos, B.G. Nutrition assessment of NPK in mint (Mentha spicata L.) cultivated in soilless system. Annu. Res. Rev. Biol. 2014, 4, 2462–2470. [Google Scholar] [CrossRef]
- Espinosa Moya, E.A.; Angel Sahagún, C.A.; Mendoza Carrillo, J.M.; Albertos Alpuche, P.J.; Álvarez-González, C.A.; Martínez-Yáñez, R. Herbaceous plants as part of biological filter for aquaponics system. Aquac. Res. 2016, 47, 1716–1726. [Google Scholar] [CrossRef]
- Silva, C.M.; Silva, L.; Oliveira, N.; Geraldes, P.; Hervías, S. Control of giant reed Arundo donax on Vila Franca do Campo Islet, Azores, Portugal. Conserv. Evid. 2011, 8, 93–99. [Google Scholar]
- Calheiros, C.S.; Quitério, P.V.; Silva, G.; Crispim LF Brix, H.; Moura, S.C.; Castro, P.M. Use of constructed wetland systems with Arundo and Sarcocornia for polishing high salinity tannery wastewater. J. Environ. Manag. 2012, 95, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Idris, S.M.; Jones, P.L.; Salzman, S.A.; Croatto, G.; Allinson, G. Evaluation of the giant reed (Arundo donax) in horizontal subsurface flow wetlands for the treatment of recirculating aquaculture system effluent. Environ. Sci. Pollut. Res. 2012, 19, 1159–1170. [Google Scholar] [CrossRef]
- Leto, C.; Tuttolomondo, T.; Bella, S.L.; Leone, R.; Licata, M. Growth of Arundo donax L. and Cyperus alternifolius L. in a horizontal subsurface flow constructed wetland using pre-treated urban wastewater—A case study in Sicily (Italy). Desalination Water Treat. 2013, 51, 7447–7459. [Google Scholar] [CrossRef]
- Sylla, A. Domestic wastewater treatment using vertical flow constructed wetlands planted with Arundo donax, and the intermittent sand filters impact. Ecohydrol. Hydrobiol. 2020, 20, 48–58. [Google Scholar] [CrossRef]
- Ayres, J.R.; Awad, J.; Burger, H.; Marzouk, J.; van Leeuwen, J. Investigation of the potential of buffalo and couch grasses to grow on AFIs and for removal of nutrients from paper mill wastewater. Water Sci. Technol. 2019, 79, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Saxena, G.; Goutam, S.P.; Mishra, A.; Mulla, S.I.; Bharagava, R.N. Emerging and ecofriendly technologies for the removal of organic and inorganic pollutants from industrial wastewaters. In Bioremediation of Industrial Waste for Environmental Safety; Springer: Singapure, 2020; pp. 113–126. [Google Scholar] [CrossRef]
- Vymazal, J. The use of sub-surface constructed wetlands for wastewater treatment in the Czech Republic. In Focus on Ecology Research; Burk, A.R., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2006; pp. 175–196. [Google Scholar]
- Wrobel, C.; Coulman, B.E.; Smith, D.L. The potential use of reed canarygrass (Phalaris arundinacea L.) as a biofuel crop. Acta Agric. Scand. Sect. B–Soil Plant Sci. 2009, 59, 1–18. [Google Scholar] [CrossRef]
- Matheson, F.E. Nitrogen Removal and the Fate of Nitrate in Riparian Buffer Zones. Ph.D. Thesis, Durham University, Durham, UK, 2001. [Google Scholar]
- Matthews, D.J.; Moran, B.M.; McCabe, P.F.; Otte, M.L. Zinc tolerance, uptake, accumulation and distribution in plants and protoplasts of five European populations of the wetland grass Glyceria Flutings. Aquat. Bot. 2004, 80, 39–52. [Google Scholar] [CrossRef]
- Al-Omari, A.; Fayyad, M. Treatment of domestic wastewater by subsurface flow constructed wetlands in Jordan. Desalination 2003, 155, 27–39. [Google Scholar] [CrossRef]
- Brown, P.J.; Cross, R.B.; McCarty, L.B.; Kerr, R.A. Control of torpedograss (Panicum repens) and Southern watergrass (Luziola fluitans) in bermudagrass turf. Weed Technol. 2019, 33, 616–619. [Google Scholar] [CrossRef]
- El-Gendy, A.S.; El-Kassas, H.I.; Razek, T.A.; Abdel-Latif, H. Phyto-dewatering of sewage sludge using Panicum repens L. Water Sci. Technol. 2017, 75, 1667–1674. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Gao, G.; Yang, J.; Yuan, J.; Yang, Z. The integrated response of torpedo grass (Panicum repens) to Cd–Pb co-exposures. Ecol. Eng. 2015, 82, 428–431. [Google Scholar] [CrossRef]
- Ghaly, F.M. Role of natural vegetation in improving salt affected soil in northern Egypt. Soil Tillage Res. 2002, 64, 173–178. [Google Scholar] [CrossRef]
- Licata, M.; Gennaro, M.C.; Tuttolomondo, T.; Leto, C.; La Bella, S. Research focusing on plant performance in constructed wetlands and agronomic application of treated wastewater–A set of experimental studies in Sicily (Italy). PLoS ONE 2019, 14, e0219445. [Google Scholar] [CrossRef]
- Nema, A.; Yadav, K.D.; Christian, R.A. A small-scale study of plant orientation in treatment performance of vertical flow constructed wetland in continuous flow. Int. J. Phytoremediation 2020, 22, 849–856. [Google Scholar] [CrossRef]



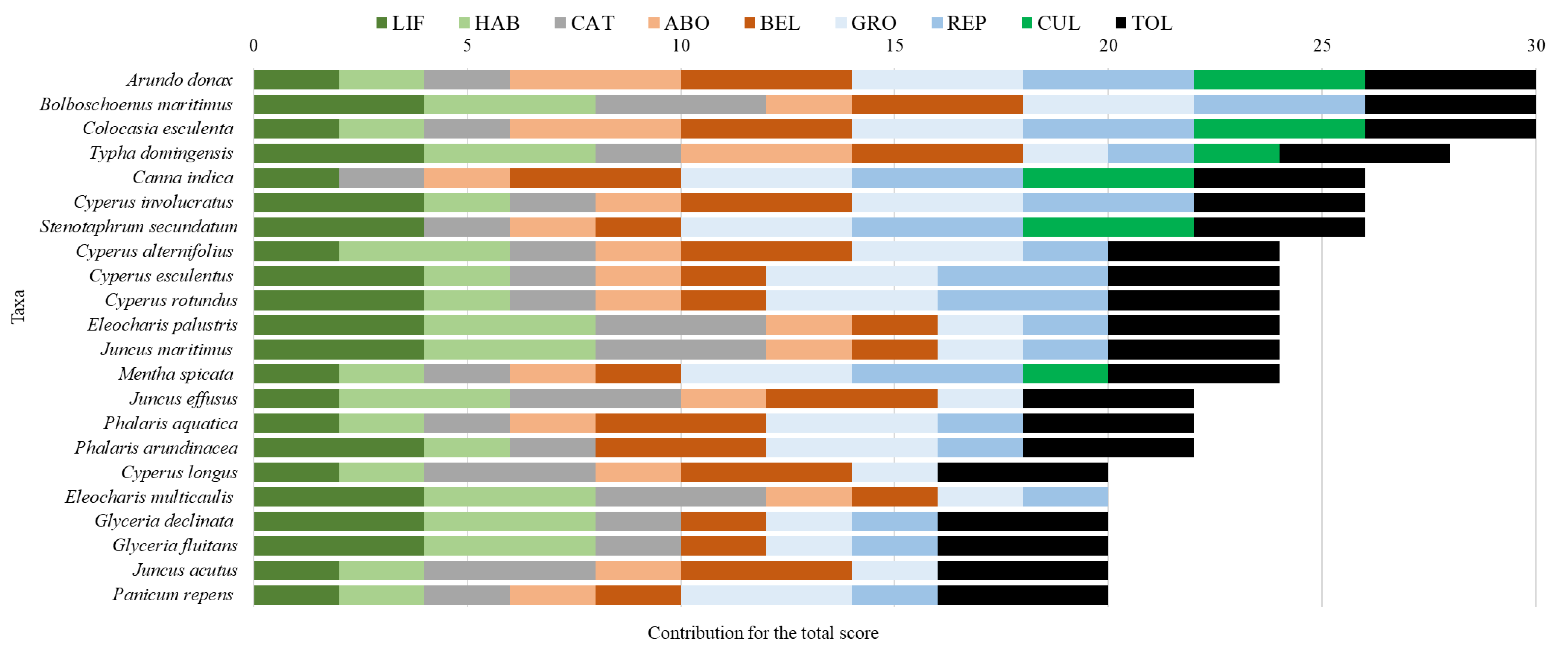
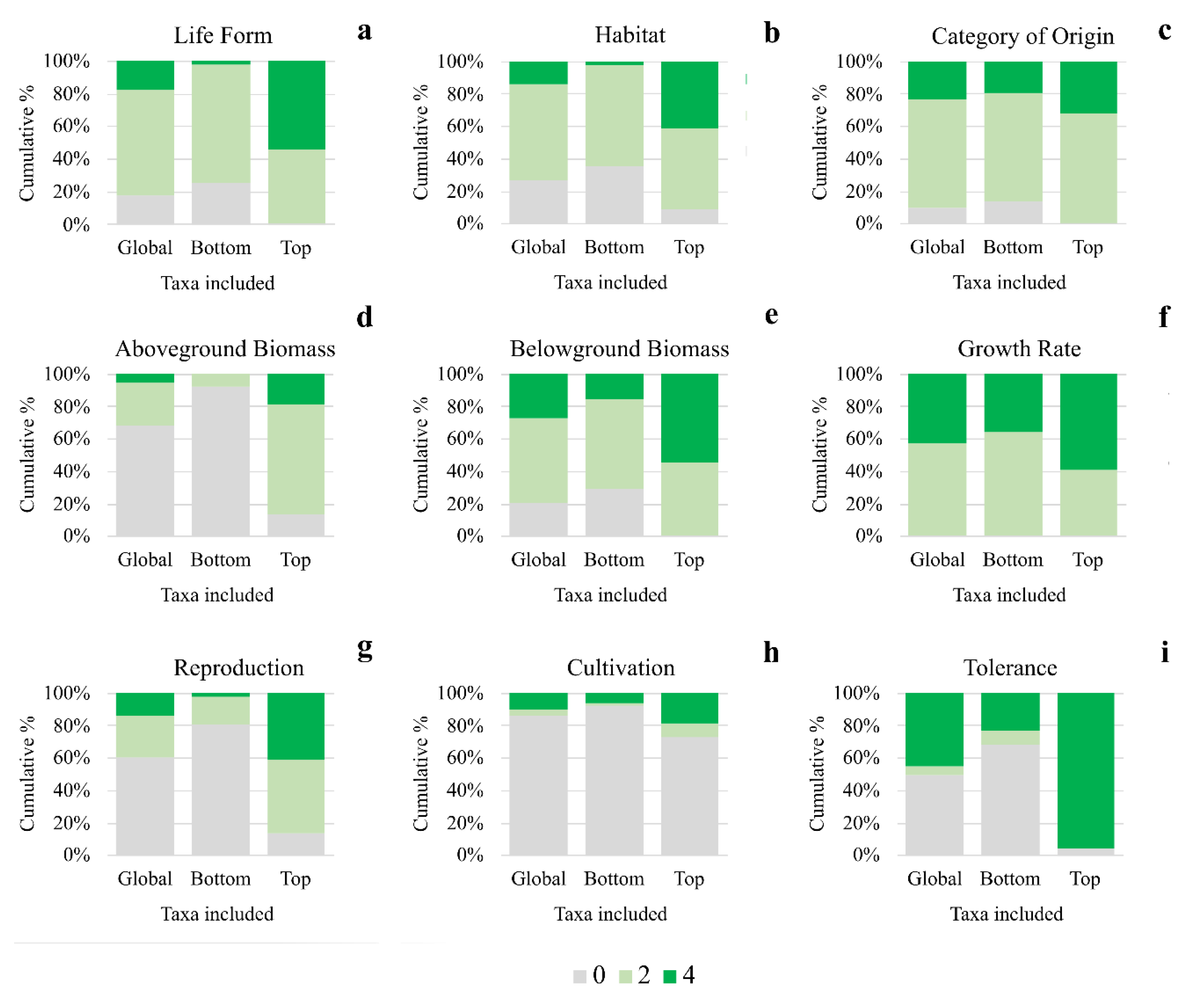
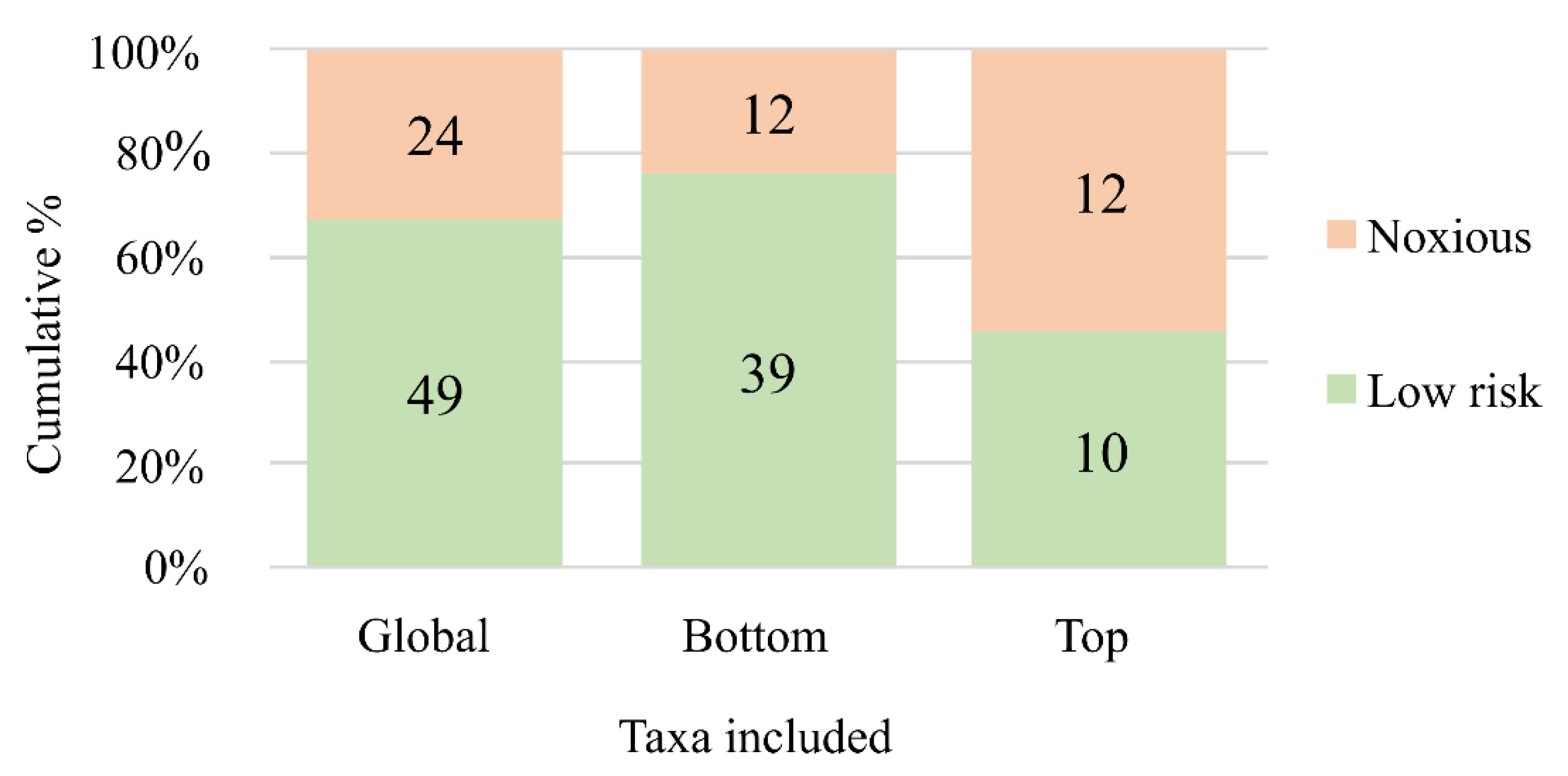
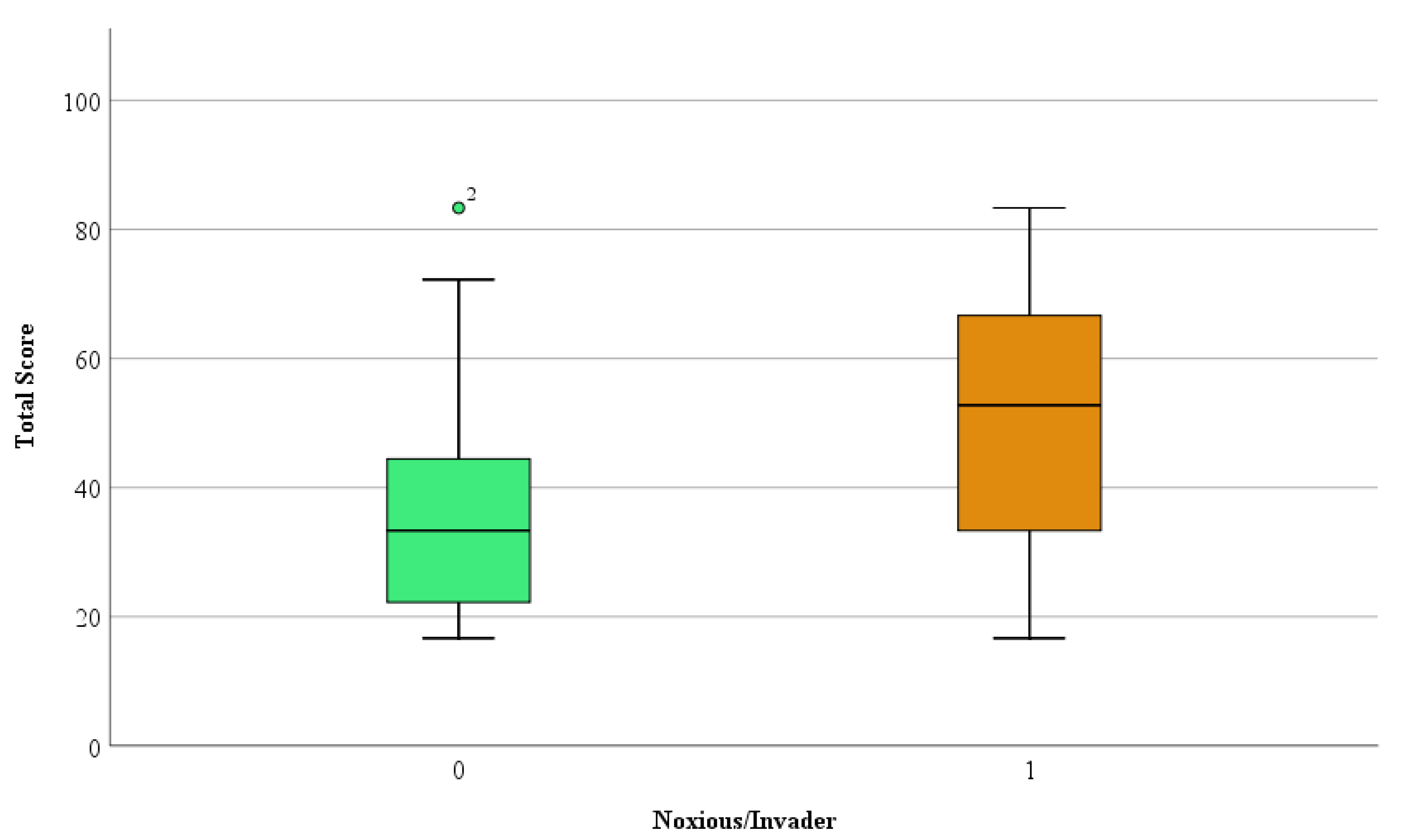

| Criteria | Parameters | Points | |
|---|---|---|---|
| 1 | Life form | Helophyte, Hydrophyte, Hygrophyte | 4 |
| Proto-hemicryptophyte, Hemicryptophyte, Microphanerophyte | 2 | ||
| Therophyte | 0 | ||
| 2 | Habitat | Wetlands | 4 |
| Terrestrial or wetlands, Terrestrial or Humid soils | 2 | ||
| Terrestrial | 0 | ||
| 3 | Category of origin | Native | 4 |
| Naturalized, Casual | 2 | ||
| Endemic | 0 | ||
| 4 | Aboveground biomass | Large aboveground organs (>1.5 m height, large leaves with >0.5 m long) | 4 |
| Medium sized aboveground organs (<1.5 m height, leaves up to 0.6 m long) | 2 | ||
| Thin, slender stems or culms and small leaves (< 1 m height, leaves up to 0.5 m and 0.1 m wide) | 0 | ||
| 5 | Belowground organs | Large rhizome, Corm | 4 |
| Rhizome, tuberose root, bulb, tuber | 2 | ||
| Short fibrous roots | 0 | ||
| 6 | Potential growth rate (In a season) | High | 4 |
| Medium | 2 | ||
| Low | 0 | ||
| 7 | Reproduction | Vegetative reproduction | 4 |
| Mainly by seed but some vegetative spread | 2 | ||
| Only by seed | 0 | ||
| 8 | Cultivation | Cultivated in large areas | 4 |
| Cultivated in small areas | 2 | ||
| Not cultivated | 0 | ||
| 9 | Tolerance to nutrient loadings and removal efficiency | High tolerance and/or high nutrient removal | 4 |
| Presence in habitats with nutrients, but no data on nutrient removal | 2 | ||
| No tolerance or no data | 0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raposo, V.B.; Silva, L.; Quadros, S. Azorean Vascular Plants with Potential Use in Constructed Wetlands with Horizontal Subsurface Flow. Sustainability 2022, 14, 14681. https://doi.org/10.3390/su142214681
Raposo VB, Silva L, Quadros S. Azorean Vascular Plants with Potential Use in Constructed Wetlands with Horizontal Subsurface Flow. Sustainability. 2022; 14(22):14681. https://doi.org/10.3390/su142214681
Chicago/Turabian StyleRaposo, Vera B., Luís Silva, and Sílvia Quadros. 2022. "Azorean Vascular Plants with Potential Use in Constructed Wetlands with Horizontal Subsurface Flow" Sustainability 14, no. 22: 14681. https://doi.org/10.3390/su142214681






