Chemometric Screening of Oregano Essential Oil Composition and Properties for the Identification of Specific Markers for Geographical Differentiation of Cultivated Greek Oregano
Abstract
1. Introduction
2. Materials and Methods
2.1. Oregano Samples
2.2. Extraction of EOs
2.3. Gas Chromatography–Mass Spectrometry (GC/MS) Instrumentation and Analysis Conditions
2.4. Determination of Total Phenolic Content (TPC) and Antioxidant Activity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Essential Oil Chemical Composition
3.2. Total Phenolic Content and Antioxidant Activity
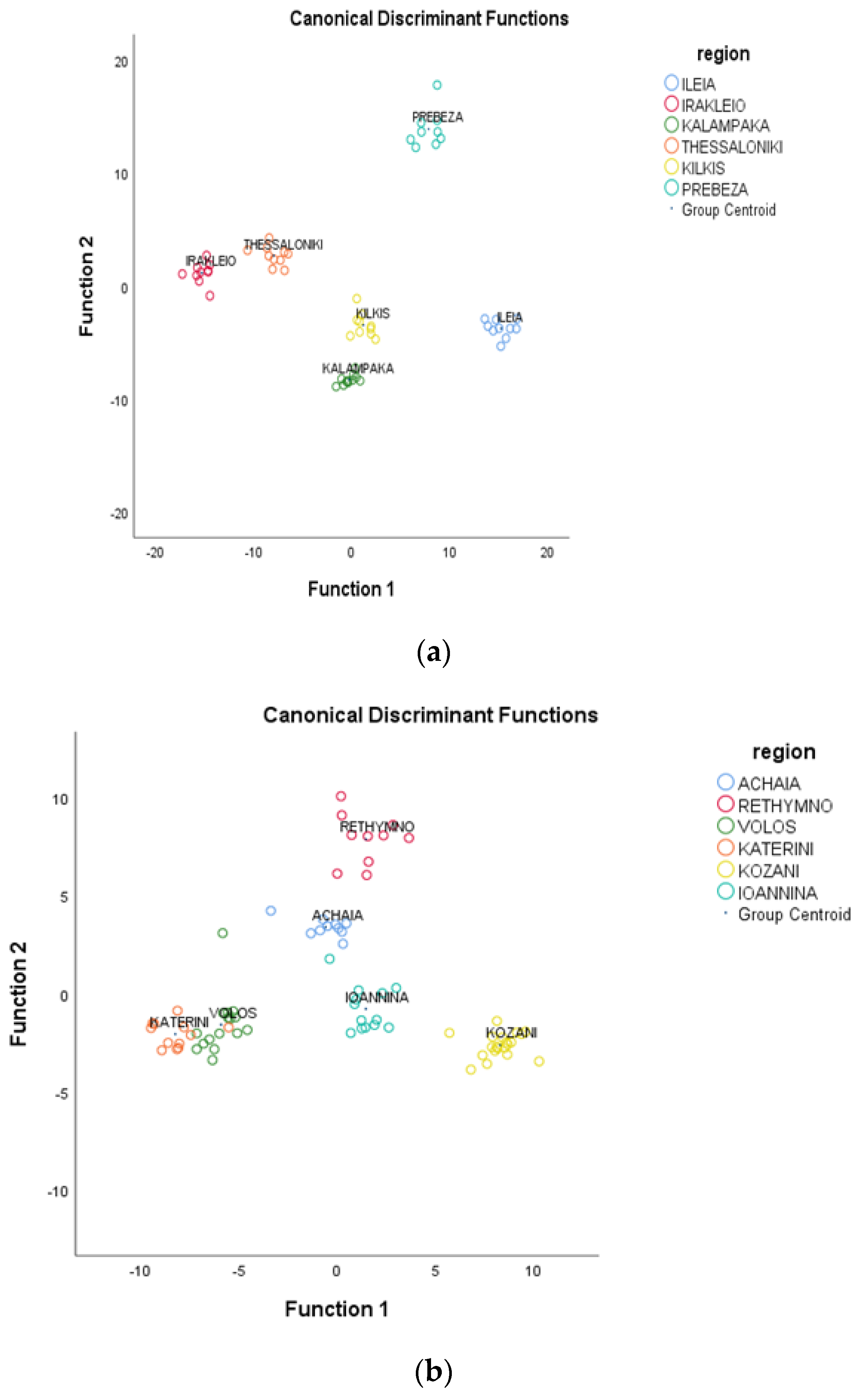
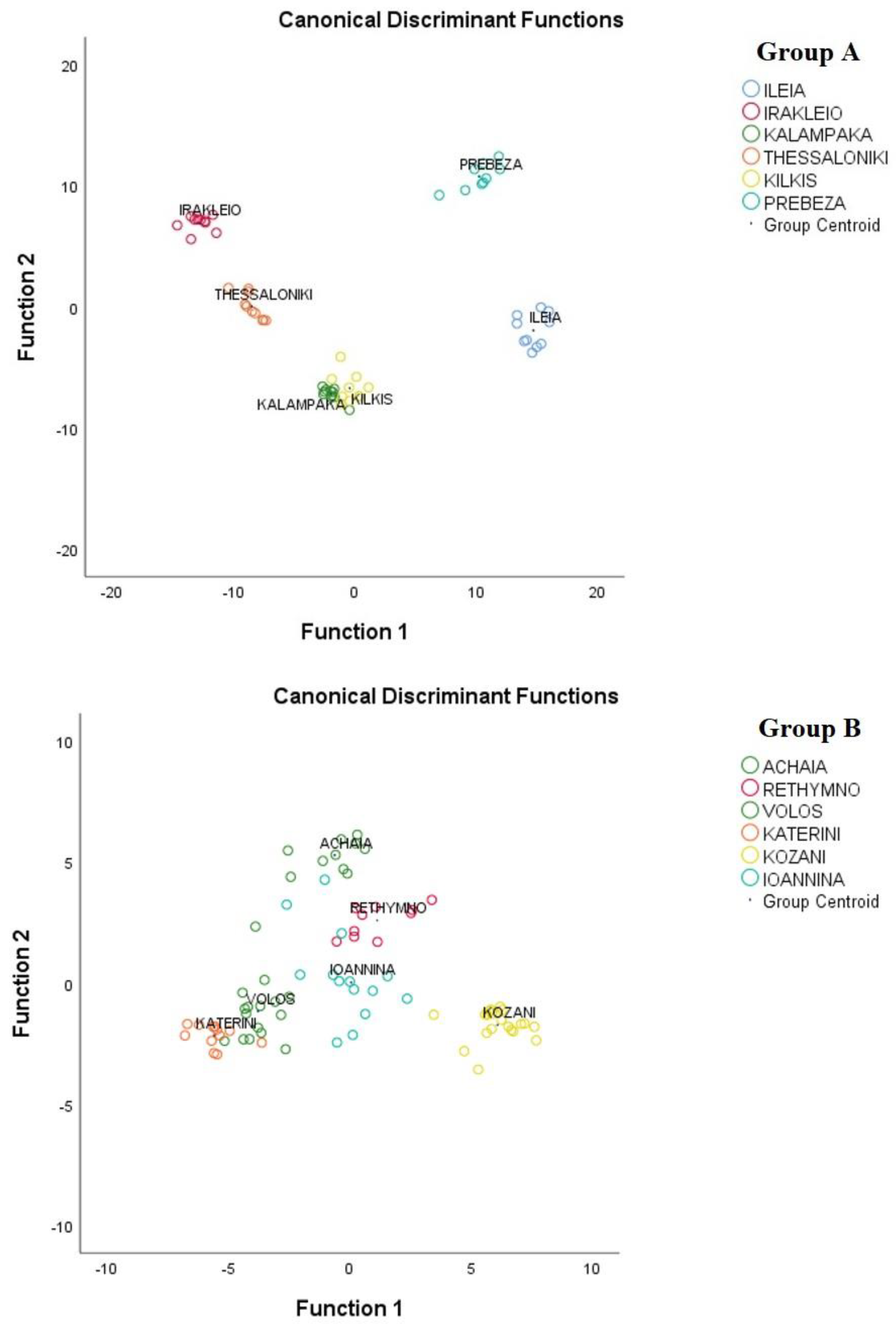
3.3. Geographical Differentiation of Greek Oregano Based on EO Composition, TPC, and Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, S.; El, S.N.; Karagözlü, N.; Şahin, S. Antioxidant and Antimicrobial Activities of Essential Oils Obtained from Oregano (Origanum vulgare ssp. hirtum) by Using Different Extraction Methods . J. Med. Food 2011, 14, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Kokkini, S. Taxonomy, Diversity and Distribution of Origanum Species. In Proceedgs of the IPGRI International Workshop on Oregano, Valenzano, Italy, 8–12 May 1996; Padulosi, S., Ed.; IPGRI: Bari, Italy, 1996; pp. 2–12. [Google Scholar]
- Skoula, M.; Harborne, J. The Taxonomy and Chemistry of Origanum. In Oregano: The Genera Origanum and Lippia; Kintzios, S.E., Ed.; Taylor & Francis: London, UK, 2002; pp. 67–108. [Google Scholar]
- Stefanakis, M.K.; Touloupakis, E.; Anastasopoulos, E.; Ghanotakis, D.; Katerinopoulos, H.E.; Makridis, P. Antibacterial Activity of Essential Oils from Plants of the Genus Origanum. Food Control 2013, 34, 539–546. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils-A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef]
- Kintzios, S.E. Profile of the multifaceted prince of the herbs. In Oregano: The Genera Origanum and Lippia; Kintzios, S.E., Ed.; Taylor & Francis: London, UK, 2002; pp. 3–8. [Google Scholar]
- Kokkini, S.; Vokou, D.D.; Karousou, R.R. Morphological and Chemical Variation of Origanum vulgare L. in Greece. Bot. Chron. 1991, 10, 337–346. [Google Scholar]
- Tucker, A.O.; Maciarello, M.J. Oregano: Botany, Chemistry, and Cultivation. Dev. Food Sci. 1994, 34, 439–456. [Google Scholar]
- Soltani, S.; Shakeri, A.; Iranshahi, M.; Boozari, M. A Review of the Phytochemistry and Antimicrobial Properties of Origanum vulgare L. and Subspecies. Iran. J. Pharm. Res. 2021, 20, 268–285. [Google Scholar] [CrossRef]
- Węglarz, Z.; Kosakowska, O.; Przybył, J.L.; Pióro-Jabrucka, E.; Bączek, K. The Quality of Greek Oregano (O. vulgare L. subsp. Hirtum (Link) Ietswaart) and Common Oregano (O. vulgare L. subsp. Vulgare) Cultivated in the Temperate Climate of Central Europe. Foods 2020, 9, 1671. [Google Scholar] [CrossRef]
- Skoufogianni, E.; Solomou, A.D.; Danalatos, N.G. View of Ecology, Cultivation and Utilization of the Aromatic Greek Oregano (Origanum vulgare L.): A Review. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 545–552. [Google Scholar] [CrossRef]
- Vokou, D.; Kokkini, S.; Bessiere, J.-M.M.; Kokkinit, S.; Bessiere, J.-M.M.; Kokkini, S.; Bessiere, J.-M.M. Geographic Variation of Greek Oregano (Origanum vulgare ssp. Hirtum) Essential Oils . Biochem. Syst. Ecol. 1993, 21, 287–295. [Google Scholar] [CrossRef]
- Kokkini, S.; Karousou, R.; Vokou, D. Pattern of Geographic Variations of Origanum vulgare Trichomes and Essential Oil Content in Greece. Biochem. Syst. Ecol. 1994, 22, 517–528. [Google Scholar] [CrossRef]
- Wogiatzi, E.; Gougoulias, N.; Papachatzis, A.; Vagelas, I.; Chouliaras, N. Greek Oregano Essential Oils Production, Phytotoxicity, and Antifungal Activity. Biotechnol. Biotechnol. Equip. 2009, 23, 1150–1152. [Google Scholar] [CrossRef]
- Skoula, M.; Gotsiou, P.; Naxakis, G.; Johnson, C.B. A Chemosystematic Investigation on the Mono- and Sesquiterpenoids in the Genus Origanum (Labiatae). Phytochemistry 1999, 52, 649–657. [Google Scholar] [CrossRef]
- Stefanaki, A.; van Andel, T. Mediterranean Aromatic Herbs and Their Culinary Use. In Aromatic Herbs in Food. Bioactive Compounds, Processing, and Applications, 1st ed.; Galanakis, C.M., Ed.; Academic Press: London, UK, 2021; Chapter 3; pp. 93–121. [Google Scholar]
- Kokkini, S.; Karousou, R.; Dardioti, A.; Krigas, N.; Lanaras, T. Autumn Essential Oils of Greek Oregano. Phytochemistry 1997, 44, 883–886. [Google Scholar] [CrossRef]
- Russo, M.; Galletti, G.C.; Bocchini, P.; Carnacini, A. Essential Oil Chemical Composition of Wild Populations of Italian Oregano Spice (Origanum vulgare ssp. Hirtum (Link) Ietswaart): A Preliminary Evaluation of Their Use in Chemotaxonomy by Cluster Analysis. 1. Inflorescences. J. Agric. Food Chem. 1998, 46, 3741–3746. [Google Scholar] [CrossRef]
- Jing, D.; Deguang, W.; Linfang, H.; Shilin, C.; Minjian, Q. Application of Chemometrics in Quality Evaluation of Medicinal Plants. J. Med. Plants Res. 2011, 5, 4001–4008. [Google Scholar]
- Andre, C.M.; Soukoulis, C. Food Quality Assessed by Chemometrics. Foods 2020, 9, 897. [Google Scholar] [CrossRef]
- Dimitrakopoulou, M.E.; Vantarakis, A. Does Traceability Lead to Food Authentication? A Systematic Review from A European Perspective. Food Rev. Int. 2021. [Google Scholar] [CrossRef]
- Nousias, P.; Karabagias, I.K.; Kontakos, S.; Riganakos, K.A. Characterization and Differentiation of Greek Commercial Thyme Honeys According to Geographical Origin Based on Quality and Some Bioactivity Parameters Using Chemometrics. J. Food Process. Preserv. 2017, 41, e13061. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Badeka, A.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Characterization and Classification of Thymus Capitatus (L.) Honey According to Geo-graphical Origin Based on Volatile Compounds, Physicochemical Parameters and Chemometrics. Food Res. Int. 2014, 55, 363–372. [Google Scholar] [CrossRef]
- Senyuva, H.Z.; Gilbert, J.; Silici, S.; Charlton, A.; Dal, C.; Gürel, N.; Cimen, D. Profiling Turkish Honeys to Determine Authenticity Using Physical and Chemical Characteristics. J. Agric. Food Chem. 2009, 57, 3911–3919. [Google Scholar] [CrossRef] [PubMed]
- Vatavali, K.A.; Kosma, I.S.; Louppis, A.P.; Badeka, A.V.; Kontominas, M.G. Analyses in Combination with Chemometrics for the Discrimination of the Geographical Origin of Mean Values and SDs of the Percentages of Fatty Acids (% Fatty Acids) for the Graviera Cheese Samples Tested. Cheeses. Molecules 2020, 25, 3507. [Google Scholar] [CrossRef] [PubMed]
- Danezis, G.; Theodorou, C.; Massouras, T.; Zoidis, E.; Hadjigeorgiou, I.; Georgiou, C.A. Greek Graviera Cheese Assessment through Elemental Metabolomics—Implications for Authentication, Safety and Nutrition. Molecules 2019, 24, 670. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, J.; Bialek, M.; Adamska, A.; Zbikowska, A. Differentiation of Geographical Origin of Cream Products in Poland According to Their Fatty Acid Profile. Food Chem. 2015, 178, 26–31. [Google Scholar] [CrossRef]
- Anastasaki, E.; Kanakis, C.; Pappas, C.; Maggi, L.; del Campo, C.P.; Carmona, M.; Alonso, G.L.; Polissiou, M.G. Geographical Differentiation of Saffron by GC-MS/FID and Chemometrics. Eur. Food Res. Technol. 2009, 229, 899–905. [Google Scholar] [CrossRef]
- Barbosa, S.; Campmajó, G.; Saurina, J.; Puignou, L.; Núñez, O. Determination of Phenolic Compounds in Paprika by Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectrometry: Application to Product Designation of Origin Authentication by Chemometrics. J. Agric. Food Chem. 2020, 68, 591–602. [Google Scholar] [CrossRef]
- Bernáth, J. Some Scientific and Practical Aspects of Production and Utilization of Oregano in Central Europe. In Proceedings of the IPGRI International Workshop on Oregano, Valenzano, Italy, 8–12 May 1996; Padulosi, S., Ed.; IPGRI: Bari, Italy, 1996; pp. 75–92. [Google Scholar]
- Goliaris, A.H.; Chatzopoulou, P.S.; Katsiotis, S.T. Production of New Greek Oregano Clones and Analysis of Their Essential Oils. J. Herbs Spices Med. Plants 2003, 10, 29–35. [Google Scholar] [CrossRef]
- Sarrou, E.; Tsivelika, N.; Chatzopoulou, P.; Tsakalidis, G.; Menexes, G.; Mavromatis, A. Conventional Breeding of Greek Oregano (Origanum vulgare ssp. hirtum) and Development of Improved Cultivars for Yield Potential and Essential Oil Quality. Euphytica 2017, 213, 104. [Google Scholar] [CrossRef]
- Farías, G.; Brutti, O.; Grau, R.; Di Leo Lira, P.; Retta, D.; van Baren, C.; Vento, S.; Bandoni, A.L. Morphological, Yielding and Quality Descriptors of Four Clones of Origanum spp. (Lamiaceae) from the Argentine Littoral Region Germplasm Bank. Ind. Crops Prod. 2010, 32, 472–480. [Google Scholar] [CrossRef]
- Torres, L.E.; Brunetti, P.C.; Baglio, C.; Bauzá, P.G.; Chaves, A.G.; Massuh, Y.; Ocaño, S.F.; Ojeda, M.S. Field Evaluation of Twelve Clones of Oregano Grown in the Main Production Areas of Argentina: Identification of Quantitative Trait with the Highest Discriminant Value. ISRN Agron. 2012, 2012, 349565. [Google Scholar] [CrossRef]
- Azizi, A.; Wagner, C.; Honermeier, B.; Friedt, W. Intraspecific Diversity and Relationship between Subspecies of Origanum vulgare Revealed by Comparative AFLP and SAMPL Marker Analysis. Plant Syst. Evol. 2009, 281, 151–160. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.T.; Khan, M.; Mousa, A.A.; Mahmood, A.; Alkhathlan, H.Z. Chemical Diversity in Leaf and Stem Essential Oils of Origanum vulgare L. and Their Effects on Microbicidal Activities. AMB Express 2019, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Esen, G.; Azaz, A.D.; Kurkcuoglu, M.; Baser, K.H.C.; Tinmaz, A. Essential Oil and Antimicrobial Activity of Wild and Cultivated Origanum vulgare L. subsp. hirtum (Link) Ietswaart from the Marmara Region, Turkey. Flavour Fragr. J. 2007, 22, 371–376. [Google Scholar] [CrossRef]
- NIST. National Institute of Standards and Technology; J. Wiley & Sons Ltd.: West Sussex, UK, 2005. [Google Scholar]
- Oil IOC Olive Oil Extraction 2009.
- Kosma, I.; Badeka, A.; Vatavali, K.; Kontakos, S.; Kontominas, M. Differentiation of Greek Extra Virgin Olive Oils According to Cultivar Based on Volatile Compound Analysis and Fatty Acid Composition. Eur. J. Lipid Sci. Technol. 2016, 118, 849–861. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Payum, T.; Das, A.K.; Shankar, R.; Tamuly, C.; Hazarika, M. Antioxidant Potential of Solanum spirale Shoot and Berry: A Medicinal Food Plant Used in Arunachal Pradesh. Am. J. Pharmtech Res. 2015, 5, 307–314. [Google Scholar]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity Using the DPPH• Free Radical Method. LWT Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- IBM SPSS Statistics 25. Available online: https://www.ibm.com/support/pages/downloading-ibm-spss-statistics-25 (accessed on 25 January 2021).
- Friendly, M.; Sigal, M. Visualizing Tests for Equality of Covariance Matrices. Am. Stat. 2020, 74, 144–155. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using SPSS ISM (London, England) Introducing Statistical Methods Series; SAGE Publications Ltd.: New York, NY, USA, 2009; Volume 2, ISBN1 1847879071. ISBN2 9781847879073. [Google Scholar]
- Pizarro, C.; Rodríguez-Tecedor, S.; Pérez-del-Notario, N.; González-Sáiz, J.M. Recognition of Volatile Compounds as Markers in Geographical Discrimination of Spanish Extra Virgin Olive Oils by Chemometric Analysis of Non-Specific Chromatography Volatile Profiles. J. Chromatogr. A 2011, 1218, 518–523. [Google Scholar] [CrossRef]
- Kosma, I.S.; Kontominas, M.G.; Badeka, A.V. The Application of Chemometrics to Volatile Compound Analysis for the Recognition of Specific Markers for Cultivar Differentiation of Greek Virgin Olive Oil Samples. Foods 2020, 9, 1672. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Khan, S.T.; Khan, N.A.; Mahmood, A.; Al-Kedhairy, A.A.; Alkhathlan, H.Z. The Composition of the Essential Oil and Aqueous Distillate of Origanum vulgare L. Growing in Saudi Arabia and Evaluation of Their Antibacterial Activity. Arab. J. Chem. 2018, 11, 1189–1200. [Google Scholar] [CrossRef]
- Kokkini, S.; Karousou, R.; Hanlidou, E.; Lanaras, T. Essential Oil Composition of Greek (Origanum vulgare ssp. hirtum) and Turkish (O. onites) Oregano: A Tool for Their Distinction. J. Essent. Oil Res. 2004, 16, 334–338. [Google Scholar] [CrossRef]
- Bosabalidis, A.; Kokkini, S. Infraspecific Variation of Leaf Anatomy in Origanum vulgare Grown Wild in Greece. Bot. J. Linn. Soc. 1997, 123, 353–362. [Google Scholar] [CrossRef]
- Karousou, R. Taxonomic Studies on the Cretan Labiatae: Distribution, Morphology and Essential Oils. In Proceedings of the X OPTIMA Meeting, Palermo, Italy, 13–19 September 2001; University of Thessaloniki: Thessaloniki, Greek, 1996. [Google Scholar]
- Lagouri, V.; Blekas, G.; Tsimidou, M.; Kokkini, S.; Boskou, D. Composition and Antioxidant Activity of Essential Oils from Oregano Plants Grown Wild in Greece. Z Leb. Unters Forsch 1993, 197, 20–23. [Google Scholar] [CrossRef]
- Sivropoulou, A.; Papanikolaou, E.; Nikolaou, C.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antimicrobial and Cytotoxic Activities of Origanum Essential Oils. J. Agric. Food Chem. 1996, 44, 1202–1205. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Decoction, Infusion and Hydroalcoholic Extract of Origanum vulgare L.: Different Performances Regarding Bioactivity and Phenolic Compounds. Food Chem. 2014, 158, 73–80. [Google Scholar] [CrossRef]
- Modnicki, D.; Balcerek, M. Estimation of Total Polyphenols Contents in Ocimum basilicum L., Origanum vulgare L. and Thymus vulgaris L. Commercial Samples. Herba Pol. 2009, 55, 35–42. [Google Scholar]
- Pasias, I.N.; Ntakoulas, D.D.; Raptopoulou, K.; Gardeli, C.; Proestos, C. Chemical Composition of Essential Oils of Aromatic and Medicinal Herbs Cultivated in Greece—Benefits and Drawbacks. Foods 2021, 10, 2354. [Google Scholar] [CrossRef]
- Semiz, G.; Semiz, A.; Mercan-Doğan, N. International Journal of Food Properties Essential Oil Composition, Total Phenolic Content, Antioxidant and Antibiofilm Activities of Four Origanum Species from Southeastern Turkey Essential Oil Composition, Total Phenolic Content, Antioxidant and Antibiofilm Activities of Four Origanum Species from Southeastern Turkey. Int. J. Food Prop. 2018, 21, 194–204. [Google Scholar] [CrossRef]
- Oniga, I.; Pus, C.; Silaghi-Dumitrescu, R.; Olah, N.K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare ssp. vulgare: Chemical Composition and Biological Studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef] [PubMed]
- Spiridon, I.; Colceru, S.; Anghel, N.; Teaca, C.A.; Bodirlau, R.; Armatu, A. Antioxidant Capacity and Total Phenolic Contents of Oregano (Origanum vulgare), Lavender (Lavandula Angustifolia) and Lemon Balm (Melissa Officinalis) from Romania. Nat. Prod. Res. 2011, 25, 1657–1661. [Google Scholar] [CrossRef] [PubMed]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of Essential Oils in Food Safety: Antimicrobial and Antioxidant Applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Kosakowska, O.; Zenon, W.; Pióro-Jabrucka, E.; Przybył, J.L.; Krasniewska, K.; Gniewosz, M.; Baczek, K. Antioxidant and Antibacterial Activity of Essential Oils and Hydroethanolic Extracts of Greek Oregano. Molecules 2021, 26, 15. [Google Scholar] [CrossRef]
- Kulisic, T.; Radonic, A.; Katalinic, V.; Milos, M. Use of Different Methods for Testing Antioxidative Activity of Oregano Essential Oil. Food Chem. 2004, 85, 633–640. [Google Scholar] [CrossRef]
- Al-Mansori, B.; El-Ageeli, W.H.; Alsagheer, S.H.; Ben-Khayal, F.A.F. Antioxidant Activity-Synergistic Effects of Thymol and Carvacrol. Al-Mukhtar J. Sci. 2020, 35, 185–194. [Google Scholar] [CrossRef]
- Rostro-Alanis, M.; Báez-González, J.; Torres-Alvarez, C.; Parra-Saldívar, R.; Rodriguez-Rodriguez, J.; Castillo, S. Chemical Composition and Biological Activities of Oregano Essential Oil and Its Fractions Obtained by Vacuum Distillation. Molecules 2019, 24, 1904. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Burton, D.; Parra, F.; López, J.; Muñoz, P.; Escobar, H.; Parra, C. Antioxidant and Antibacterial Capacities of Origanum vulgare L. Essential Oil from the Arid Andean Region of Chile and Its Chemical Characterization by Gc-Ms. Metabolites 2020, 10, 414. [Google Scholar] [CrossRef]
- Yan, F.; Azizi, A.; Janke, S.; Schwarz, M.; Zeller, S.; Honermeier, B. Antioxidant Capacity Variation in the Oregano (Origanum vulgare L.) Collection of the German National Genebank. Ind. Crops Prod. 2016, 92, 19–25. [Google Scholar] [CrossRef]
- Katerinopoulou, K.; Kontogeorgos, A.; Salmas, C.E.; Patakas, A.; Ladavos, A. Geographical Origin Authentication of Agri-Food Products: A Review. Foods 2020, 9, 489. [Google Scholar] [CrossRef] [PubMed]
| S/N a | Compounds | RIEXP | RILIT | THESSALONIKI | KATERINI | KILKIS | KOZANI | VOLOS | KALAMBAKA | IOANNINA | PREVEZA | ILEIA | ACHAEA | RETHYMNO | HERAKLION |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | α-pinene | 1012 | 1012 | 0.26 | 0.62 | 0.60 | 0.75 | 0.53 | 0.85 | 0.62 | 0.73 | 0.90 | 0.54 | 0.71 | 0.80 |
| 2 | α-thujene | 1019 | 1017 | 0.09 | 0.56 | 0.90 | 0.45 | 0.59 | 0.70 | 0.96 | 1.03 | 0.68 | 0.67 | 0.06 | |
| 3 | camphene | 1054 | 1055 | 0.16 | tr b | 0.13 | 0.12 | 0.16 | 0.07 | ||||||
| 4 | β-pinene | 1096 | 1100 | 0.15 | 0.11 | 0.12 | 0.17 | 0.07 | 0.06 | ||||||
| 5 | δ-3-carene | 1143 | 1138 | 0.06 | 0.09 | ||||||||||
| 6 | α-phellandrene | 1166 | 1166 | 0.16 | 0.19 | 0.05 | 0.18 | 0.22 | 0.29 | 0.22 | 0.07 | ||||
| 7 | β-myrcene | 1175 | 1175 | 0.55 | 0.91 | 1.39 | 1.46 | 1.20 | 1.32 | 1.51 | 1.51 | 2.01 | 1.47 | 1.65 | 1.15 |
| 8 | α-terpinene | 1191 | 1201 | 0.67 | 1.09 | 1.45 | 1.63 | 1.22 | 1.57 | 1.46 | 1.58 | 2.48 | 1.63 | 1.56 | 1.26 |
| 9 | limonene | 1199 | 1208 | 0.14 | 0.16 | 0.25 | 0.12 | 0.22 | 0.24 | 0.26 | 0.38 | 0.29 | 0.29 | 0.19 | |
| 10 | β-phellandrene | 1221 | 1228 | 0.10 | 0.18 | 0.29 | 0.08 | 0.11 | 0.28 | 0.28 | 0.29 | 0.28 | 0.24 | 0.07 | |
| 11 | γ-terpinene | 1243 | 1246 | 2.72 | 3.13 | 5.76 | 7.05 | 5.40 | 6.67 | 6.96 | 7.00 | 10.49 | 8.18 | 7.28 | 4.13 |
| 12 | 3-octanone | 1255 | 1252 | 0.09 | 0.21 | 0.14 | 0.22 | 0.24 | 0.22 | 0.23 | 0.24 | ||||
| 13 | p-cymene | 1270 | 1269 | 6.60 | 11.10 | 8.32 | 9.77 | 8.93 | 12.26 | 8.47 | 10.45 | 11.98 | 9.98 | 12.03 | 12.53 |
| 14 | α-terpinolene | 1280 | 1282 | tr | 0.14 | tr | 0.15 | 0.15 | 0.16 | 0.12 | 0.11 | tr | |||
| 15 | 1-octen-3-ol | 1448 | 1447 | 0.41 | 0.57 | 0.50 | 0.34 | 0.52 | 1.07 | 0.54 | 0.45 | 0.32 | 0.41 | 0.10 | 0.43 |
| 16 | cis-sabinene hydrate | 1462 | 1471 | 0.49 | 0.51 | 0.67 | 0.58 | 0.56 | 0.36 | 0.50 | 0.55 | 0.49 | 0.68 | 0.43 | 0.52 |
| 17 | trans-sabinene hydrate | 1546 | 1556 | 0.39 | 0.43 | 0.50 | 0.46 | 0.50 | 0.44 | 0.37 | 0.39 | 0.34 | 0.47 | 0.38 | 0.54 |
| 18 | bornyl acetate | 1561 | 1566 | tr | |||||||||||
| 19 | caryophyllene | 1598 | 1590 | 1.75 | 2.43 | 2.08 | 1.92 | 1.77 | 1.96 | 1.72 | 1.74 | 1.50 | 1.13 | 0.74 | 1.45 |
| 20 | 4-terpineol | 1604 | 1605 | 1.22 | 0.85 | 0.82 | 1.28 | 1.20 | 0.33 | 1.00 | 0.88 | 0.17 | 0.89 | 0.80 | 1.25 |
| 21 | carvacrol methyl ether | 1610 | 1601 | 0.79 | 0.88 | 0.61 | 0.43 | 0.41 | 0.64 | 0.20 | 0.27 | 0.27 | 0.70 | ||
| 22 | cis-dihydrocarvone | 1629 | - | 0.05 | 0.16 | 0.05 | |||||||||
| 23 | α-humulene | 1668 | 1677 | 0.09 | 0.10 | 0.27 | 0.05 | 0.23 | 0.21 | 0.10 | 0.09 | ||||
| 24 | α-terpineol | 1698 | 1698 | tr | |||||||||||
| 25 | borneol | 1702 | 1717 | 1.41 | 1.16 | 1.06 | 0.90 | 1.20 | 1.03 | 0.80 | 0.75 | 0.51 | 0.65 | 0.51 | 0.84 |
| 26 | β-bisabolene | 1726 | 1722 | 1.33 | 1.45 | 0.87 | 1.24 | 1.19 | 1.53 | 1.06 | 1.10 | 1.32 | 1.22 | 1.45 | 2.07 |
| 27 | δ-cadinene | 1733 | 1756 | 0.25 | 0.13 | 0.17 | |||||||||
| 28 | p-cymen-8-ol | 1851 | 1865 | 0.06 | tr | tr | |||||||||
| 29 | carvacryl acetate | 1875 | 1880 | tr | tr | ||||||||||
| 30 | caryophyllene oxide | 1973 | 1994 | 1.72 | 1.31 | 0.96 | 0.68 | 1.00 | 0.72 | 0.66 | 0.68 | 0.37 | 0.52 | 0.40 | 0.98 |
| 31 | spathulenol | 2122 | 2136 | 0.08 | tr | tr | 0.06 | ||||||||
| 32 | 4-isopropyl-m-cresol | 2155 | - | 0.08 | tr | 0.14 | 0.20 | 0.17 | 0.13 | ||||||
| 33 | thymol | 2173 | 2186 | 10.89 | 10.22 | 12.85 | 8.41 | 13.03 | 16.45 | 10.64 | 13.65 | 35.22 | 34.68 | 24.01 | 7.39 |
| 34 | 5-isopropyl-m-cresol | 2208 | - | 0.10 | tr | 0.10 | 0.18 | 0.26 | 0.14 | ||||||
| 35 | carvacrol | 2211 | 2212 | 68.79 | 62.95 | 60.28 | 59.89 | 60.04 | 51.62 | 60.05 | 55.09 | 28.74 | 34.77 | 46.19 | 63.55 |
| Main compounds c | 89.00 | 87.40 | 87.21 | 85.12 | 87.40 | 87.00 | 86.12 | 86.19 | 86.43 | 87.61 | 89.51 | 87.60 | |||
| Monoterpene hydrocarbons | 10.80 | 17.18 | 18.58 | 22.74 | 17.98 | 23.59 | 20.87 | 23.38 | 30.43 | 23.53 | 24.60 | 20.26 | |||
| Oxygenated Monoterpenes | 83.98 | 77.00 | 76.79 | 72.24 | 76.94 | 70.87 | 73.82 | 71.95 | 65.67 | 72.84 | 72.59 | 74.79 | |||
| Sesquiterpene hydrocarbons | 3.08 | 3.97 | 3.05 | 3.68 | 3.01 | 3.49 | 3.14 | 3.22 | 2.92 | 2.44 | 2.19 | 3.52 | |||
| Oxygenated Sesquiterpenes | 1.72 | 1.31 | 0.96 | 0.76 | 1.00 | 0.72 | 0.66 | 0.68 | 0.43 | 0.52 | 0.40 | 0.98 | |||
| Miscellaneous | 0.41 | 0.57 | 0.59 | 0.55 | 0.66 | 1.29 | 0.78 | 0.67 | 0.55 | 0.65 | 0.10 | 0.43 |
| Location | TPC (mg GAE/g EO) | TEAC (μmol TE/g EO) |
|---|---|---|
| Thessaloniki | 88.25 ± 7.92 | 395.84 ± 12.03 |
| Katerini | 83.20 ± 9.24 | 387.79 ± 13.65 |
| Kilkis | 86.48 ± 5.15 | 382.51 ± 11.37 |
| Kozani | 79.92 ± 7.39 | 457.00 ± 7.42 |
| Volos | 83.92 ± 6.98 | 375.81 ± 9.37 |
| Kalambaka | 85.92 ± 6.41 | 321.89 ± 7.53 |
| Ioannina | 84.17 ± 6.93 | 410.71 ± 10.95 |
| Preveza | 81.58 ± 7.28 | 397.06 ± 6.71 |
| Ileia | 85.38 ± 3.32 | 382.29 ± 20.33 |
| Achaea | 89.03 ± 4.76 | 306.83 ± 5.01 |
| Rethymno | 75.27 ± 3.31 | 461.32 ± 7.27 |
| Heraklion | 74.49 ± 3.57 | 361.43 ± 16.06 |
| Group A | Group B | |||
|---|---|---|---|---|
| Dependent Variables | F | Sig. | F | Sig. |
| α-pinene | 3.747 | 0.006 | 0.707 | 0.620 |
| α-thujene | 16.479 | 0.000 | 7.269 | 0.000 |
| camphene | 19.979 | 0.000 | 6.349 | 0.000 |
| β-pinene | 43.232 | 0.000 | 12.184 | 0.000 |
| δ-3-carene | 19.328 | 0.000 | 7.860 | 0.000 |
| α-phellandrene | 16.598 | 0.000 | 16.736 | 0.000 |
| β-myrcene | 7.314 | 0.000 | 3.602 | 0.006 |
| α-terpinene | 12.833 | 0.000 | 2.341 | 0.050 |
| limonene | 12.281 | 0.000 | 4.912 | 0.001 |
| β-phellandrene | 8.004 | 0.000 | 12.216 | 0.000 |
| γ-terpinene | 17.102 | 0.000 | 8.733 | 0.000 |
| 3-octanone | 9.956 | 0.000 | 10.266 | 0.000 |
| p-cymene | 12.625 | 0.000 | 4.350 | 0.002 |
| α-terpinolene | 11.586 | 0.000 | 23.839 | 0.000 |
| 1-octen-3-ol | 13.330 | 0.000 | 10.677 | 0.000 |
| cis-sabinene hydrate | 3.399 | 0.010 | 2.573 | 0.033 |
| trans-sabinene hydrate | 1.948 | 0.101 | 2.545 | 0.035 |
| bornyl acetate | 1.049 | 0.395 | ||
| caryophyllene | 4.710 | 0.001 | 7.956 | 0.000 |
| 4-terpineol | 14.092 | 0.000 | 1.743 | 0.135 |
| carvacrol methyl ether | 5.480 | 0.000 | 8.447 | 0.000 |
| cis-dihydrocarvone | 2.623 | 0.034 | 9.498 | 0.000 |
| α-humulene | 8.666 | 0.000 | 16.284 | 0.000 |
| α-terpineol | 1.253 | 0.293 | ||
| borneol | 17.003 | 0.000 | 18.737 | 0.000 |
| β-bisabolene | 40.163 | 0.000 | 2.540 | 0.035 |
| δ-Cadinene | 11.646 | 0.000 | 46.986 | 0.000 |
| p-cymen-8-ol | 10.009 | 0.000 | 9.047 | 0.000 |
| carvacryl acetate | 2.416 | 0.044 | ||
| caryophyllene oxide | 20.672 | 0.000 | 13.091 | 0.000 |
| spathulenol | 3.750 | 0.005 | 5.293 | 0.000 |
| 4-isopropyl-m-cresol | 64.496 | 0.000 | 20.953 | 0.000 |
| thymol | 19.504 | 0.000 | 40.470 | 0.000 |
| 5-isopropyl-m-cresol | 28.198 | 0.000 | 22.509 | 0.000 |
| carvacrol | 59.721 | 0.000 | 33.509 | 0.000 |
| TPC | 6.587 | 0.000 | 4.570 | 0.001 |
| TEAC | 2.726 | 0.029 | 26.044 | 0.000 |
| Discriminant Function | Original Method (%) | Cross-Validation (%) | |
|---|---|---|---|
| GROUP A | EO compounds | 100 | 93.3 |
| TPC and TEAC | 38.3 | 33.3 | |
| GROUP B | EO compounds | 96.3% | 81.5% |
| TPC and TEAC | 43.9 | 39.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsoumani, E.S.; Kosma, I.S.; Badeka, A.V. Chemometric Screening of Oregano Essential Oil Composition and Properties for the Identification of Specific Markers for Geographical Differentiation of Cultivated Greek Oregano. Sustainability 2022, 14, 14762. https://doi.org/10.3390/su142214762
Tsoumani ES, Kosma IS, Badeka AV. Chemometric Screening of Oregano Essential Oil Composition and Properties for the Identification of Specific Markers for Geographical Differentiation of Cultivated Greek Oregano. Sustainability. 2022; 14(22):14762. https://doi.org/10.3390/su142214762
Chicago/Turabian StyleTsoumani, Eleftheria S., Ioanna S. Kosma, and Anastasia V. Badeka. 2022. "Chemometric Screening of Oregano Essential Oil Composition and Properties for the Identification of Specific Markers for Geographical Differentiation of Cultivated Greek Oregano" Sustainability 14, no. 22: 14762. https://doi.org/10.3390/su142214762
APA StyleTsoumani, E. S., Kosma, I. S., & Badeka, A. V. (2022). Chemometric Screening of Oregano Essential Oil Composition and Properties for the Identification of Specific Markers for Geographical Differentiation of Cultivated Greek Oregano. Sustainability, 14(22), 14762. https://doi.org/10.3390/su142214762






