Identifying and Characterizing Critical Source Areas of Organic and Inorganic Pollutants in Urban Agglomeration in Lake Baikal Watershed
Abstract
1. Introduction
2. Materials and Methods
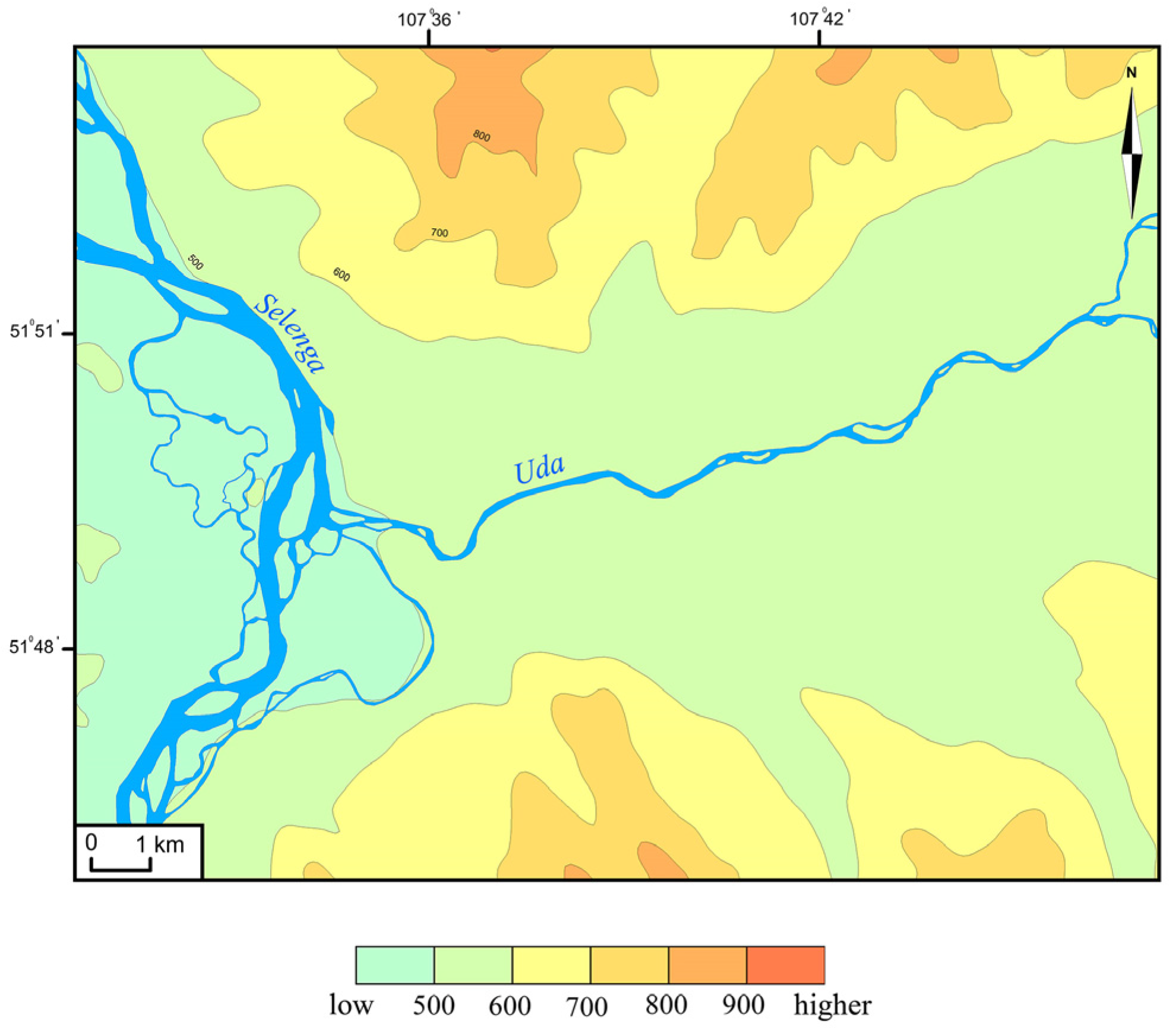
2.1. Study Area
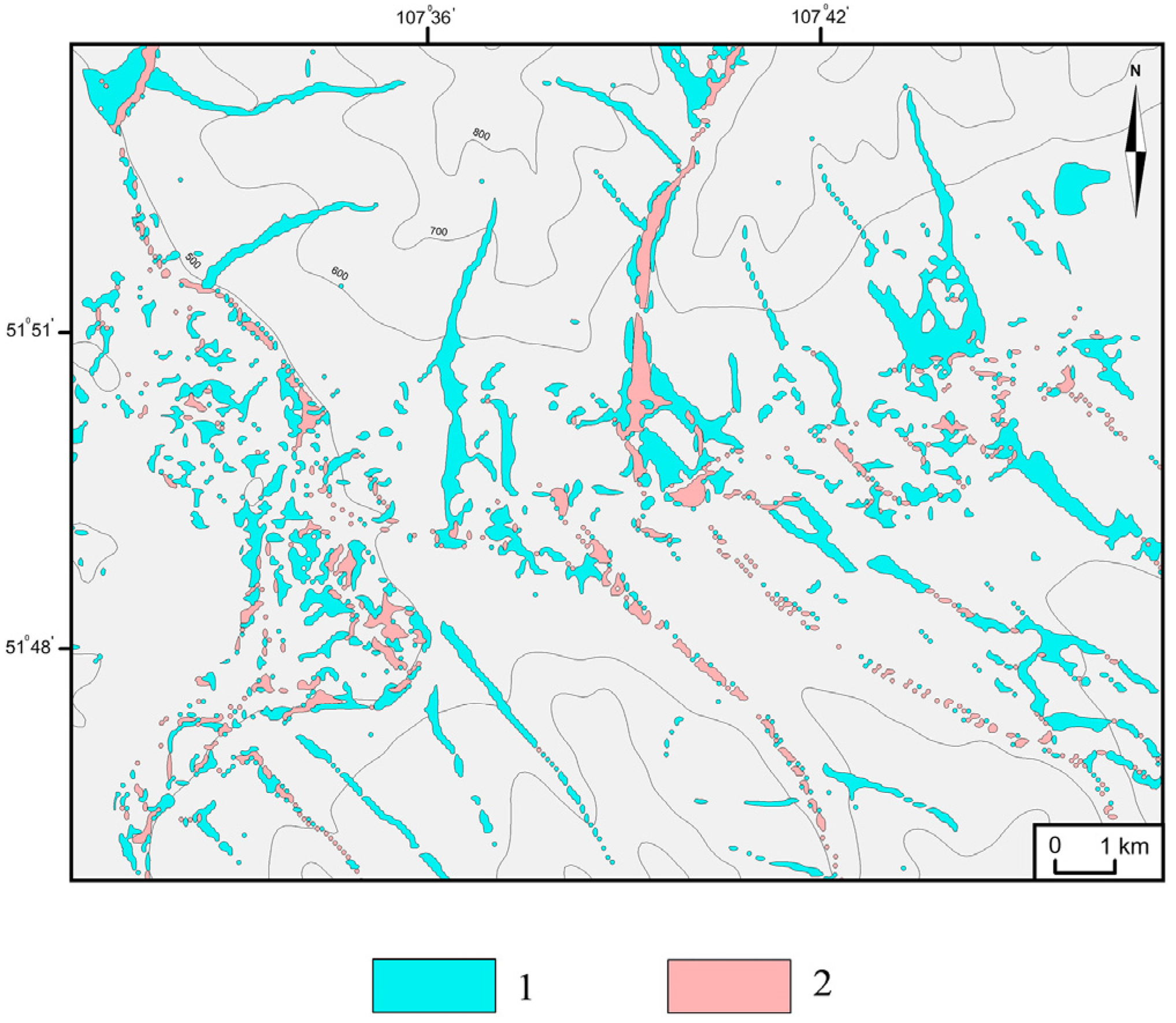
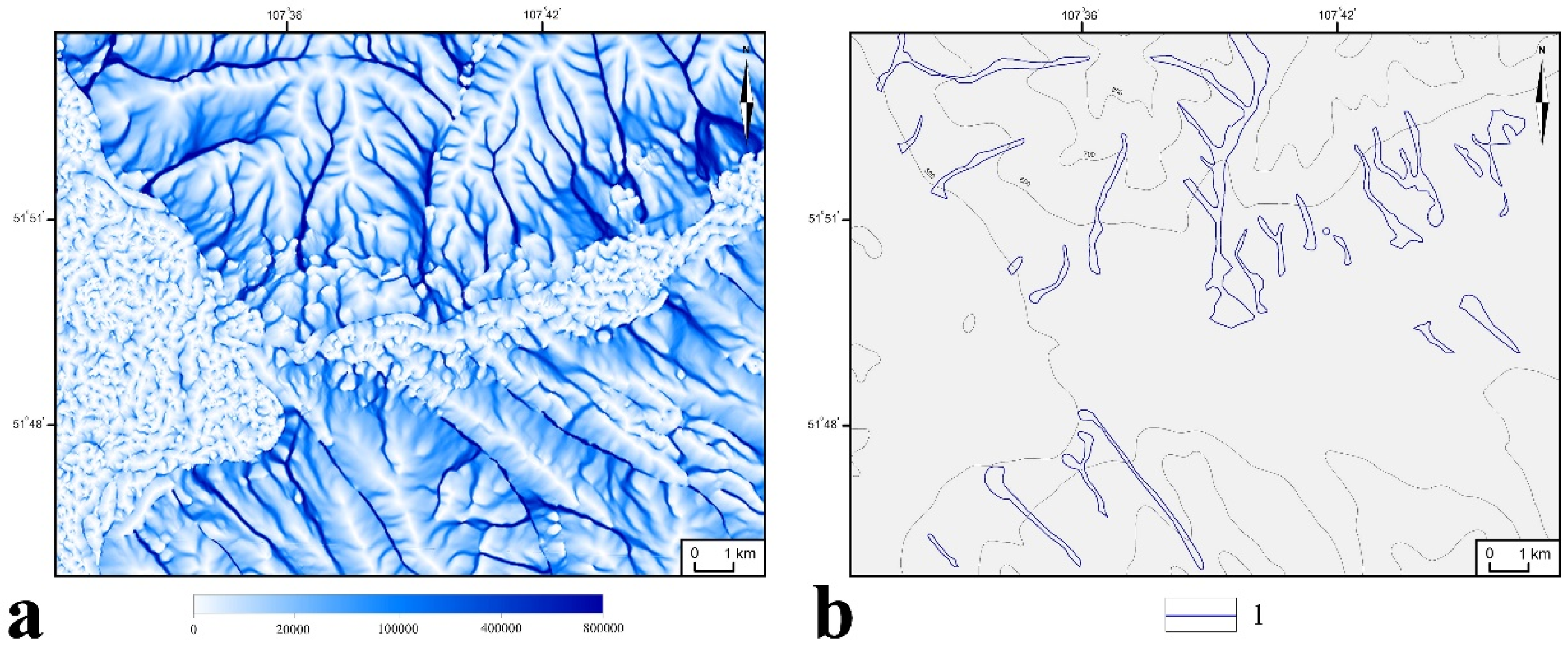
2.2. Digital Mapping
2.3. Soil Sampling and Chemical Determinations
2.3.1. Polycyclic Aromatic Hydrocarbons
2.3.2. Trace Metals
2.4. Chemical Data Processing
3. Results and Discussion
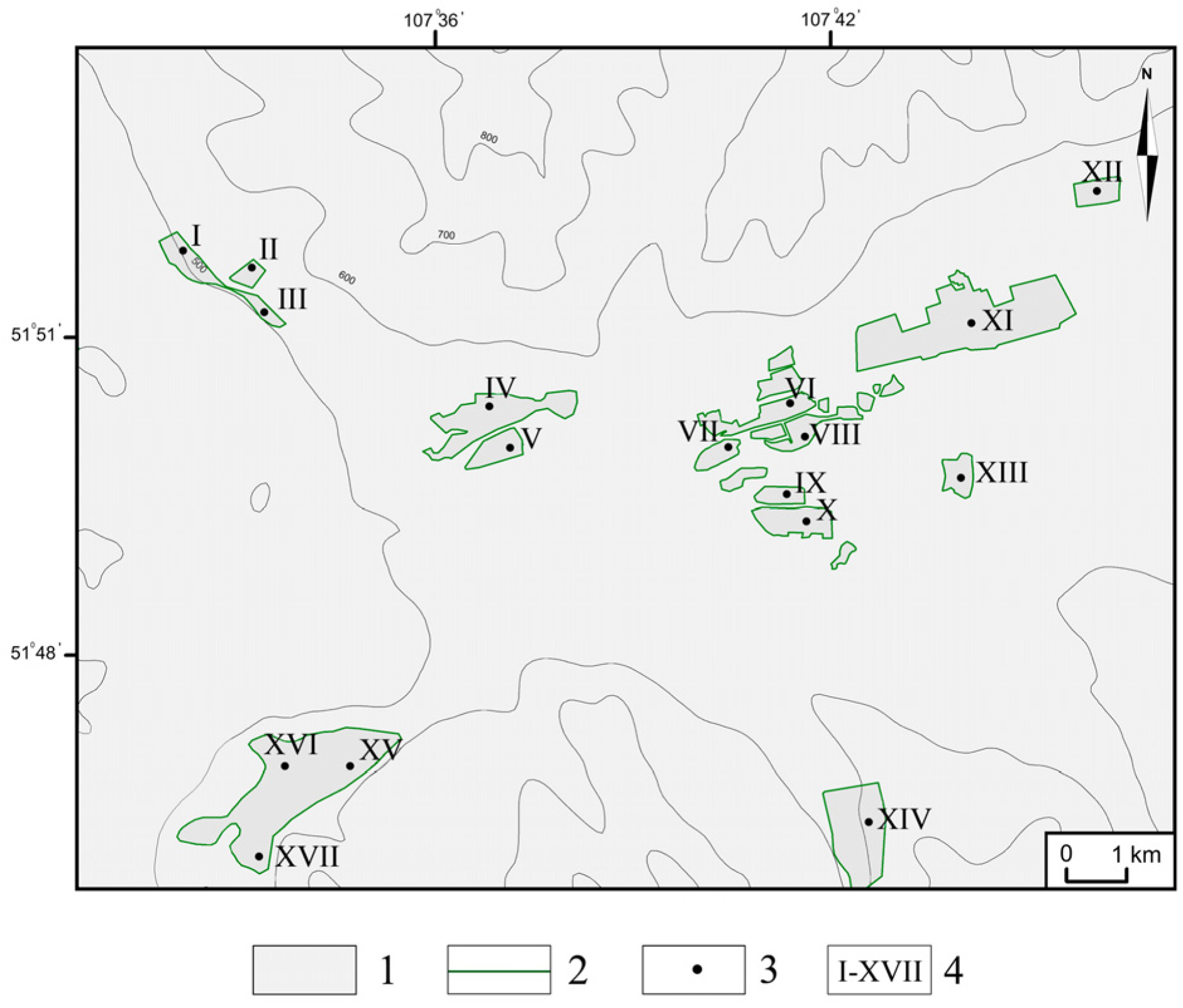
3.1. Identification of HSAs and CSAs Using Digital Mapping Techniques
3.2. Assessing the Level of Soil Pollution in Identified CSAs
3.2.1. Polycyclic Aromatic Hydrocarbons
3.2.2. Trace Metals
3.3. Source Apportionment of Soil Pollutants in Identified CSAs
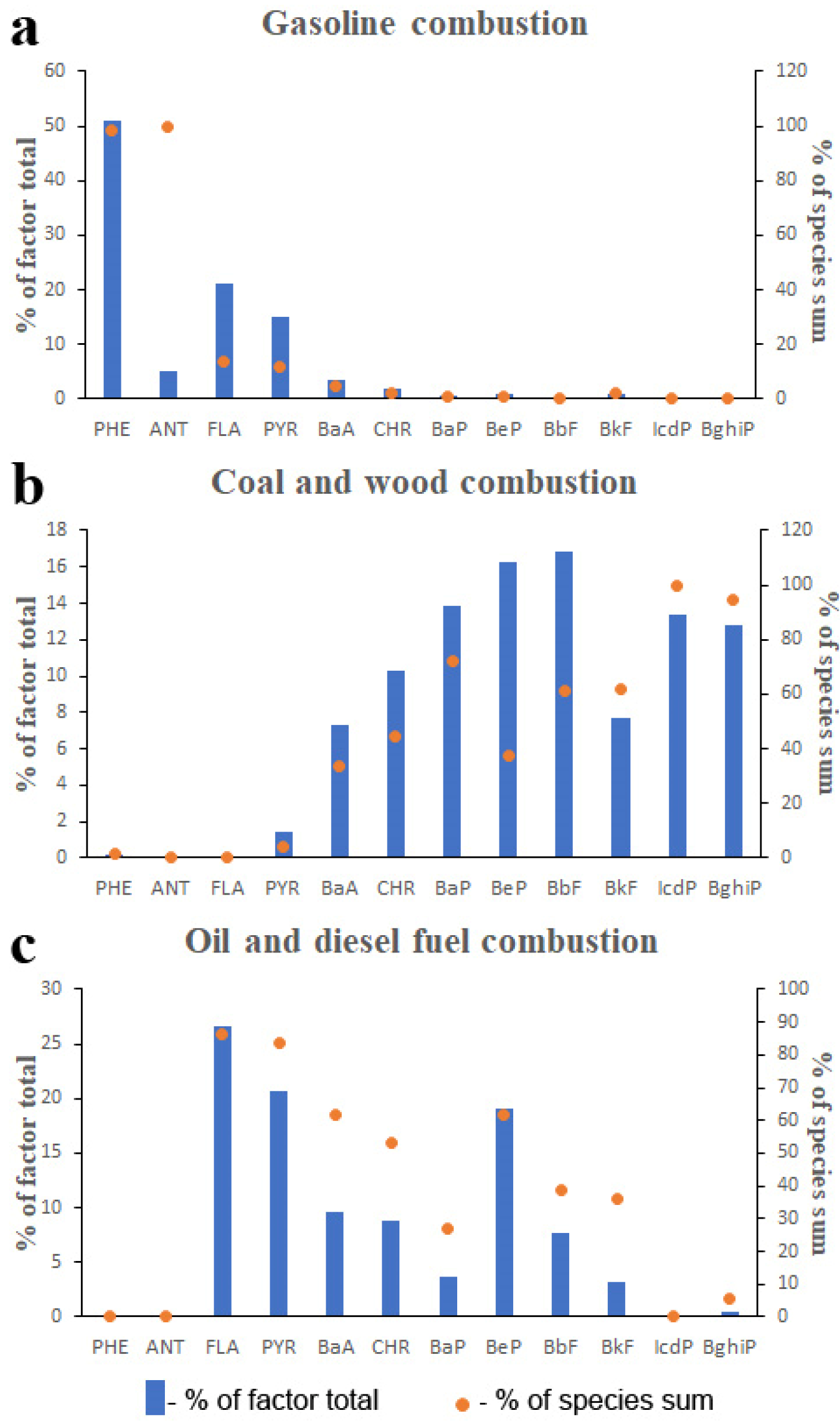
3.3.1. Polycyclic Aromatic Hydrocarbons
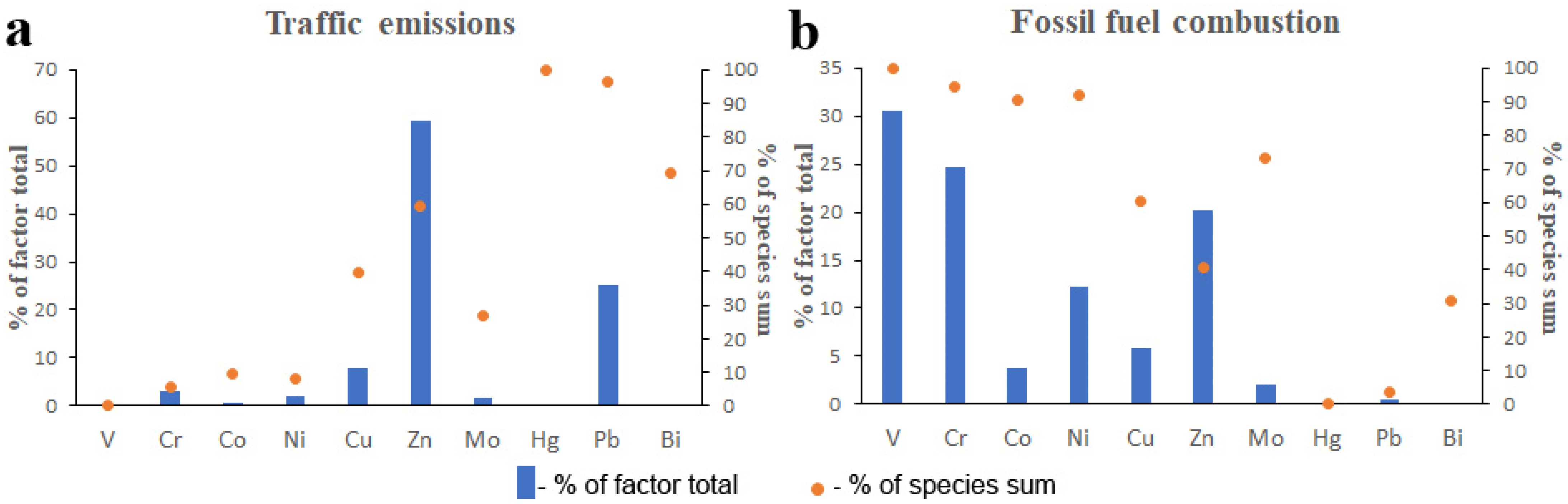
3.3.2. Trace Metals
3.4. Revealing the Link between Pollution of Soils and Aquatic Environment
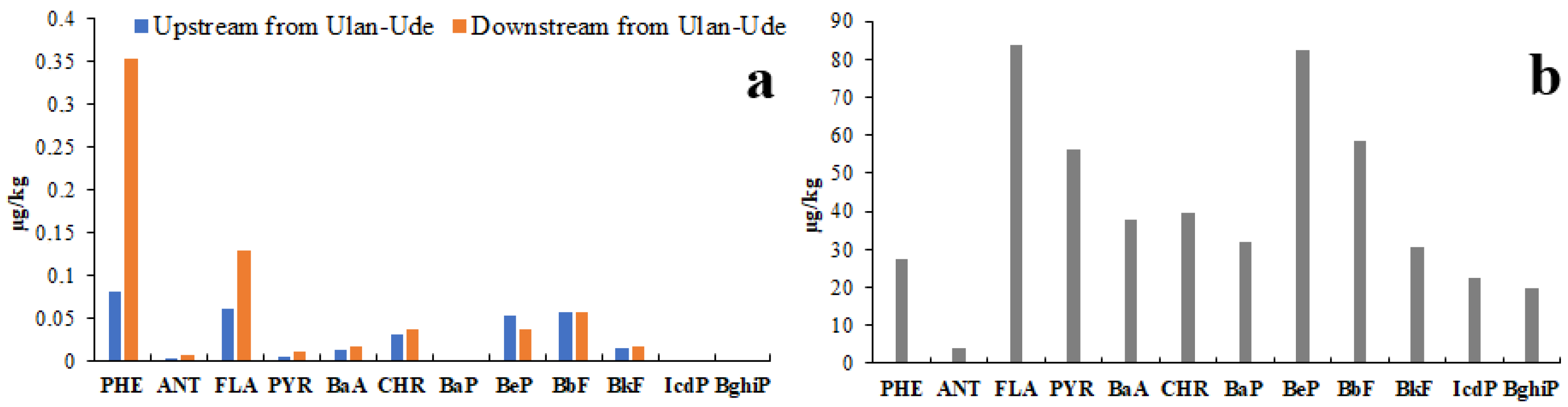
3.4.1. Polycyclic Aromatic Hydrocarbons
3.4.2. Trace Metals
4. Conclusions
- Due to different operating modes and different production capacities of the enterprises, the soil pollution levels were highly variable among critical source areas. The average pollution level of the PAHs and TMs in the soils of CSAs was approximately two times higher than those outside the CSAs.
- Due to high concentrations of specified metals in native preindustrial soils, the pollution levels of the PAHs and TMs in CSA soils do not correlate with each other. In contrast to PAHs that are mostly of anthropogenic origin, the trace metals are ubiquitous and may occur in high concentrations in both natural and anthropogenic soils.
- The major sources of both PAHs and TMs in soils of critical source areas were traffic emissions and coal-fired thermal power stations.
- Due to PAH fractionation during the water movement through the soil, the PAH composition of riverine bottom sediments is distinctly different from that of soils. The similar TM composition in soils and sediments was due to the fact that TMs in mineral particles are embedded within water-insoluble compounds that do not undergo any changes neither in soil nor in sediments.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peters, N.E.; Meybeck, M. Water quality degradation effects on freshwater availability: Impacts of human activities. Water Int. 2000, 25, 185–193. [Google Scholar] [CrossRef]
- Wan, L.; Wang, X.H.; Peirson, W. Impacts of climate change and non-point-source pollution on water quality and algal blooms in the Shoalhaven River estuary, NSW, Australia. Water 2022, 14, 1914. [Google Scholar] [CrossRef]
- Khodzher, T.V.; Domysheva, V.M.; Sorokovikova, L.M.; Sakirko, M.V.; Tomberg, I.V. Current chemical composition of Lake Baikal water. Inland Waters 2017, 7, 250–258. [Google Scholar] [CrossRef]
- Semenov, M.Y.; Semenov, Y.M.; Silaev, A.V.; Begunova, L.A. Source apportionment of inorganic solutes in surface waters of Lake Baikal watershed. Sustainability 2021, 13, 5389. [Google Scholar] [CrossRef]
- Kravtsova, L.S.; Izhboldina, L.A.; Khanaev, I.V.; Pomazkina, G.V.; Rodionova, E.V.; Domysheva, V.M.; Sakirko, M.V.; Tomberg, I.V.; Kostornova, T.Y.; Kravchenko, O.S.; et al. Nearshore benthic blooms of filamentous green algae in Lake Baikal. J. Great Lakes Res. 2014, 40, 441–448. [Google Scholar] [CrossRef]
- Timoshkin, O.A.; Moore, M.V.; Kulikova, N.N.; Tomberg, I.V.; Malnik, V.V.; Shimaraev, M.N.; Troitskaya, E.S.; Shirokaya, A.A.; Sinyukovich, V.N.; Zaitseva, E.P.; et al. Groundwater contamination by sewage causes benthic algal outbreaks in the littoral zone of Lake Baikal (East Siberia). J. Great Lakes Res. 2018, 44, 230–244. [Google Scholar] [CrossRef]
- Chebykin, E.P.; Sorokovikova, L.M.; Tomberg, I.V.; Rasskazov, S.V.; Khodzher, T.V.; Grachev, M.A. Current state of the Selenga River waters in the Russian territory concerning major components and trace elements. Chem. Sustain. Dev. 2012, 20, 561–580. [Google Scholar]
- Khazheeva, Z.I.; Plyusnin, A.M. The regime of dissolved gases and organic matter in Selenga basin rivers. Water Resour. 2013, 40, 61–73. [Google Scholar] [CrossRef]
- Semenov, M.Y.; Semenov, Y.M.; Silaev, A.V.; Begunova, L.A. Assessing the self-purification capacity of surface waters in Lake Baikal watershed. Water 2019, 11, 1505. [Google Scholar] [CrossRef]
- Sasakova, N.; Gregova, G.; Takacova, D.; Mojzisova, J.; Papajova, I.; Venglovsky, J.; Szaboova, T.; Kovacova, S. Pollution of surface and ground water by sources related to agricultural activities. Front. Sustain. Food Syst. 2018, 2, 42. [Google Scholar] [CrossRef]
- Pérez-Lucas, G.; Vela, N.; Aatik, A.E.; Navarro, S. Environmental risk of groundwater pollution by pesticide leaching through the soil profile. In Pesticides—Use and Misuse and Their Impact in the Environment; Larramendy, M., Soloneski, S., Eds.; Intech Open: London, UK, 2018. [Google Scholar] [CrossRef]
- Sweetman, A.J.; Valle, M.D.; Prevedouros, K.; Jones, K.C. The role of soil organic carbon in the global cycling of persistent organic pollutants (POPs): Interpreting and modelling field data. Chemosphere 2005, 60, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.G.; Srinivasan, R.; Muttiah, R.S.; Williams, J.R. Large area hydrologic modeling and assessment part I: Model development. J. Am. Water Resour. Assoc. 1998, 34, 73–89. [Google Scholar] [CrossRef]
- Young, R.A.; Onstad, C.A.; Boesch, D.D.; Anderson, W.P. AGNPS—A nonpoint source pollution model for evaluating agricultural watersheds. J. Soil Water Conserv. 1989, 44, 168–173. [Google Scholar]
- Haith, D.A.; Shoemaker, L.L. Generalized watershed loading functions for stream-flow nutrients. Water Resour. Res. 1987, 23, 471–478. [Google Scholar] [CrossRef]
- Mockus, V. National Engineering Handbook, Section 4: Hydrology; US Soil Conservation Service, Department of Agriculture: Washington DC, USA, 1972; 762p. [Google Scholar]
- Agnew, L.J.; Lyon, S.; Gérard-Marchan, T.P.; Collins, V.B.; Lembo, A.J.; Steenhuis, T.S.; Walter, M.T. Identifying hydrologically sensitive areas: Bridging the gap between science and application. J. Environ. Manag. 2006, 78, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.T.; Walter, M.F.; Brooks, E.S.; Steenhuis, T.S.; Boll, J.; Weiler, K. Hydrologically sensitive areas: Variable source area hydrology implications for water quality risk assessment. J. Soil Water Conserv. 2000, 55, 277–284. [Google Scholar]
- Sharpley, A.N.; Daniel, T.C.; Edwards, D.R. Phosphorus movement in the landscape. J. Prod. Agric. 1993, 6, 492–500. [Google Scholar] [CrossRef]
- Patterson, C.L.; Haught, R.C. Regulatory considerations to ensure clean and safe drinking water. In Handbook of Water Purity and Quality, 2nd ed.; Ahuja, S., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 6, pp. 93–135. [Google Scholar] [CrossRef]
- Horton, R.E. An approach toward a physical interpretation of infiltration capacity. Soil Sci. Soc. Am. Proc. 1940, 4, 399–417. [Google Scholar] [CrossRef]
- Hewlett, J.D.; Hibbert, A.R. Factors affecting the response of small watersheds to precipitation in humid regions. For. Hydrol. 1967, 1, 275–290. [Google Scholar]
- Dunne, T.; Black, R.D. Partial area contributions to storm runoff in a small New England watershed. Water Resour. Res. 1970, 6, 1296–1311. [Google Scholar] [CrossRef]
- Beven, K. TOPMODEL: A critique. Hydrol. Process. 1997, 11, 1069–1085. [Google Scholar] [CrossRef]
- Beven, K.J.; Kirkby, M.J. A physically based, variable contributing area model of basin hydrology. Hydrol. Sci. Bull. 1979, 24, 43–69. [Google Scholar] [CrossRef]
- Hillel, D. Fundamentals of Soil Physics; Academic Press: New York, NY, USA, 1980. [Google Scholar] [CrossRef]
- Khalikov, I.S.; Luk’yanova, N.N.; Makarenko, A.A. Analysis of soil pollution by polyarenes based on the results of the reconnaissance survey of Buryatia in 2014. Sci. Alm. 2016, 4-3, 434–438. (In Russian) [Google Scholar]
- Korlyakov, I.; Kasimov, N.; Kosheleva, N. Heavy metals and metalloids in the soil cover of the city of Ulan-Ude. PNRPU. Applied Ecology. Urban Dev. 2019, 3, 120–137. (In Russian) [Google Scholar] [CrossRef]
- Kasimov, N.; Kosheleva, N.; Gunin, P.; Korlyakov, I.; Sorokina, O.; Timofeev, I. State of the environment of urban and mining areas in the Selenga Transboundary River Basin (Mongolia Russia). Environ. Earth Sci. 2016, 75, 1283. [Google Scholar] [CrossRef]
- Kasimov, N.S.; Korlyakov, I.D.; Kosheleva, N.E. Distribution and factors of accumulation of heavy metals and metalloids in river bottom sediments in the territory of the Ulan-Ude city. RUDN J. Ecol. Life Saf. 2017, 25, 380–395. (In Russian) [Google Scholar] [CrossRef]
- Conrad, O.; Bechtel, B.; Bock, M.; Dietrich, H.; Fischer, E.; Gerlitz, L.; Wehberg, J.; Wichmann, V.; Böhner, J. System for automated geoscientific analyses (SAGA) v.2.1.4. Geosci. Model Dev. 2015, 8, 107–122. [Google Scholar] [CrossRef]
- Gallant, J.C.; Hutchinson, M.F. A Differential equation for specific catchment area. Water Resour. Res. 2011, 47, 28–33. [Google Scholar] [CrossRef]
- Drusch, M.; Del Bello, U.; Carlier, S.; Colin, O.; Fernandez, V.; Gascon, F.; Hoersch, B.; Isola, C.; Laberinti, P.; Martimort, P.; et al. Sentinel-2: ESA’s optical high-resolution mission for GMES operational services. Remote Sens. Environ. 2012, 20, 25–36. [Google Scholar] [CrossRef]
- Batoev, V.B.; Vaisflot, L.; Ventsel, K.D.; Tsydenova, O.V.; Palitsyna, S.S. Pollution of the basin of the Lake Baikal: Polyaromatic hydrocarbons. Khimiya v Interesakh Ustoichivogo Razvitiya. Chem. Sustain. Dev. 2003, 11, 829–834. (In Russian) [Google Scholar]
- Semenov, M.Y.; Marinaite, I.I.; Golobokova, L.P.; Khuriganova, O.I.; Khodzher, T.V.; Semenov, Y.M. Source apportionment of polycyclic aromatic hydrocarbons in Lake Baikal water and adjacent air layer. Chem. Ecol. 2017, 33, 977–990. [Google Scholar] [CrossRef]
- Liang, S.X.; Wu, H.; Sun, H.W. Determination of trace elements in airborne PM10 by inductively coupled plasma mass spectrometry. Int. J. Environ. Sci. Technol. 2015, 12, 1373–1378. [Google Scholar] [CrossRef]
- Semenov, M.Y.; Silaev, A.V.; Semenov, Y.M.; Begunova, L.A. Using Si, Al and Fe as tracers for source apportionment of air pollutants in Lake Baikal snowpack. Sustainability 2020, 12, 3392. [Google Scholar] [CrossRef]
- Lucarelli, F.; Calzolai, G.; Chiari, M.; Giardi, F.; Czelusniak, C.; Nava, S. Hourly elemental composition and source identification by positive matrix factorization (PMF) of fine and coarse particulate matter in the high polluted industrial area of Taranto (Italy). Atmosphere 2020, 11, 419. [Google Scholar] [CrossRef]
- Belis, C.A.; Favez, O.; Mircea, M.; Diapouli, E.; Manousakas, M.-I.; Vratolis, S.; Gilardoni, S.; Paglione, M.; Decesari, S.; Mocnik, G.; et al. European Guide on Air Pollution Source Apportionment with Receptor Models—Revised Version 2019, EUR 29816 EN; Publications office of the European Union: Luxembourg, 2019; ISBN 978-92-76-09001-4. [Google Scholar]
- Javed, W.; Guo, B. Chemical characterization and source apportionment of fine and coarse atmospheric particulate matter in Doha, Qatar. Atmos. Pollut. Res. 2020, 12, 122–136. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Z.; Zhang, T.; Wang, F.; Tao, Y.; Zhang, X.; Wang, F.; Huang, J.; Cheng, T.; Jiang, H.; et al. Chemical nature and predominant sources of PM10 and PM2.5 from multiple sites on the Silk Road, Northwest China. Atmos. Pollut. Res. 2020, 12, 425–436. [Google Scholar] [CrossRef]
- Qiu, Z.; Lyon, S.W.; Creveling, E. Defining a topographic index threshold to delineate hydrologically sensitive areas for water resources planning and management. Water Resour. Manag. 2020, 34, 3675–3688. [Google Scholar] [CrossRef]
- Arnold, N. A New approach for dealing with depressions in digital elevation models when calculating flow accumulation values. Prog. Phys. Geogr. 2010, 34, 781–809. [Google Scholar] [CrossRef]
- Semenov, M.Y.; Marinaite, I.I.; Golobokova, L.P.; Semenov, Y.M.; Khodzher, T.V. Revealing the chemical profiles of airborne particulate matter sources in Lake Baikal area: A Combination of Three Techniques. Sustainability 2022, 14, 6170. [Google Scholar] [CrossRef]
- Mi, H.H.; Lee, W.J.; Tsai, P.J.; Chen, C.B. A comparison on the emission of polycyclic aromatic hydrocarbons and their corresponding carcinogenic potencies from a vehicle engine using leaded and lead-free gasoline. Environ. Health Perspect. 2001, 109, 1285–1290. [Google Scholar] [CrossRef]
- Dao, L.; Morrison, L.; Zhang, H.; Zhang, C. Influences of traffic on Pb, Cu and Zn concentrations in roadside soils of an urban park in Dublin, Ireland. Environ. Geochem. Health 2014, 36, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Bartkowiak, A.; Lemanowicz, J.; Breza-Boruta, B. Evaluation of the content of Zn, Cu, Ni and Pb as well as the enzymatic activity of forest soils exposed to the effect of road traffic pollution. Environ. Sci. Pollut. Res. 2017, 24, 23893–23902. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H. Toxic metal concentrations and Cu–Zn–Pb isotopic compositions in tires. J. Anal. Sci. Technol. 2022, 13, 1–10. [Google Scholar] [CrossRef]
- Root, R.A. Analysis of a study of lead wheel weight deposition and abrasion in New Jersey. Water Air Soil Pollut. 2015, 226, 381. [Google Scholar] [CrossRef][Green Version]
- Taylor, M.; Kruger, N. Tyre weights an overlooked diffuse source of lead and antimony to road runoff. Sustainability 2020, 12, 6790. [Google Scholar] [CrossRef]
- Navarro, R.; Guzman, J.; Saucedo, I.; Revilla, J.; Guibal, E. Vanadium recovery from oil fly ash by leaching, precipitation and solvent extraction processes. Waste Manag. 2007, 27, 425–438. [Google Scholar] [CrossRef]
- Jung, M.; Mishra, B. Vanadium recovery from oil fly ash by carbon removal and roast-leach process. JOM 2018, 70, 168–172. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Dan, S.R.; Das, A.K. Determination of vanadium in coal fly ash by electrothermal atomic absorption spectrometry. Anal. Lett. 1993, 26, 295–308. [Google Scholar] [CrossRef]
- Aydin, F.; Saydut, A.; Gunduz, B.; Aydin, I.; Hamamci, C. Chemical speciation of vanadium in coal bottom ash. Clean—Soil Air Water 2012, 40, 444–448. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Al-Degs, Y.S.; Ghrair, A.; Khoury, H.; Ziedan, M. Extraction and separation of vanadium and nickel from fly ash produced in heavy fuel power plants. Chem. Eng. J. 2011, 173, 191–197. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Q.; Xiao, Z.; Fan, L.; Wang, D.; Li, X.; Du, J.; Cheng, J. Behaviors of chromium in coal-fired power plants and associated atmospheric emissions in Guizhou, Southwest China. Atmosphere 2020, 11, 951. [Google Scholar] [CrossRef]
- Tang, Q.; Chang, L.; He, F.; Miao, C.; Zheng, L.; Ma, D.; Wang, R.; Fu, B. Impact of ultra-low emission retrofitting on partitioning and emission behavior of chromium in a Chinese coal-fired power plant. Chemosphere 2022, 302, 134859. [Google Scholar] [CrossRef] [PubMed]
- Semenov, M.Y.; Onishchuk, N.A.; Netsvetaeva, O.G.; Khodzher, T.V. Source apportionment of particulate matter in urban snowpack using end-member mixing analysis and positive matrix factorization model. Sustainability 2021, 13, 13584. [Google Scholar] [CrossRef]




| CSA | PHE | ANT | FLA | PYR | BaA | CHR | BaP | BeP | BbF | BkF | IcdP | BghiP | Sum |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 42.4 | 2.04 | 109 | 79.9 | 38.6 | 43.3 | 28.8 | 86.0 | 44.6 | 18.6 | 14.4 | 18.1 | 526 |
| 2 | 173 | 24.3 | 634 | 525 | 412 | 360 | 352 | 701 | 417 | 198 | 224 | 183 | 4201 |
| 3 | 20.9 | 2.76 | 22.3 | 16.0 | 8.52 | 8.88 | 6.96 | 14.9 | 10.8 | 5.52 | 5.52 | 5.16 | 128 |
| 4 | 8.64 | 1.08 | 34.0 | 26.0 | 18.5 | 17.8 | 16.6 | 37.8 | 21.6 | 9.7 | 10.9 | 10.6 | 213 |
| 5 | 10.7 | 0.48 | 26.4 | 19.1 | 9.60 | 10.7 | 5.40 | 20.5 | 10.0 | 4.32 | 2.16 | 2.76 | 122 |
| 6 | 22.6 | 1.92 | 45.1 | 35.0 | 22.9 | 22.9 | 19.8 | 42.7 | 27.2 | 12.7 | 15.8 | 15.1 | 284 |
| 7 | 14.2 | 1.20 | 64.8 | 47.4 | 20.9 | 23.6 | 15.7 | 52.1 | 27.2 | 10.9 | 7.80 | 8.16 | 294 |
| 8 | 30.5 | 2.76 | 103 | 73.9 | 34.6 | 29.0 | 23.9 | 68.4 | 35.9 | 13.9 | 15.4 | 16.7 | 448 |
| 9 | 31.6 | 7.20 | 141 | 107 | 47.4 | 75.8 | 32.6 | 141 | 85.9 | 33.8 | 19.3 | 18.0 | 741 |
| 10 | 48.5 | 4.32 | 70.1 | 55.8 | 45.8 | 39.2 | 35.8 | 77.4 | 46.2 | 21.8 | 30.8 | 31.4 | 507 |
| 11 | 24.1 | 2.64 | 37.6 | 28.7 | 15.6 | 18.2 | 13.3 | 33.5 | 22.0 | 9.24 | 7.80 | 8.16 | 221 |
| Average | 38.8 | 4.6 | 117.0 | 92.2 | 61.3 | 59.1 | 50.0 | 116 | 68.0 | 30.7 | 32.2 | 28.8 | 699 |
| STD * | 46.1 | 6.79 | 176 | 146 | 117 | 102 | 100 | 197 | 118 | 56.0 | 64.16 | 51.6 | 1177 |
| PHE | ANT | FLA | PYR | BaA | CHR | BaP | BeP | BbF | BkF | IcdP | BghiP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | 0.07 | 0.03 | 0.89 | 1.21 | 0.59 | 0.46 | 0.32 | 0.61 | 0.49 | 0.21 | 0.39 | 0.45 |
| 25th * | 4.87 | 0.38 | 4.86 | 3.77 | 2.69 | 3.15 | 2.67 | 3.41 | 2.79 | 1.32 | 2.14 | 1.42 |
| Med | 10.3 | 1.27 | 17.4 | 16.7 | 9.09 | 8.87 | 10.1 | 14.2 | 11.3 | 4.33 | 5.51 | 7.33 |
| 75th * | 22.6 | 2.97 | 67.5 | 56.9 | 28.2 | 31.8 | 21.4 | 47.0 | 29.9 | 13.7 | 16.3 | 13.1 |
| Max | 189 | 34.1 | 811 | 716 | 535 | 388 | 501 | 798 | 489 | 205 | 301 | 252 |
| Mean | 27.4 | 4.01 | 83.9 | 56.4 | 37.9 | 39.5 | 31.9 | 82.4 | 58.6 | 30.4 | 22.3 | 19.6 |
| STD ** | 41.5 | 4.88 | 127 | 99.8 | 78.7 | 91.2 | 76.3 | 132 | 98.7 | 42.9 | 45.4 | 34.6 |
| CSA | V | Cr | Co | Ni | Cu | Zn | Mo | Hg | Pb | Bi | Sum |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 54.7 | 42.6 | 8.09 | 22.0 | 18.5 | 70.8 | 1.54 | 0.02 | 22.8 | 0.12 | 244 |
| 2 | 64.8 | 83.3 | 9.67 | 28.8 | 55.4 | 210 | 2.38 | 0.07 | 61.2 | 0.25 | 522 |
| 3 | 90.0 | 47.4 | 8.47 | 28.3 | 17.5 | 133 | 2.51 | 0.04 | 24.7 | 0.13 | 358 |
| 4 | 48.8 | 58.4 | 8.21 | 23.0 | 17.8 | 79.6 | 2.07 | 0.03 | 24.0 | 0.10 | 266 |
| 5 | 71.4 | 46.7 | 6.28 | 23.8 | 13.7 | 76.2 | 1.65 | 0.01 | 22.6 | 0.11 | 267 |
| 6 | 46.6 | 43.0 | 5.29 | 24.4 | 13.7 | 66.6 | 1.52 | 0.03 | 24.0 | 0.08 | 229 |
| 7 | 50.0 | 41.2 | 8.03 | 18.8 | 22.3 | 93.7 | 1.83 | 0.03 | 23.4 | 0.18 | 265 |
| 8 | 40.1 | 31.9 | 7.16 | 17.9 | 13.6 | 57.6 | 2.83 | 0.03 | 18.8 | 0.10 | 196 |
| 9 | 73.3 | 84.4 | 32.6 | 37.7 | 35.6 | 176 | 2.09 | 0.07 | 57.1 | 0.84 | 506 |
| 10 | 51.6 | 36.2 | 6.11 | 21.1 | 14.4 | 137 | 1.40 | 0.06 | 27.0 | 0.18 | 298 |
| 11 | 69.5 | 66.6 | 9.91 | 26.9 | 16.6 | 75.8 | 1.46 | 0.02 | 15.7 | 0.14 | 287 |
| Average | 60.1 | 52.9 | 10.0 | 24.8 | 21.7 | 107 | 1.93 | 0.04 | 29.2 | 0.20 | 313 |
| STD * | 14.9 | 18.1 | 7.64 | 5.56 | 12.8 | 50.2 | 0.48 | 0.02 | 15.1 | 0.22 | 108 |
| V | Cr | Co | Ni | Cu | Zn | Mo | Hg | Pb | Bi | |
|---|---|---|---|---|---|---|---|---|---|---|
| Min | 29.3 | 24.3 | 3.12 | 12.2 | 6.75 | 25.8 | 1.75 | 0.004 | 7.61 | 0.03 |
| 25th * | 38.4 | 29.5 | 4.93 | 16.4 | 10.5 | 46.7 | 1.90 | 0.01 | 10.5 | 0.06 |
| Median | 45.1 | 37.0 | 6.17 | 19.7 | 13.8 | 66.3 | 2.44 | 0.02 | 14.7 | 0.09 |
| 75th * | 57.5 | 46.9 | 7.78 | 23.9 | 18.2 | 103 | 3.43 | 0.04 | 19.7 | 0.18 |
| Max | 91.1 | 85.2 | 33.6 | 39.9 | 56.1 | 220 | 6.70 | 0.15 | 88.9 | 0.92 |
| Mean | 49.6 | 43.8 | 7.38 | 21.8 | 16.4 | 86.3 | 2.72 | 0.03 | 18.6 | 0.16 |
| STD ** | 8.74 | 11.9 | 2.87 | 6.07 | 8.15 | 34.2 | 1.14 | 0.02 | 13.1 | 0.09 |
| CSA | Gasoline Combustion | Coal and Wood Combustion | Oil and Diesel Fuel Combustion |
|---|---|---|---|
| 1 | 0.33 | 0.21 | 0.46 |
| 2 | 0.28 | 0.33 | 0.39 |
| 3 | 0.70 | 0.17 | 0.13 |
| 4 | 0.29 | 0.35 | 0.35 |
| 5 | 0.33 | 0.14 | 0.53 |
| 6 | 0.42 | 0.32 | 0.27 |
| 7 | 0.31 | 0.19 | 0.49 |
| 8 | 0.42 | 0.21 | 0.37 |
| 9 | 0.35 | 0.19 | 0.47 |
| 10 | 0.47 | 0.32 | 0.21 |
| 11 | 0.55 | 0.20 | 0.25 |
| Mean | 0.40 | 0.24 | 0.36 |
| CSA | Nonexhaust Traffic Emissions | Fossil Fuel Combustion |
|---|---|---|
| 1 | 46 | 54 |
| 2 | 69 | 31 |
| 3 | 50 | 50 |
| 4 | 48 | 52 |
| 5 | 36 | 64 |
| 6 | 52 | 48 |
| 7 | 56 | 44 |
| 8 | 63 | 37 |
| 9 | 53 | 47 |
| 10 | 65 | 35 |
| 11 | 32 | 68 |
| Mean | 52 | 48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenov, M.Y.; Silaev, A.V.; Semenov, Y.M.; Begunova, L.A.; Semenov, Y.M. Identifying and Characterizing Critical Source Areas of Organic and Inorganic Pollutants in Urban Agglomeration in Lake Baikal Watershed. Sustainability 2022, 14, 14827. https://doi.org/10.3390/su142214827
Semenov MY, Silaev AV, Semenov YM, Begunova LA, Semenov YM. Identifying and Characterizing Critical Source Areas of Organic and Inorganic Pollutants in Urban Agglomeration in Lake Baikal Watershed. Sustainability. 2022; 14(22):14827. https://doi.org/10.3390/su142214827
Chicago/Turabian StyleSemenov, Mikhail Y., Anton V. Silaev, Yuri M. Semenov, Larisa A. Begunova, and Yuri M. Semenov. 2022. "Identifying and Characterizing Critical Source Areas of Organic and Inorganic Pollutants in Urban Agglomeration in Lake Baikal Watershed" Sustainability 14, no. 22: 14827. https://doi.org/10.3390/su142214827
APA StyleSemenov, M. Y., Silaev, A. V., Semenov, Y. M., Begunova, L. A., & Semenov, Y. M. (2022). Identifying and Characterizing Critical Source Areas of Organic and Inorganic Pollutants in Urban Agglomeration in Lake Baikal Watershed. Sustainability, 14(22), 14827. https://doi.org/10.3390/su142214827





