Constructed Wetlands as Nature-Based Solutions for the Removal of Antibiotics: Performance, Microbial Response, and Emergence of Antimicrobial Resistance (AMR)
Abstract
:1. Introduction
2. Materials and Methods
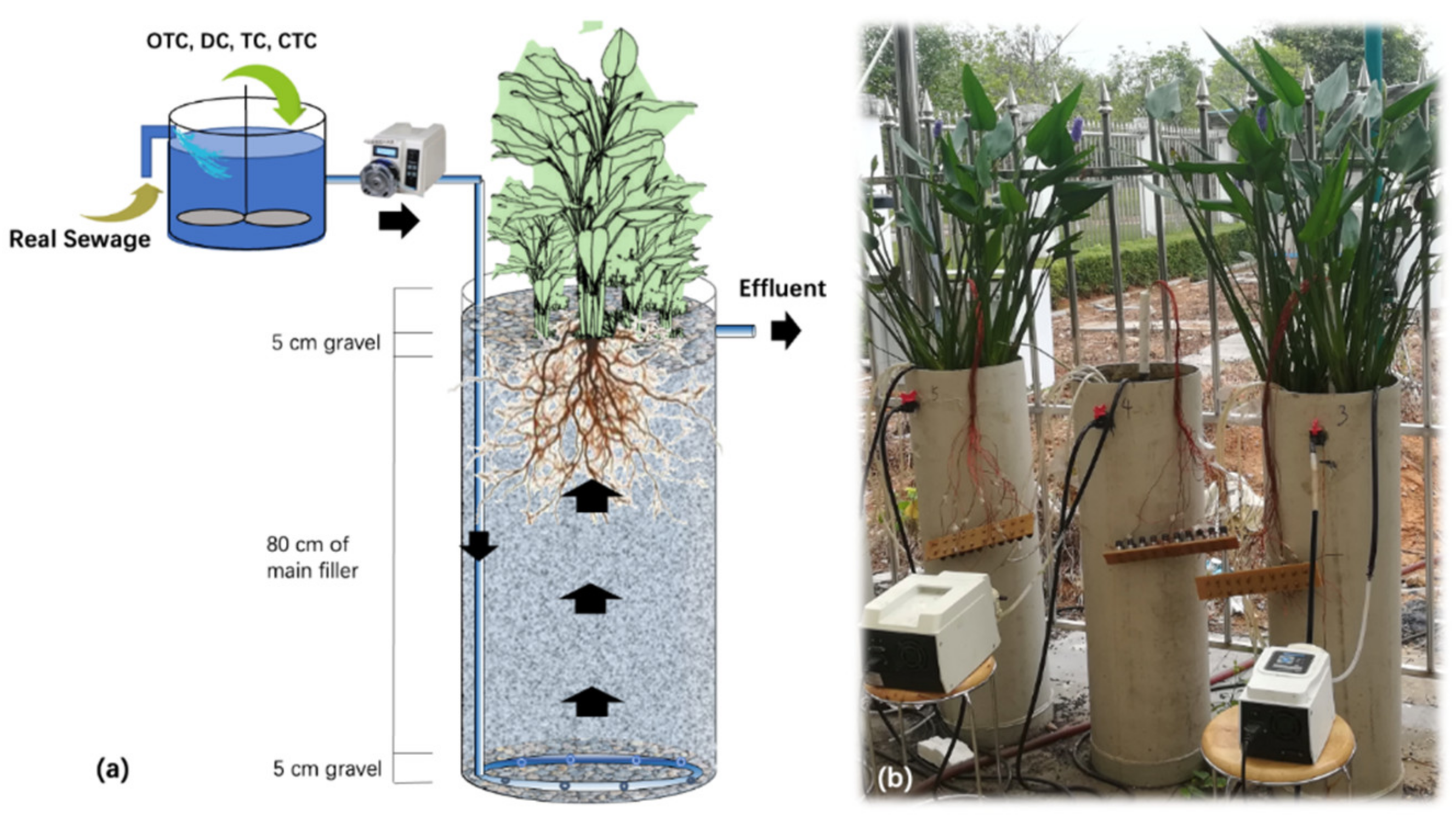
2.1. Experimental Setup and Operation
2.2. Sampling and Data Analysis
2.2.1. Analysis of Water Samples
2.2.2. Quantification of ARGs
2.2.3. Microbial Community Analysis
2.3. Statistical Analysis
3. Results
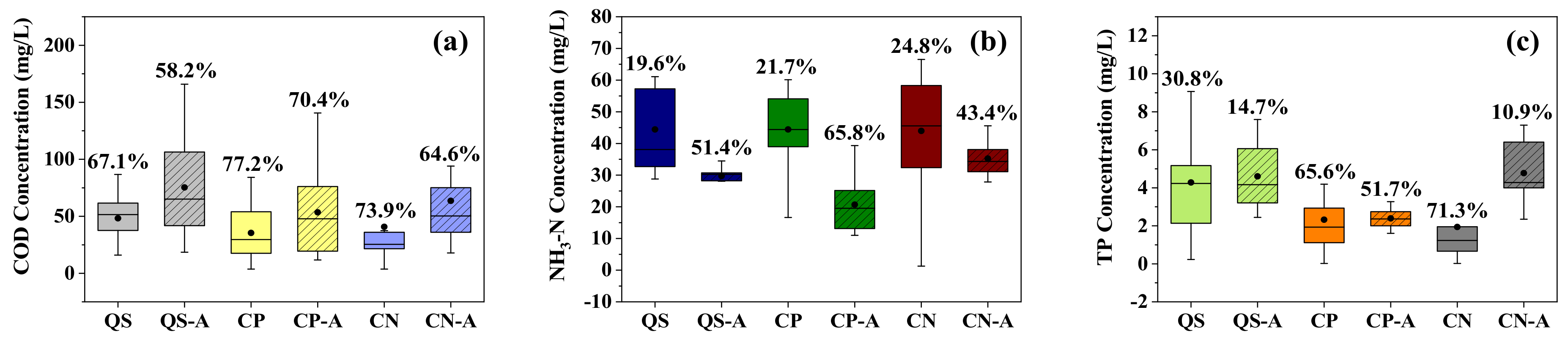
3.1. The Removal of COD and Nutrients
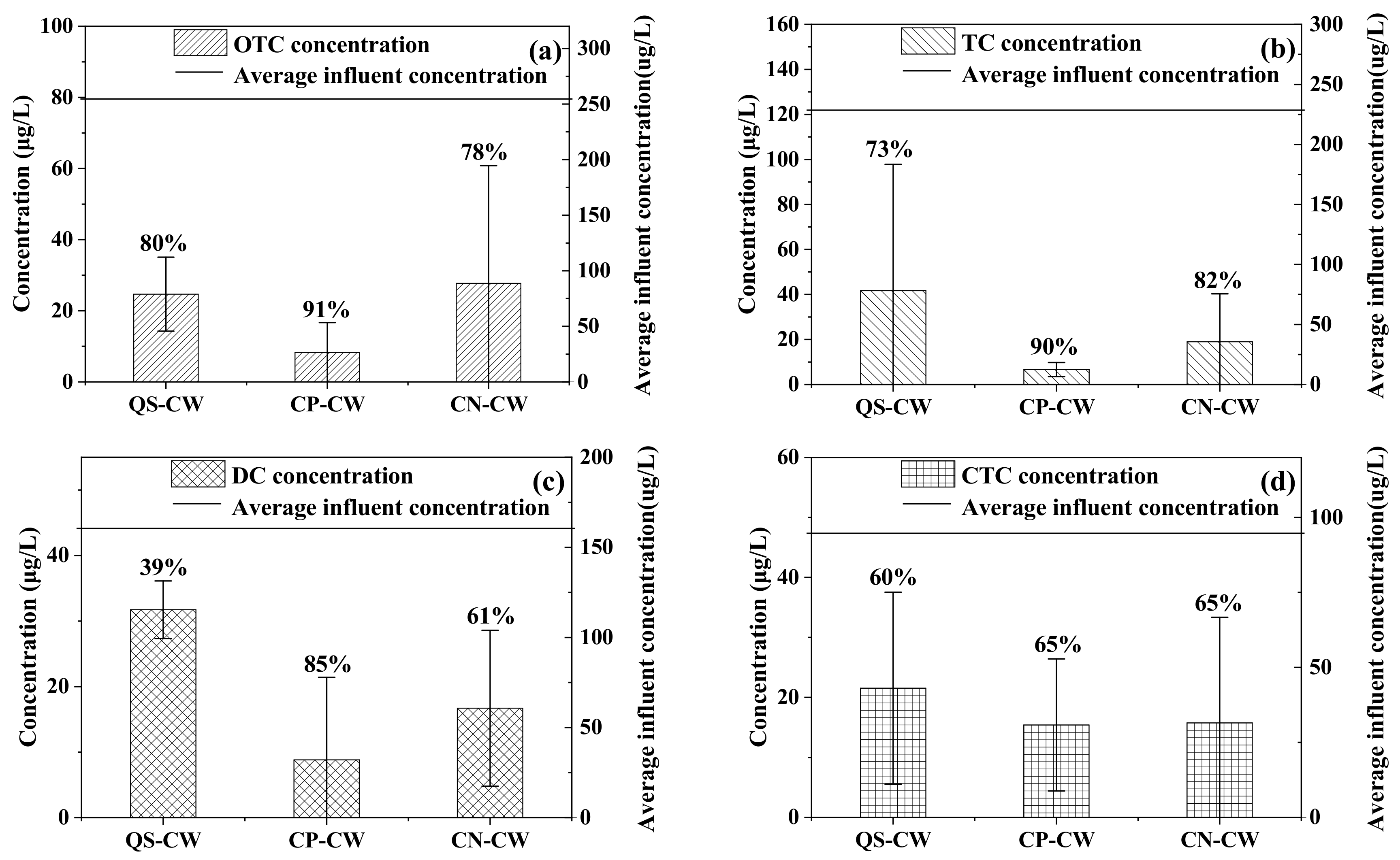
3.2. The Removal of Antibiotics
3.3. The Emergence of ARGs in the Substrate
3.4. The Responses of the Microbial Community
4. Discussion
4.1. The Effects of Substrate on the Removal of Nutrients and Antibiotics
4.2. The Effects of the Plant on the Removal of Nutrients and Antibiotics
4.3. Microbial Responses of CWs to Antibiotics
4.4. The Potential Risks of ARGs Accumulation
| Wetland Type | Substrate | Plant | Antibiotic Type | Removal (%) | ARGs Abundance | References |
|---|---|---|---|---|---|---|
| VFCW | volcanic rocks | hybrid pennisetum | Oxytetracycline | 91 | decrease | [45] |
| VFCW | zeolite | hybrid pennisetum | Oxytetracycline | 95 | increase | [45] |
| VFCW | sand | Phragmites australis | Lincosamides Clindamycin | <−200 | decrease | [18] |
| VFCW | zeolite | Phragmites communis | Sulfamethazine | 49 | decrease | [21] |
| VFCW | biochar | Phragmites communis | Sulfamethazine | 57 | decrease | [21] |
| VFCW | sand | Phragmites australis (Cav.) Trin. Ex Steud. | CIP | 95 | - | [26] |
| VFCW | sand | unplanted | CIP | 93 | - | [26] |
| HSCW | light expanded clay aggregates | unplanted | Tetracyclines | - | decrease | [30] |
| HSCW | - | Phragmites australis | Oxytetracycline | 28~100 | decrease | [46] |
| HSCW | oyster shell | Cyperus alternifolius L. | ETM-H2O, MON, OFX, SMR, SMZ, NOV | 10~60 | decrease | [33] |
| HSCW | zeolite | Cyperus alternifolius L. | ETM-H2O, MON, OFX, SMR, SMZ, NOV | 66~99 | decrease | [33] |
| HSCW | zeolite, gravel | Iris pseudacorus | Sulfamethazine | 69 | decrease (69%) | [16] |
| HSCW | zeolite, gravel | Phragmites australis | Sulfamethazine | 65 | decrease (65%) | [16] |
| SFCW | - | Phragmites australis | - | - | decrease (60~78%) | [43] |
| SFCW | heavy clay | Phragmites australis, Typha latifolia | Ciprofloxacin | 59 | unchanged | [28] |
| SFCW | sandy clay loam | Typha orientalis Presl | Monensin, Salinomycin and Narasin | 27 | - | [47] |
| SFCW | sandy soils | Typha orientalis Presl | Monensin, Salinomycin, and Narasin | 32 | - | [47] |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gothwal, R.S.; Shashidhar, T. Antibiotic Pollution in the Environment: A Review. CLEAN Soil Air Water 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Jeong, J.; Song, W.; Cooper, W.J.; Jung, J.; Greaves, J. Degradation of tetracycline antibiotics: Mechanisms and kinetic studies for advanced oxidation/reduction processes. Chemosphere 2010, 78, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.P.; Mitzel, M.R.; Slawson, R.M.; Legge, R.L. Effect of ciprofloxacin on microbiological development in wetland mesocosms. Water Res. 2011, 45, 3185–3196. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Carvalho, P.N.; Zhang, L.; Zhang, Y.; Button, M.; Arias, C.A.; Weber, K.P.; Brix, H. Functionality of microbial communities in constructed wetlands used for pesticide remediation: Influence of system design and sampling strategy. Water Res. 2017, 110, 241–251. [Google Scholar] [CrossRef]
- Taubes, G. The bacteria fight back. Science 2008, 321, 356–361. [Google Scholar] [CrossRef]
- Nnadozie, C.F.; Kumari, S.; Bux, F. Status of pathogens, antibiotic resistance genes and antibiotic residues in wastewater treatment systems. Rev. Env. Sci. Biotechnol. 2017, 16, 491–515. [Google Scholar] [CrossRef]
- Teshome, A.; Alemayehu, T.; Deriba, W.; Ayele, Y. Antibiotic Resistance Profile of Bacteria Isolated from Wastewater Systems in Eastern Ethiopia. J. Environ. Public Health 2020, 2020, 2796365. [Google Scholar] [CrossRef]
- Bai, S.; Lyu, T.; Ding, Y.; Li, Z.; Wang, D.; You, S.; Xie, Q. Campus Sewage Treatment in Multilayer Horizontal Subsurface Flow Constructed Wetlands: Nitrogen Removal and Microbial Community Distribution. CLEAN Soil Air Water 2017, 45, 1700254. [Google Scholar] [CrossRef] [Green Version]
- Hijosa-Valsero, M.; Fink, G.; Schlusener, M.P.; Sidrach-Cardona, R.; Martin-Villacorta, J.; Ternes, T.; Becares, E. Removal of antibiotics from urban wastewater by constructed wetland optimization. Chemosphere 2011, 83, 713–719. [Google Scholar] [CrossRef]
- Lyu, T.; He, K.; Dong, R.; Wu, S. The intensified constructed wetlands are promising for treatment of ammonia stripped effluent: Nitrogen transformations and removal pathways. Environ. Pollut. 2018, 236, 273–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, F.; Almeida, C.M.R.; Ribeiro, I.; Mucha, A.P. Potential of constructed wetland for the removal of antibiotics and antibiotic resistant bacteria from livestock wastewater. Ecol. Eng. 2019, 129, 45–53. [Google Scholar] [CrossRef]
- Zhang, L.; Lyu, T.; Zhang, Y.; Button, M.; Arias, C.A.; Weber, K.P.; Brix, H.; Carvalho, P.N. Impacts of design configuration and plants on the functionality of the microbial community of mesocosm-scale constructed wetlands treating ibuprofen. Water Res. 2018, 131, 228–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Liu, C.; Li, K.; Su, J.; Zhu, G.; Liu, L. Performance of vertical up-flow constructed wetlands on swine wastewater containing tetracyclines and tet genes. Water Res. 2015, 70, 109–117. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Y.X.; Zhang, J.J.; Liu, S.; Song, H.L.; Yang, X.L. Constructed Wetland Revealed Efficient Sulfamethoxazole Removal but Enhanced the Spread of Antibiotic Resistance Genes. Molecules 2020, 25, 834. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.F.; Ye, G.-Y.; Yi, N.K.; Lu, L.-J.; Zhang, L.; Yang, L.Y.; Xiao, L.; Liu, J. Effect of plant physiological characteristics on the removal of conventional and emerging pollutants from aquaculture wastewater by constructed wetlands. Ecol. Eng. 2019, 135, 45–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Lyu, T.; Zhang, L.; Button, M.; Arias, C.A.; Weber, K.P.; Shi, J.; Chen, Z.; Brix, H.; Carvalho, P.N. Microbial community metabolic profiles in saturated constructed wetlands treating iohexol and ibuprofen. Sci. Total Environ. 2019, 651, 1926–1934. [Google Scholar] [CrossRef] [Green Version]
- Avila, C.; Garcia-Galan, M.J.; Borrego, C.M.; Rodriguez-Mozaz, S.; Garcia, J.; Barcelo, D. New insights on the combined removal of antibiotics and ARGs in urban wastewater through the use of two configurations of vertical subsurface flow constructed wetlands. Sci. Total Environ. 2021, 755, 142554. [Google Scholar] [CrossRef]
- Du, L.; Zhao, Y.; Wang, C.; Zhang, H.; Chen, Q.; Zhang, X.; Zhang, L.; Wu, J.; Wu, Z.; Zhou, Q. Removal performance of antibiotics and antibiotic resistance genes in swine wastewater by integrated vertical-flow constructed wetlands with zeolite substrate. Sci. Total Environ. 2020, 721, 137765. [Google Scholar] [CrossRef]
- Bai, S.; Qin, L.; Liu, L.; Gao, X.; Ding, Y.; Li, Y. Effect of substrate types on contaminant removals, electrochemical characteristics and microbial community in vertical flow constructed wetlands for treatment of urban sewage. J. Environ. Manag. 2021, 280, 111682. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, B.; Wang, H.; Lai, X.; Li, F.; Salam, M.M.A.; Pan, F.; Zhao, Y. The simultaneous antibiotics and nitrogen removal in vertical flow constructed wetlands: Effects of substrates and responses of microbial functions. Bioresour. Technol. 2020, 310, 123419. [Google Scholar] [CrossRef] [PubMed]
- de Nicolás, A.P.; Berenguer, R.; Esteve-Núñez, A. Evaluating bioelectrochemically-assisted constructed wetland (METland®) for treating wastewater: Analysis of materials, performance and electroactive communities. Chem. Eng. J. 2022, 440, 135748. [Google Scholar] [CrossRef]
- Lei, Y.; Langenhoff, A.; Bruning, H.; Rijnaarts, H. Sorption of micropollutants on selected constructed wetland support matrices. Chemosphere 2021, 275, 130050. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Zhang, Y.; Casas, M.E.; Carvalho, P.N.; Arias, C.A.; Best, K.; Brix, H. Phytoremediation of imazalil and tebuconazole by four emergent wetland plant species in hydroponic medium. Chemosphere 2016, 148, 459–466. [Google Scholar] [CrossRef]
- Tai, Y.; Tam, N.F.-Y.; Dai, Y.; Yang, Y.; Lin, J.; Tao, R.; Yang, Y.; Wang, J.; Wang, R.; Huang, W.; et al. Assessment of rhizosphere processes for removing water-borne macrolide antibiotics in constructed wetlands. Plant Soil 2017, 419, 489–502. [Google Scholar] [CrossRef]
- Wang, S.; Cui, Y.; Li, A.; Zhang, W.; Wang, D.; Ma, J. Fate of antibiotics in three distinct sludge treatment wetlands under different operating conditions. Sci. Total Environ. 2019, 671, 443–451. [Google Scholar] [CrossRef]
- Wen, H.; Zhu, H.; Yan, B.; Xu, Y.; Shutes, B. Treatment of typical antibiotics in constructed wetlands integrated with microbial fuel cells: Roles of plant and circuit operation mode. Chemosphere 2020, 250, 126252. [Google Scholar] [CrossRef]
- Berglund, B.; Khan, G.A.; Weisner, S.E.; Ehde, P.M.; Fick, J.; Lindgren, P.E. Efficient removal of antibiotics in surface-flow constructed wetlands, with no observed impact on antibiotic resistance genes. Sci. Total Environ. 2014, 476–477, 29–37. [Google Scholar] [CrossRef]
- Wei, Z.; Feng, K.; Wang, Z.; Zhang, Y.; Yang, M.; Zhu, Y.G.; Virta, M.P.J.; Deng, Y. High-Throughput Single-Cell Technology Reveals the Contribution of Horizontal Gene Transfer to Typical Antibiotic Resistance Gene Dissemination in Wastewater Treatment Plants. Environ. Sci. Technol. 2021, 55, 11824–11834. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, Q.; Nie, X.; Chen, B.; Xiao, Y.; Zhou, Q.; Liao, W.; Liang, X. Occurrence and elimination of antibiotic resistance genes in a long-term operation integrated surface flow constructed wetland. Chemosphere 2017, 173, 99–106. [Google Scholar] [CrossRef]
- Ma, J.; Cui, Y.; Li, A.; Zou, X.; Ma, C.; Chen, Z. Antibiotics and antibiotic resistance genes from wastewater treated in constructed wetlands. Ecol. Eng. 2022, 177, 106548. [Google Scholar] [CrossRef]
- Pan, M.; Lyu, T.; Zhan, L.; Matamoros, V.; Angelidaki, I.; Cooper, M.; Pan, G. Mitigating antibiotic pollution using cyanobacteria: Removal efficiency, pathways and metabolism. Water Res. 2021, 190, 116735. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, X.D.; Liu, Y.S.; Ying, G.G.; Liu, S.S.; He, L.Y.; Su, H.C.; Hu, L.X.; Chen, F.R.; Yang, Y.Q. Removal of antibiotics and antibiotic resistance genes from domestic sewage by constructed wetlands: Optimization of wetland substrates and hydraulic loading. Sci. Total Environ. 2016, 565, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Song, H.L.; Zhang, S.; Guo, J.; Yang, Y.L.; Zhang, L.M.; Li, H.; Yang, X.L.; Liu, X. Vertical up-flow constructed wetlands exhibited efficient antibiotic removal but induced antibiotic resistance genes in effluent. Chemosphere 2018, 203, 434–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Chen, X.; Tang, J.; Zhang, L.; Zhang, C.; Perry, D.C.; You, W. External carbon addition increases nitrate removal and decreases nitrous oxide emission in a restored wetland. Ecol. Eng. 2019, 138, 200–208. [Google Scholar] [CrossRef]
- Dai, X.; Su, C.; Chen, Z.; Li, X.; Lu, P.; Qi, Z.; Luo, Z.; Chen, M. Sulfonamide and quinolone antibiotics contaminated wastewater treatment by constructed rapid infiltration: Efficiency and microbial community structure. Process Saf. Environ. Prot. 2022, 161, 542–555. [Google Scholar] [CrossRef]
- Man, Y.; Wang, J.; Tam, N.F.; Wan, X.; Huang, W.; Zheng, Y.; Tang, J.; Tao, R.; Yang, Y. Responses of rhizosphere and bulk substrate microbiome to wastewater-borne sulfonamides in constructed wetlands with different plant species. Sci. Total Environ. 2020, 706, 135955. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, R.; Zhang, H.; Yang, M.; Zhang, Y. Developmental dynamics of antibiotic resistome in aerobic biofilm microbiota treating wastewater under stepwise increasing tigecycline concentrations. Environ. Int. 2019, 131, 105008. [Google Scholar] [CrossRef]
- Chen, H.; Chen, R.; Jing, L.; Bai, X.; Teng, Y. A metagenomic analysis framework for characterization of antibiotic resistomes in river environment: Application to an urban river in Beijing. Environ. Pollut. 2019, 245, 398–407. [Google Scholar] [CrossRef]
- Islam, G.M.; Gilbride, K.A. The effect of tetracycline on the structure of the bacterial community in a wastewater treatment system and its effects on nitrogen removal. J. Hazard. Mater. 2019, 371, 130–137. [Google Scholar] [CrossRef]
- Meng, F.; Gao, G.; Yang, T.-T.; Chen, X.; Chao, Y.; Na, G.; Ge, L.; Huang, L.-N. Effects of fluoroquinolone antibiotics on reactor performance and microbial community structure of a membrane bioreactor. Chem. Eng. J. 2015, 280, 448–458. [Google Scholar] [CrossRef]
- Chen, J.; Ying, G.G.; Wei, X.D.; Liu, Y.S.; Liu, S.S.; Hu, L.X.; He, L.Y.; Chen, Z.F.; Chen, F.R.; Yang, Y.Q. Removal of antibiotics and antibiotic resistance genes from domestic sewage by constructed wetlands: Effect of flow configuration and plant species. Sci. Total Environ. 2016, 571, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Nolvak, H.; Truu, M.; Tiirik, K.; Oopkaup, K.; Sildvee, T.; Kaasik, A.; Mander, U.; Truu, J. Dynamics of antibiotic resistance genes and their relationships with system treatment efficiency in a horizontal subsurface flow constructed wetland. Sci. Total Environ. 2013, 461–462, 636–644. [Google Scholar] [CrossRef]
- Imchen, M.; Kumavath, R. Metagenomic insights into the antibiotic resistome of mangrove sediments and their association to socioeconomic status. Environ. Pollut. 2021, 268, 115795. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, C.; Zheng, J.; Huang, X.; Wang, Z.; Liu, Y.; Zhu, G. Elimination of veterinary antibiotics and antibiotic resistance genes from swine wastewater in the vertical flow constructed wetlands. Chemosphere 2013, 91, 1088–1093. [Google Scholar] [CrossRef]
- Sabri, N.A.; Schmitt, H.; van der Zaan, B.M.; Gerritsen, H.W.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Performance of full scale constructed wetlands in removing antibiotics and antibiotic resistance genes. Sci. Total Environ. 2021, 786, 147368. [Google Scholar] [CrossRef]
- Hussain, S.A.; Prasher, S.O.; Patel, R.M. Removal of ionophoric antibiotics in free water surface constructed wetlands. Ecol. Eng. 2012, 41, 13–21. [Google Scholar] [CrossRef]


| Observed Genus | Diversity Index | ||
|---|---|---|---|
| Shannon–Wiener Index | Simpson’s Diversity Index | ||
| QS-CW | 2229 | 4.98 | 0.9843 |
| CP-CW | 2228 | 4.90 | 0.9903 |
| CN-CW | 2237 | 5.16 | 0.9932 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, S.; Wang, X.; Zhang, Y.; Liu, F.; Shi, L.; Ding, Y.; Wang, M.; Lyu, T. Constructed Wetlands as Nature-Based Solutions for the Removal of Antibiotics: Performance, Microbial Response, and Emergence of Antimicrobial Resistance (AMR). Sustainability 2022, 14, 14989. https://doi.org/10.3390/su142214989
Bai S, Wang X, Zhang Y, Liu F, Shi L, Ding Y, Wang M, Lyu T. Constructed Wetlands as Nature-Based Solutions for the Removal of Antibiotics: Performance, Microbial Response, and Emergence of Antimicrobial Resistance (AMR). Sustainability. 2022; 14(22):14989. https://doi.org/10.3390/su142214989
Chicago/Turabian StyleBai, Shaoyuan, Xin Wang, Yang Zhang, Fang Liu, Lulu Shi, Yanli Ding, Mei Wang, and Tao Lyu. 2022. "Constructed Wetlands as Nature-Based Solutions for the Removal of Antibiotics: Performance, Microbial Response, and Emergence of Antimicrobial Resistance (AMR)" Sustainability 14, no. 22: 14989. https://doi.org/10.3390/su142214989






