Distinct Changes in Abundance of Culturable Microbial Community and Respiration Activities in Response to Mineral–Organic Mixture Application in Contaminated Soil
Abstract
1. Introduction
2. Material and Methods
2.1. Soil Material Properties
2.2. Design and Conduct of the Pot Experiment
2.3. Chemical Analyses in Soil Material
2.4. Microbiological Analyses of Soil
2.5. Respiration Activity
2.6. Statistical Analyses
3. Results
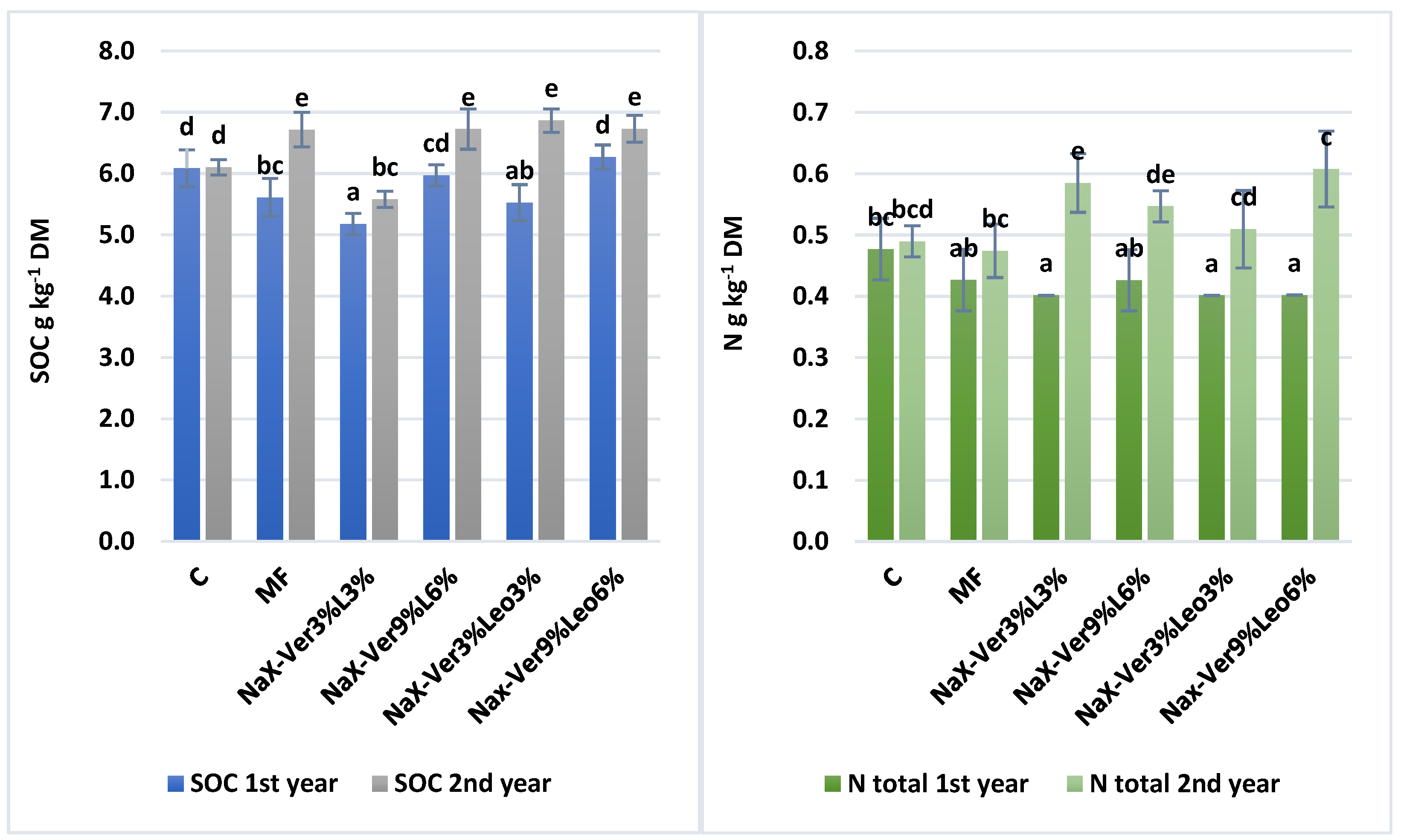
3.1. Changes in Soil Physicochemical Properties
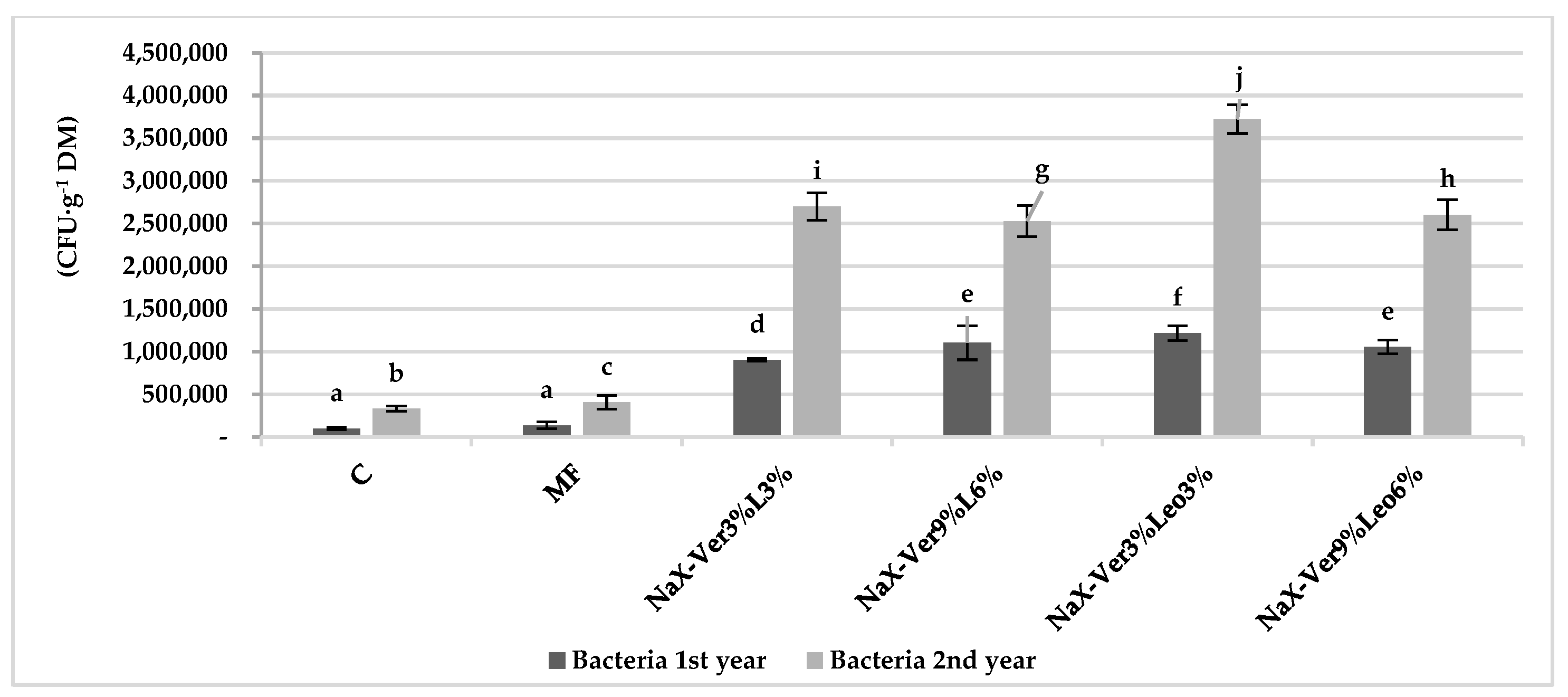
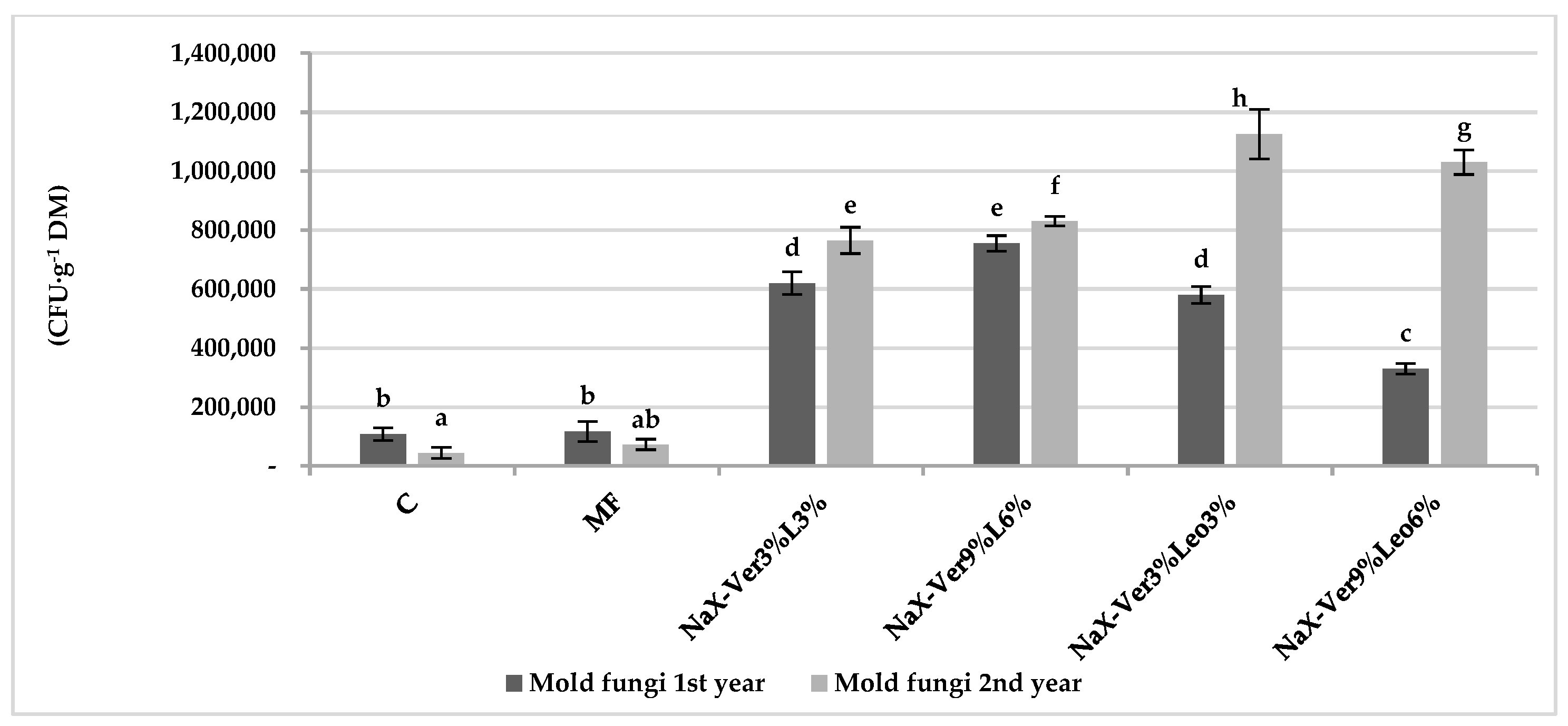
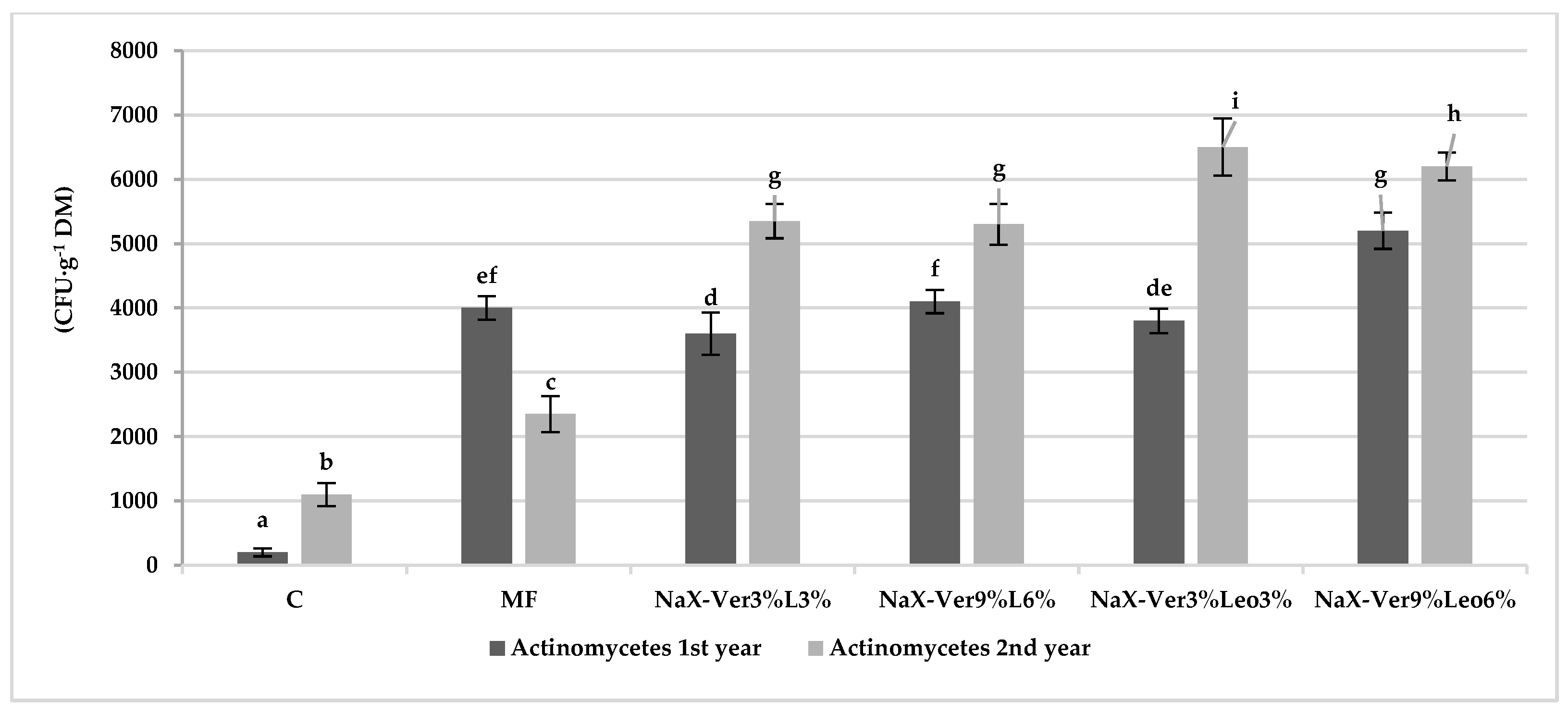
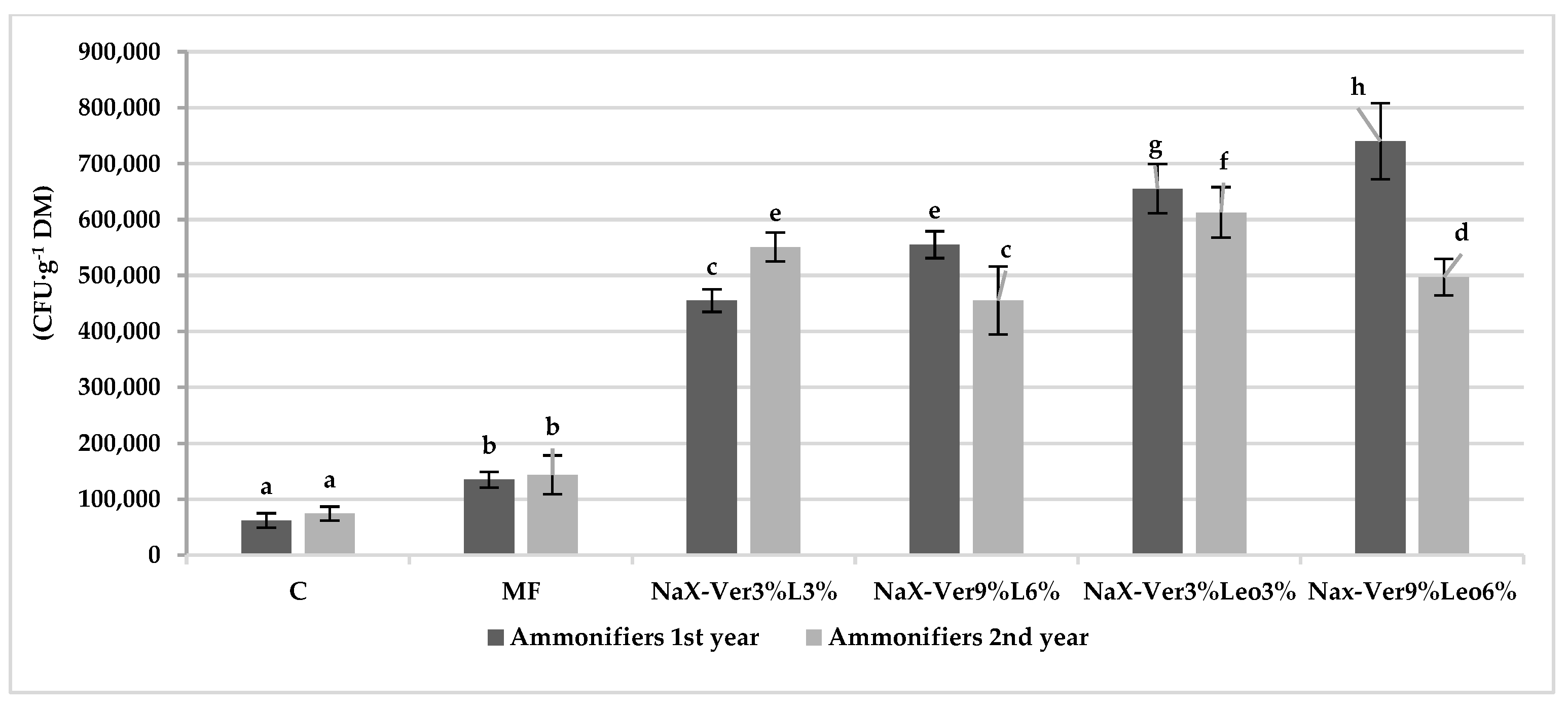
3.2. Abundance of Culturable Microorganisms in Soil
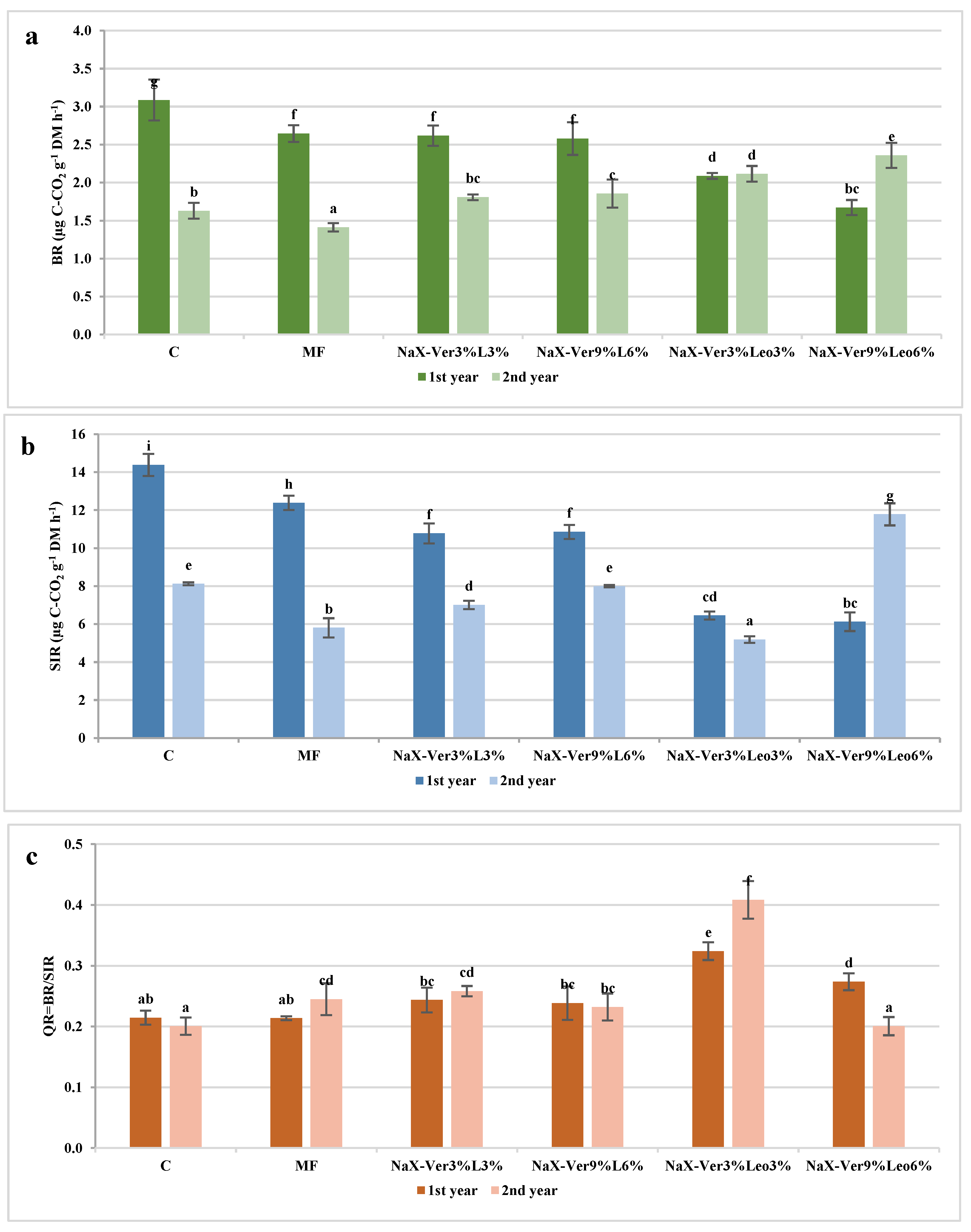
3.3. Changes in Soil Respiration Activity
4. Discussion
4.1. Mineral–Organic Mixture Application Affects Soil Physicochemical Properties
4.2. Mineral–Organic Mixture Application Impact on Microorganism Abundance
4.3. Changes in Respiration Activity in Soil
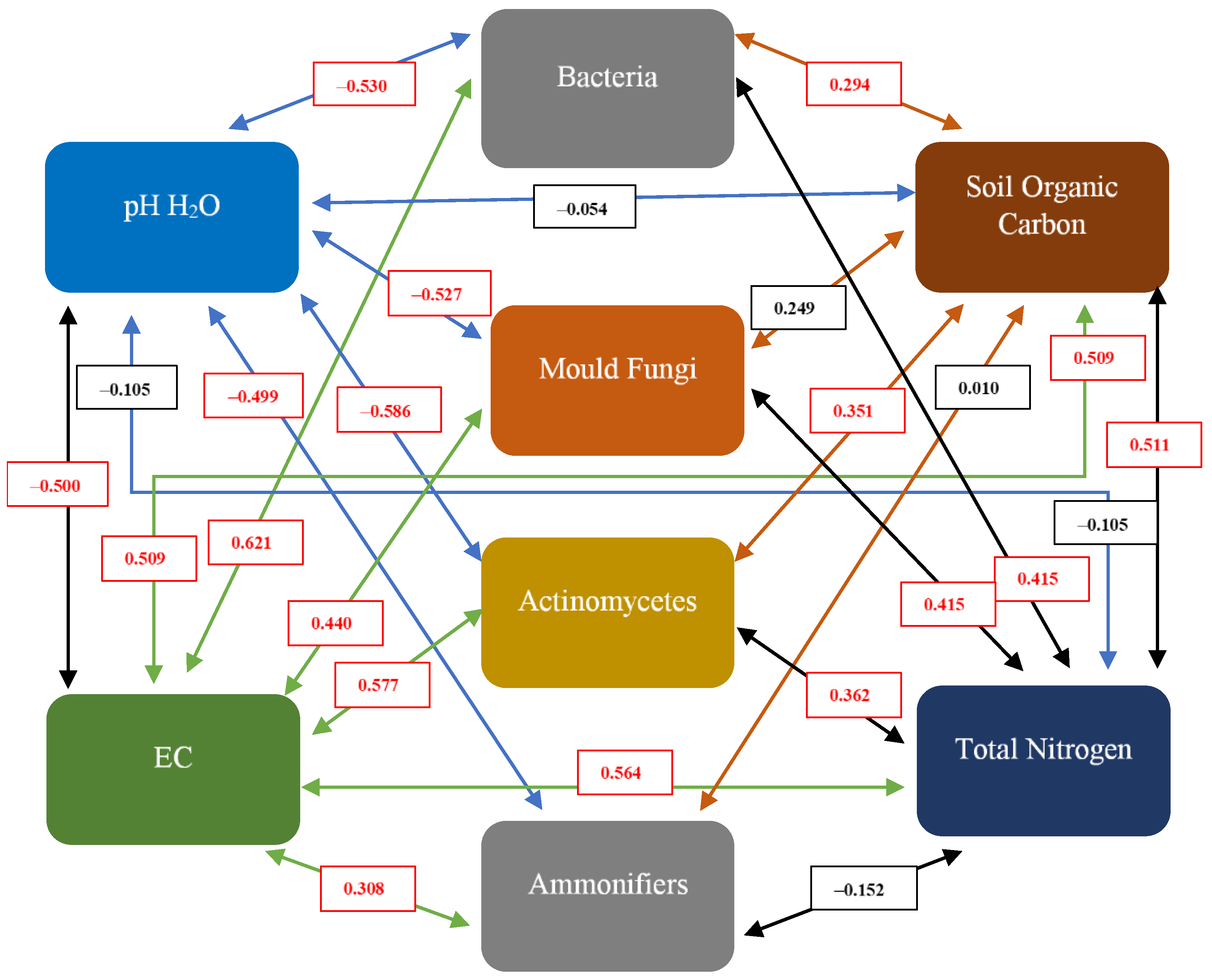
4.4. Correlation between Physicochemical Properties and Culturable Microorganisms’ Population
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeffery, S.; Gardi, C.; Jones, A.; Montanarella, L.; Marmo, L.; Miko, L.; Ritz, K.; Pérès, G.; Römbke, J.; van der Putten, W.H. European Atlas of Soil Biodiversity. Eur. Commision. Publ. Off. 2010, 128, 94222. [Google Scholar] [CrossRef]
- Paul, E.A.; Clark, F.E. Soil Microbiology and Biochemistry; UMCS: Lublin, Poland, 2000. (In Polish) [Google Scholar]
- Torsvik, V.; Øvreås, L. Microbial Diversity and Function in Soil: From Genes to Ecosystems. Curr. Opin. Microbiol. 2002, 5, 240–245. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial Diversity and Soil Functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The Rhizosphere Microbiome: Significance of Plant Beneficial, Plant Pathogenic, and Human Pathogenic Microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Jones, D.L.; Magthab, E.A.; Gleeson, D.B.; Hill, P.W.; Sánchez-Rodríguez, A.R.; Roberts, P.; Ge, T.; Murphy, D.V. Microbial Competition for Nitrogen and Carbon Is as Intense in the Subsoil as in the Topsoil. Soil Biol. Biochem. 2018, 117, 72–82. [Google Scholar] [CrossRef]
- Ishaq, S.L.; Ishaq, S.L. Plant-Microbial Interactions in Agriculture and the Use of Farming Systems to Improve Diversity and Productivity. AIMS Microbiol. 2017, 3, 335–353. [Google Scholar] [CrossRef]
- Radziemska, M. Study of Applying Naturally Occurring Mineral Sorbents of Poland (Dolomite Halloysite, Chalcedonite) for Aided Phytostabilization of Soil Polluted with Heavy Metals. Catena 2018, 163, 123–129. [Google Scholar] [CrossRef]
- Jin, Q.; Kirk, M.F. PH as a Primary Control in Environmental Microbiology: 2. Kinetic Perspective. Front. Environ. Sci. 2018, 6, 101. [Google Scholar] [CrossRef]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G.; et al. A Communal Catalogue Reveals Earth’s Multiscale Microbial Diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Yang, T.; Chu, H. Threshold Effects of Soil PH on Microbial Co-Occurrence Structure in Acidic and Alkaline Arable Lands. Sci. Total Environ. 2021, 800, 149592. [Google Scholar] [CrossRef]
- Fijałkowski, K.; Kacprzak, M.; Grobelak, A.; Placek, A. The Influence of Selected Soil Parameters on the Mobility of Heavy Metals in Soils. Inżynieria Ekol. 2012, 15, 81–92. [Google Scholar]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Masciandaro, G.; Manzi, D.; Masini, C.M.; Mattii, G.B. Application of Zeolites in Agriculture and Other Potential Uses: A Review. Agronomy 2021, 11, 1547. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Xie, Y.; Luo, Y.; Sheng, M.; Xu, F.; Xu, H. Ecological Responses of Soil Microbial Abundance and Diversity to Cadmium and Soil Properties in Farmland around an Enterprise-Intensive Region. J. Hazard. Mater. 2020, 392, 122478. [Google Scholar] [CrossRef]
- Belviso, C. Zeolite for Potential Toxic Metal Uptake from Contaminated Soil: A Brief Review. Processes 2020, 8, 820. [Google Scholar] [CrossRef]
- Jarosz, R.; Szerement, J.; Gondek, K.; Mierzwa-Hersztek, M. The Use of Zeolites as an Addition to Fertilisers—A Review. Catena 2022, 213, 106125. [Google Scholar] [CrossRef]
- Lenart, A.; Wolny-Koładka, K. The Effect of Heavy Metal Concentration and Soil Ph on the Abundance of Selected Microbial Groups within Arcelormittal Poland Steelworks in Cracow. Bull. Environ. Contam. Toxicol. 2013, 90, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, I.V.N.; Megharaj, M.; Bolan, N.; Naidu, R. Tolerance of Heavy Metals by Gram Positive Soil Bacteria. Int. J. Bioeng. Life Sci. 2009, 3, 270–274. [Google Scholar] [CrossRef]
- Xie, Y.; Fan, J.; Zhu, W.; Amombo, E.; Lou, Y.; Chen, L.; Fu, J. Effect of Heavy Metals Pollution on Soil Microbial Diversity and Bermudagrass Genetic Variation. Front. Plant Sci. 2016, 7, 755. [Google Scholar] [CrossRef]
- Good, A.G.; Beatty, P.H. Fertilizing Nature: A Tragedy of Excess in the Commons. PLoS Biol. 2011, 9, 1001124. [Google Scholar] [CrossRef]
- Thompson, R.L.; Lassaletta, L.; Patra, P.K.; Wilson, C.; Wells, K.C.; Gressent, A.; Koffi, E.N.; Chipperfield, M.P.; Winiwarter, W.; Davidson, E.A.; et al. Acceleration of Global N2O Emissions Seen from Two Decades of Atmospheric Inversion. Nat. Clim. Change 2019, 9, 993–998. [Google Scholar] [CrossRef]
- Mondal, M.; Biswas, B.; Garai, S.; Sarkar, S.; Banerjee, H.; Brahmachari, K.; Bandyopadhyay, P.K.; Maitra, S.; Brestic, M.; Skalicky, M.; et al. Zeolites Enhance Soil Health, Crop Productivity and Environmental Safety. Agronomy 2021, 11, 448. [Google Scholar] [CrossRef]
- Doni, S.; Gispert, M.; Peruzzi, E.; Macci, C.; Mattii, G.B.; Manzi, D.; Masini, C.M.; Grazia, M. Impact of Natural Zeolite on Chemical and Biochemical Properties of Vineyard Soils. Soil Use Manag. 2020, 2, 12665. [Google Scholar] [CrossRef]
- Lahori, A.H.; Mierzwa-Hersztek, M.; Demiraj, E.; Sajjad, R.U.; Ali, I.; Shehnaz, H.; Aziz, A.; Zuberi, M.H.; Pirzada, A.M.; Hassan, K.; et al. Direct and Residual Impacts of Zeolite on the Remediation of Harmful Elements in Multiple Contaminated Soils Using Cabbage in Rotation with Corn. Chemosphere 2020, 250, 126317. [Google Scholar] [CrossRef]
- Głąb, T.; Gondek, K.; Mierzwa–Hersztek, M. Biological Effects of Biochar and Zeolite Used for Remediation of Soil Contaminated with Toxic Heavy Metals. Sci. Rep. 2021, 11, 6998. [Google Scholar] [CrossRef]
- Mühlbachová, G.; Šimon, T. Effects of Zeolite Amendment on Microbial Biomass and Respiratory Activity in Heavy Metal Contaminated Soils. Plant Soil Environ. 2003, 49, 536–541. [Google Scholar] [CrossRef]
- Han, G.; Zhou, G.; Xu, Z.; Yang, Y.; Liu, J.; Shi, K. Soil Temperature and Biotic Factors Drive the Seasonal Variation of Soil Respiration in a Maize (Zea Mays L.) Agricultural Ecosystem. Plant Soil 2007, 291, 15–26. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Klimkowicz-Pawlas, A.; Gondek, K. Influence of Poultry Litter and Poultry Litter Biochar on Soil Microbial Respiration and Nitrifying Bacteria Activity. Waste Biomass Valorization 2018, 9, 379–389. [Google Scholar] [CrossRef]
- Álvarez-Ayuso, E.; Abad-Valle, P. Application of Different Alkaline Materials as Polluted Soil Amendments: A Comparative Assessment of Their Impact on Trace Element Mobility and Microbial Functions. Ecotoxicol. Environ. Saf. 2021, 227, 112927. [Google Scholar] [CrossRef]
- Eisentraeger, A.; Maxam, G.; Rila, J.P.; Dott, W. A Stepwise Procedure for Assessment of the Microbial Respiratory Activity of Soil Samples Contaminated with Organic Compounds. Ecotoxicol. Environ. Saf. 2000, 47, 65–73. [Google Scholar] [CrossRef]
- WRB. 2015 World Reference Base for Soil Resources. Available online: https://www.fao.org/3/i3794en/I3794en.pdf (accessed on 11 October 2022).
- Oleszczuk, N.; Castro, J.T.; da Silva, M.M.; Korn, M.; Das, G.A.; Welz, B.; Vale, M.G.R. Method Development for the Determination of Manganese, Cobalt and Copper in Green Coffee Comparing Direct Solid Sampling Electrothermal Atomic Absorption Spectrometry and Inductively Coupled Plasma Optical Emission Spectrometry. Talanta 2007, 73, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Wolny-Koładka, K.; Jarosz, R.; Marcińska-Mazur, L.; Lošák, T.; Mierzwa-Hersztek, M. Effect of Mineral and Organic Additions on Soil Microbial Composition. Int. Agrophysics 2022, 36, 131–138. [Google Scholar] [CrossRef]
- ISO. 16072 International Organization for Standardization 16072 (ISO 16072 2002—Szukaj w Google). Available online: https://www.google.com/search?q=International+Organization+for+Standardization+16072+(ISO+16072+2002&rlz=1C1AVFC_enPL885PL885&oq=International+Organization+for+Standardization+16072+(ISO+16072+2002&aqs=chrome..69i57.744j0j4&sourceid=chrome&ie=UTF-8 (accessed on 11 October 2022).
- Singer, S.; Ewing, M. ISO 14240-1:1997; Soil Quality—Determination of Soil Microbial Biomass. Part 1: Substrate-Induced Respiration Method. ISO: Geneva, Switzerland, 2000; pp. G271–G278.
- ISO 17155:2012; Soil Quality—Determination of Abundance and Activity of Soil Microflora Using Respiration Curves. ISO: Geneva, Switzerland, 2012.
- Moeen, M.; Qi, T.; Hussain, Z.; Ge, Q.; Maqbool, Z.; Jianjie, X.; Kaiqing, F. Use of Zeolite to Reduce the Bioavailability of Heavy Metals in a Contaminated Soil. J. Ecol. Eng. 2020, 21, 186–196. [Google Scholar] [CrossRef]
- Malinowski, M.; Wolny-Koładka, K.; Vaverková, M.D. Effect of Biochar Addition on the OFMSW Composting Process under Real Conditions. Waste Manag. 2019, 84, 364–372. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bååth, E. Contrasting Soil PH Effects on Fungal and Bacterial Growth Suggest Functional Redundancy in Carbon Mineralization. Appl. Environ. Microbiol. 2009, 75, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.J.; Chen, M.; Wang, J.F.; Liu, Y.; Liao, Y.Q.; Liu, Y.C. Assessment of Zeolite, Biochar, and Their Combination for Stabilization of Multimetal-Contaminated Soil. ACS Omega 2020, 5, 27374–27382. [Google Scholar] [CrossRef] [PubMed]
- Badora, A. The Influence of Zeolites on Quality Indicators of Soil-Plant Connection and Food Safety. In Zeolites—Useful Miner; IntechOpen: London, UK, 2016; p. 64590. [Google Scholar] [CrossRef]
- Ravali, C.; Rao, J.K.; Anjaiah, T.; Suresh, K. Effect of Zeolite on Soil Physical and Physico-Chemical Properties. J. Sci. Agric. Eng. 2020, 10, 776–781. [Google Scholar]
- Ramesh, K.; Biswas, A.K.; Patra, A.K. Zeolitic Farming. Indian J. Agron. 2015, 60, 185–191. [Google Scholar]
- Feng, W.; Wan, Z.; Daniels, J.; Li, Z.; Xiao, G.; Yu, J.; Xu, D.; Guo, H.; Zhang, D.; May, E.F.; et al. Synthesis of High Quality Zeolites from Coal Fly Ash: Mobility of Hazardous Elements and Environmental Applications. J. Clean. Prod. 2018, 202, 390–400. [Google Scholar] [CrossRef]
- Zaidun, S.W.; Jalloh, M.B.; Awang, A.; Sam, L.M.; Besar, N.A.; Musta, B.; Ahmed, O.H.; Omar, L. Biochar and Clinoptilolite Zeolite on Selected Chemical Properties of Soil Cultivated with Maize (Zea Mays L.). Eurasian J. Soil Sci. 2019, 8, 468100. [Google Scholar] [CrossRef]
- Yousefian, M.; Jafari, M.; Tavili, A.; Arzani, H.; Jafarian, Z. The Effects of Superabsorbent Polymer on Atriplex Lentiformis Growth and Soil Characteristics under Drought Stress (Case Study: Desert Research Station, Semnan, Iran). J. Rangel. Sci. 2018, 8, 65–76. [Google Scholar]
- Santoso, B.; Cholid, M.; Wijayanto, R.A. Effect of Zeolite and Cow Manure Application on Soil Nitrogen Content in Ramie ( Boehmeria Nivea) Plant Growth. IOP Conf. Ser. Earth Environ. Sci. 2022, 974, 012073. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Wolny-Koładka, K.; Gondek, K.; Gałązka, A.; Gawryjołek, K. Effect of Coapplication of Biochar and Nutrients on Microbiocenotic Composition, Dehydrogenase Activity Index and Chemical Properties of Sandy Soil. Waste Biomass Valorization 2020, 11, 3911–3923. [Google Scholar] [CrossRef]
- Wolny-Koładka, K.; Żukowski, W. Mixed Municipal Solid Waste Hygienisation for Refuse-Derived Fuel Production by Ozonation in the Novel Configuration Using Fluidized Bed and Horizontal Reactor. Waste Biomass Valorization 2019, 10, 575–583. [Google Scholar] [CrossRef]
- Qin, J.; Liu, H.; Zhao, J.; Wang, H.; Zhang, H.; Yang, D.; Zhang, N. The Roles of Bacteria in Soil Organic Carbon Accumulation under Nitrogen Deposition in Stipa Baicalensis Steppe. Microorganisms 2020, 8, 326. [Google Scholar] [CrossRef]
- Tian, J.; Wang, J.; Dippold, M.; Gao, Y.; Blagodatskaya, E.; Kuzyakov, Y. Biochar Affects Soil Organic Matter Cycling and Microbial Functions but Does Not Alter Microbial Community Structure in a Paddy Soil. Sci. Total Environ. 2016, 556, 89–97. [Google Scholar] [CrossRef]
- Sivojiene, D.; Kacergius, A.; Baksiene, E.; Maseviciene, A.; Zickiene, L. The Influence of Organic Fertilizers on the Abundance of Soil Microorganism Communities, Agrochemical Indicators, and Yield in East Lithuanian Light Soils. Plants 2021, 10, 2648. [Google Scholar] [CrossRef]
- Triberti, L.; Nastri, A.; Baldoni, G. Long-Term Effects of Crop Rotation, Manure and Mineral Fertilisation on Carbon Sequestration and Soil Fertility. Eur. J. Agron. 2016, 74, 47–55. [Google Scholar] [CrossRef]
- Qu, Y.; Tang, J.; Li, Z.; Zhou, Z.; Wang, J.; Wang, S.; Cao, Y. Soil Enzyme Activity and Microbial Metabolic Function Diversity in Soda Saline–Alkali Rice Paddy Fields of Northeast China. Sustainability 2020, 12, 10095. [Google Scholar] [CrossRef]
- Ramsey, P.W.; Rillig, M.C.; Feris, K.P.; Gordon, N.S.; Moore, J.N.; Holben, W.E.; Gannon, J.E. Relationship between Communities and Processes; New Insights from a Field Study of a Contaminated Ecosystem. Ecol. Lett. 2005, 8, 1201–1210. [Google Scholar] [CrossRef]
- Eisentraeger, A.; Hund-Rinke, K.; Roembke, J. Assessment of Ecotoxicity of Contaminated Soil Using Bioassays. Monit. Assess. Soil Bioremed. 2005, 5, 321–360. [Google Scholar] [CrossRef]
- Bikkinina, L.M.-H.; Ezhkov, V.O.; Faizrakhmanov, R.N.; Gazizov, R.R.; Ezhkova, A.M. Effect of Zeolites on Soil Modification and Productivity. BIO Web Conf. 2020, 17, 00117. [Google Scholar] [CrossRef][Green Version]
- Xiao, D.; Huang, Y.; Feng, S.; Ge, Y.; Zhang, W.; He, X.; Wang, K. Soil Organic Carbon Mineralization with Fresh Organic Substrate and Inorganic Carbon Additions in a Red Soil Is Controlled by Fungal Diversity along a PH Gradient. Geoderma 2018, 321, 79–89. [Google Scholar] [CrossRef]
- Feketeová, Z.; Hrabovský, A.; Šimkovic, I. Microbial Features Indicating the Recovery of Soil Ecosystem Strongly Affected by Mining and Ore Processing. Int. J. Environ. Res. Public Health 2021, 18, 3240. [Google Scholar] [CrossRef]
| Determinant | Value | Determinant | Value |
|---|---|---|---|
| Fraction 2–0.5 mm | 85% | N total | 0.40 g∙kg−1 DM |
| Fraction 0.05–0.002 mm | 12% | C total | 5.74 g∙kg−1 DM |
| Fraction < 0.002 | 3% | S total | 0.118 g∙kg−1 DM |
| pH H2O | 5.24 | Pb total | 188 ± 15 mg∙kg−1 DM |
| pH KCl | 5.03 | Cd total | 1.15 ± 0.08 mg∙kg−1 DM |
| EC | 273 µS∙cm−1 | Zn total | 267 ± 18 mg∙kg−1 DM |
| Cr total | 5.32 ± 0.73 mg∙kg−1 DM | ||
| Cu total | 5.14 ± 1.33 mg∙kg−1 DM | ||
| Ni total | 2.22 ± 0.18 mg∙kg−1 DM |
| Symbol | Mineral Salt | Zeolite–Vermiculite Composite | Lignite | Leonardite |
|---|---|---|---|---|
| C | - | - | - | - |
| MF | NPK | - | - | - |
| NaX–Ver3%L3% | NPK | 3% | 3% | - |
| NaX–Ver9%L6% | NPK | 9% | 6% | - |
| NaX–Ver3%Leo3% | NPK | 3% | - | 3% |
| NaX–Ver9%Leo6% | NPK | 9% | - | 6% |
| pH H2O | pH KCl | EC (µS·cm−1) | ||||
|---|---|---|---|---|---|---|
| Treatment | 1st year | 2nd year | 1st year | 2nd year | 1st year | 2nd year |
| C | 5.91 c ± 0.10 | 5.97 c ± 0.12 | 5.24 d ± 0.05 | 5.34 d ± 0.08 | 269 b ± 23.3 | 341 c ± 9.18 |
| MF | 5.28 ab ± 0.06 | 5.34 b ± 0.16 | 4.81 bc ± 0.09 | 4.79 ab ± 0.15 | 351 c ± 7.59 | 763 h ± 20.8 |
| NaX–Ver3%L3% | 5.22 ab ± 0.12 | 5.17 ab ± 0.13 | 4.90 bc ± 0.06 | 4.65 a ± 0.05 | 400 d ± 2.05 | 710 g ± 15.1 |
| NaX–Ver6%L6% | 5.30 ab ± 0.07 | 5.25 ab ± 0.18 | 4.89 c ± 0.06 | 4.74 a ± 0.16 | 231 a ± 5.88 | 641 f ± 49.1 |
| NaX–Ver3%Leo3% | 5.31 b ± 0.16 | 5.24 ab ± 0.13 | 4.84 c ± 0.07 | 4.67 a ± 0.12 | 434 d ± 4.92 | 699 g ± 52.9 |
| NaX–Ver6%Leo6% | 5.23 ab ± 0.05 | 5.09 a ± 0.14 | 4.89 c ± 0.12 | 4.61 a ± 0.17 | 559 e ± 6.94 | 1112 i ± 33.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolny-Koładka, K.; Jarosz, R.; Juda, M.; Mierzwa-Hersztek, M. Distinct Changes in Abundance of Culturable Microbial Community and Respiration Activities in Response to Mineral–Organic Mixture Application in Contaminated Soil. Sustainability 2022, 14, 15004. https://doi.org/10.3390/su142215004
Wolny-Koładka K, Jarosz R, Juda M, Mierzwa-Hersztek M. Distinct Changes in Abundance of Culturable Microbial Community and Respiration Activities in Response to Mineral–Organic Mixture Application in Contaminated Soil. Sustainability. 2022; 14(22):15004. https://doi.org/10.3390/su142215004
Chicago/Turabian StyleWolny-Koładka, Katarzyna, Renata Jarosz, Michał Juda, and Monika Mierzwa-Hersztek. 2022. "Distinct Changes in Abundance of Culturable Microbial Community and Respiration Activities in Response to Mineral–Organic Mixture Application in Contaminated Soil" Sustainability 14, no. 22: 15004. https://doi.org/10.3390/su142215004
APA StyleWolny-Koładka, K., Jarosz, R., Juda, M., & Mierzwa-Hersztek, M. (2022). Distinct Changes in Abundance of Culturable Microbial Community and Respiration Activities in Response to Mineral–Organic Mixture Application in Contaminated Soil. Sustainability, 14(22), 15004. https://doi.org/10.3390/su142215004








