Heterocysts of Rivularia Type for Interpreting a Palaeoenvironmental Context of the Late Quaternary in Northern Italy
Abstract
:1. Introduction
1.1. Cyanobacteria: An Ecologically Important Group of Phototrophic Bacteria
1.2. Rivularia as a Palaeoecological Indicator and Aim of the Paper
2. Materials and Methods
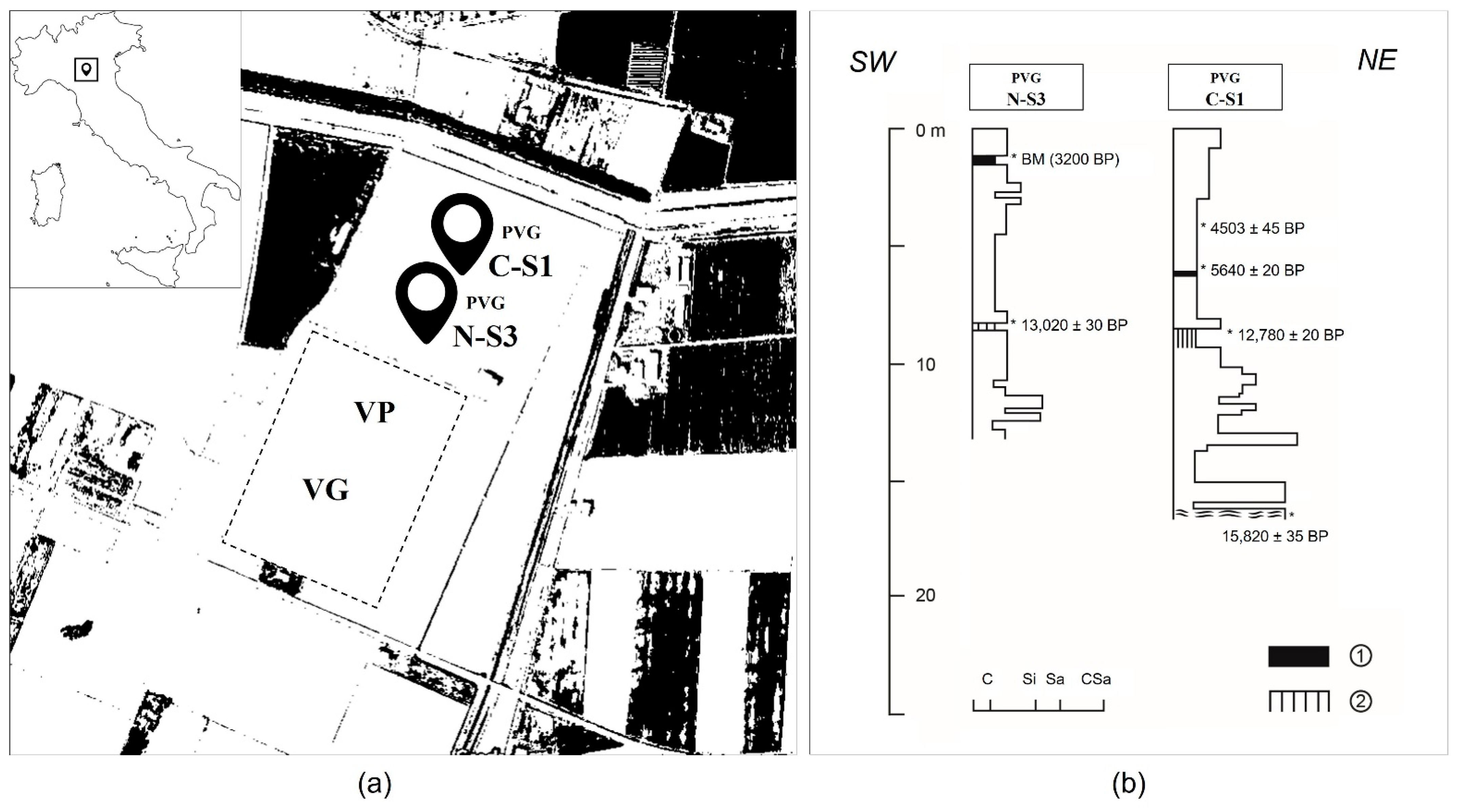
2.1. Palaeoenvironmental and Archaeological Context
2.2. Coring and Pollen Sampling
2.3. Pollen and Non-Pollen Palynomorphs Extraction
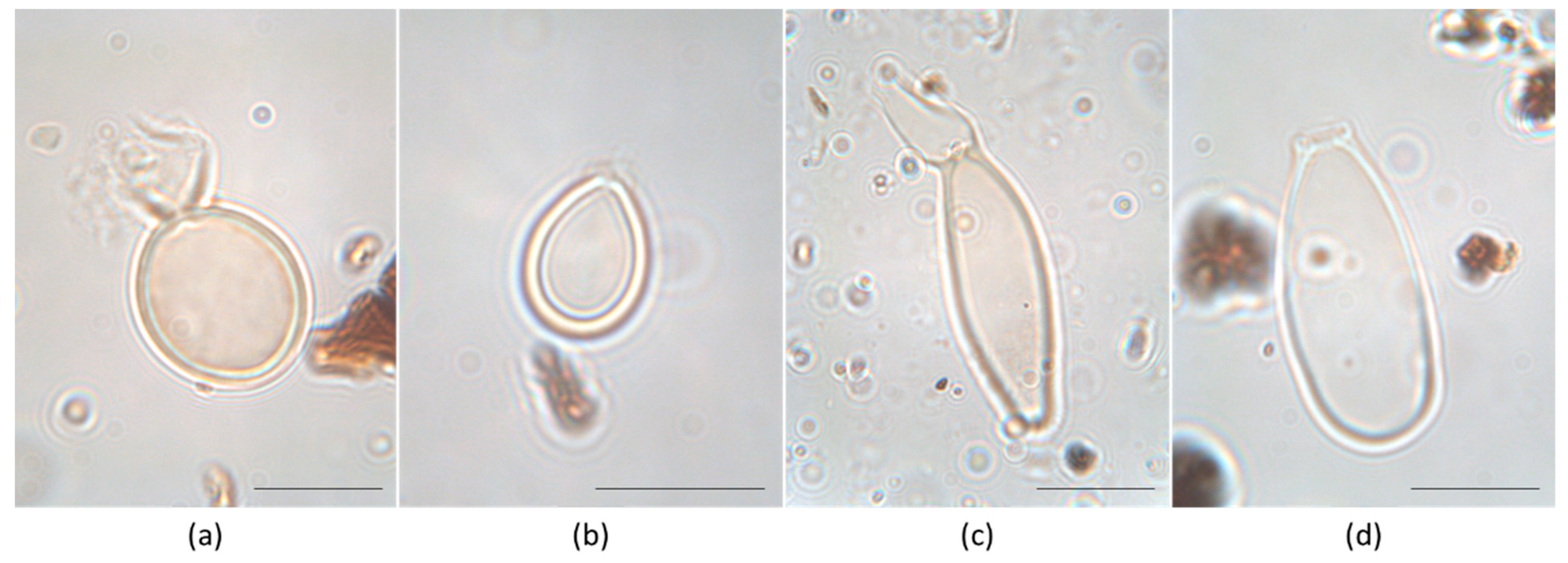
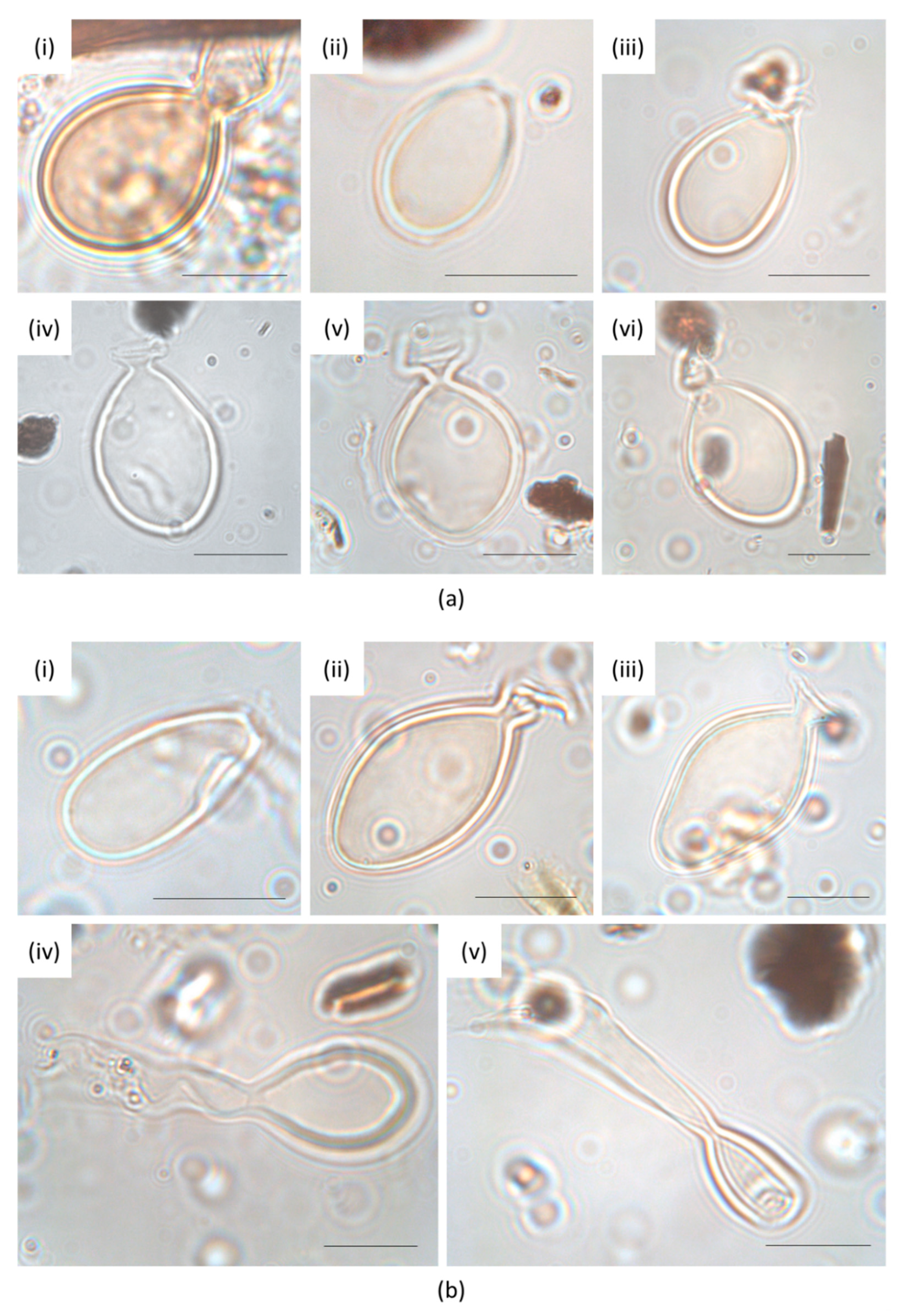
2.4. Identification of Heterocysts of the Rivularia Type and Data Elaboration
- from 1 to <1.6 = ellipsoidal heterocysts
- from ≥1.6 to higher values = elongated heterocysts
3. Results
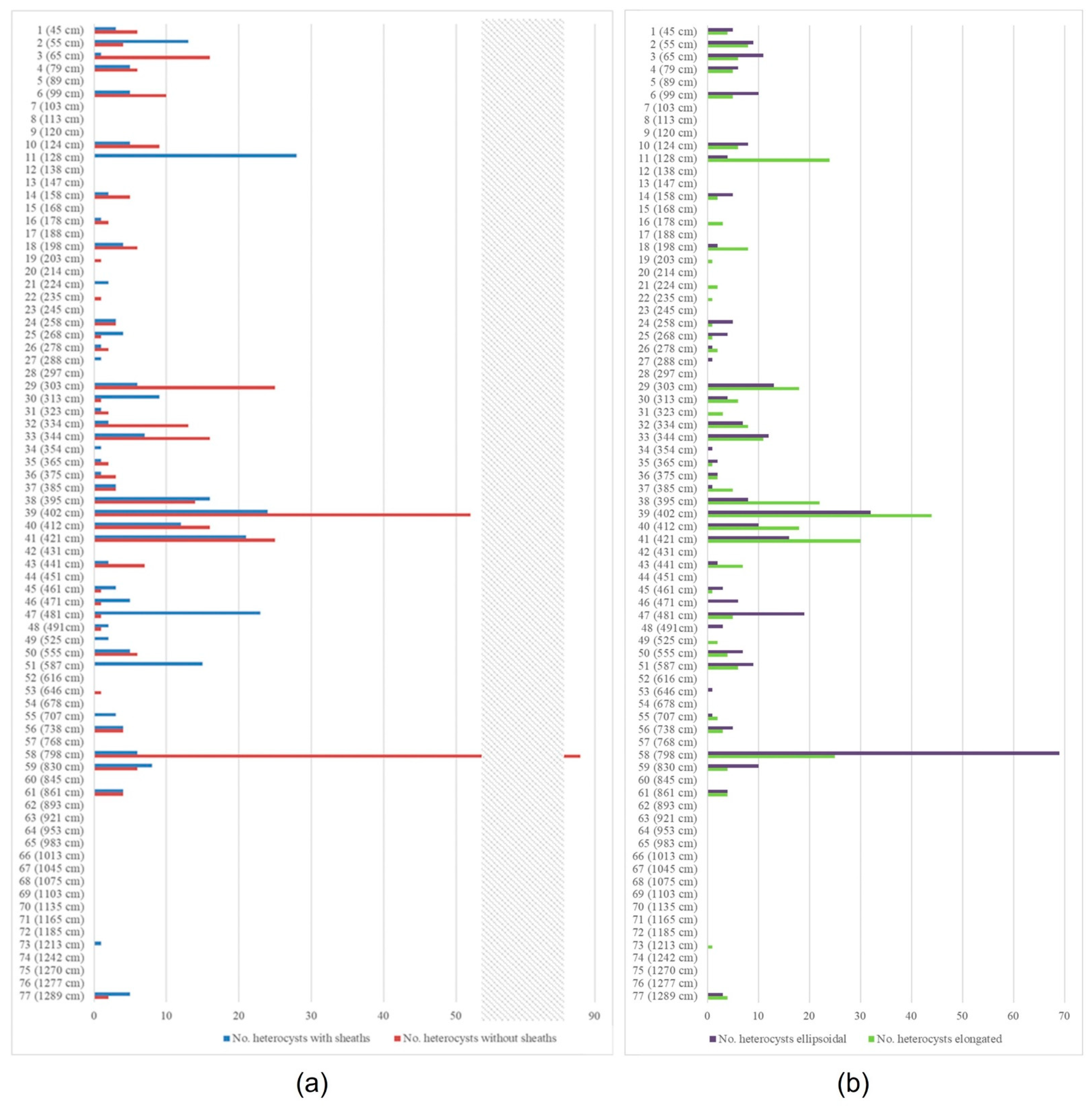
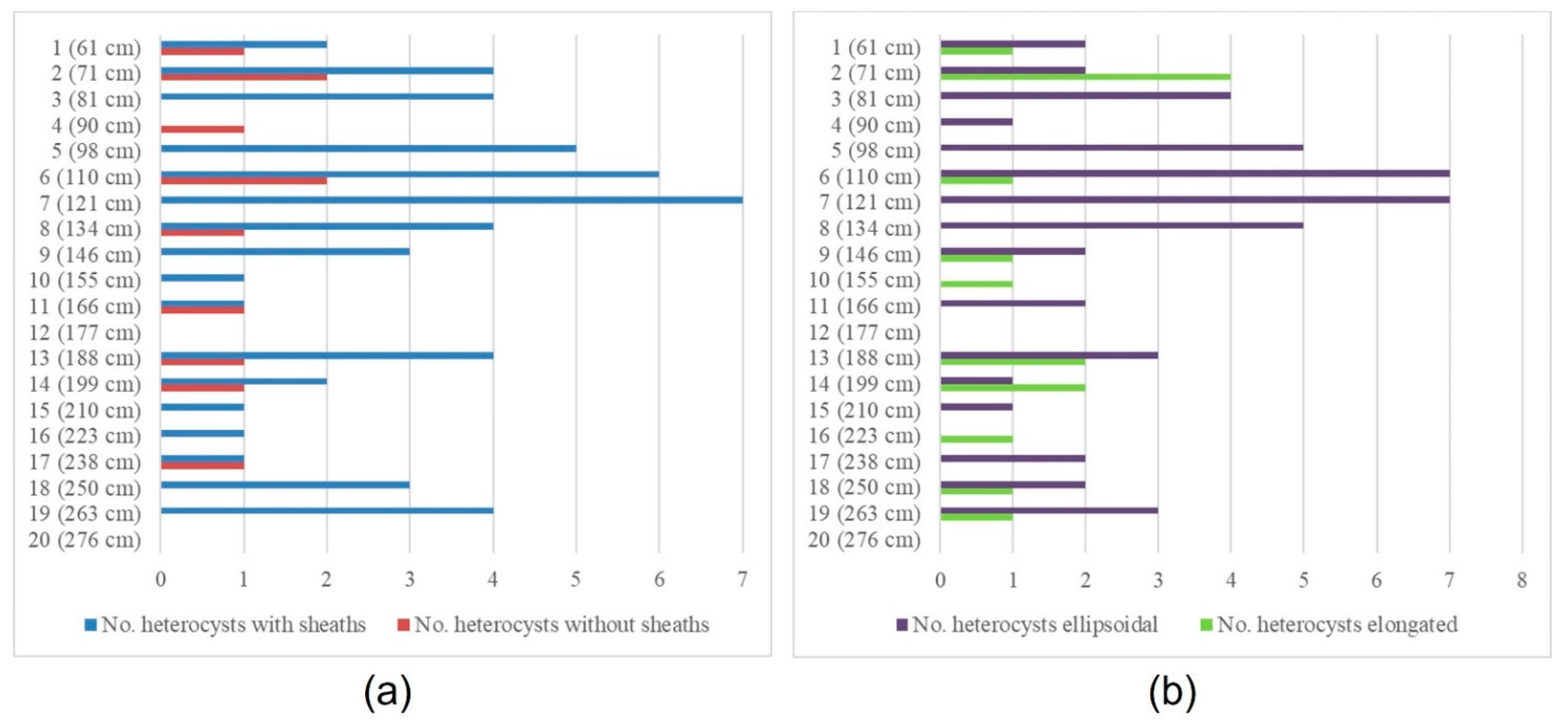
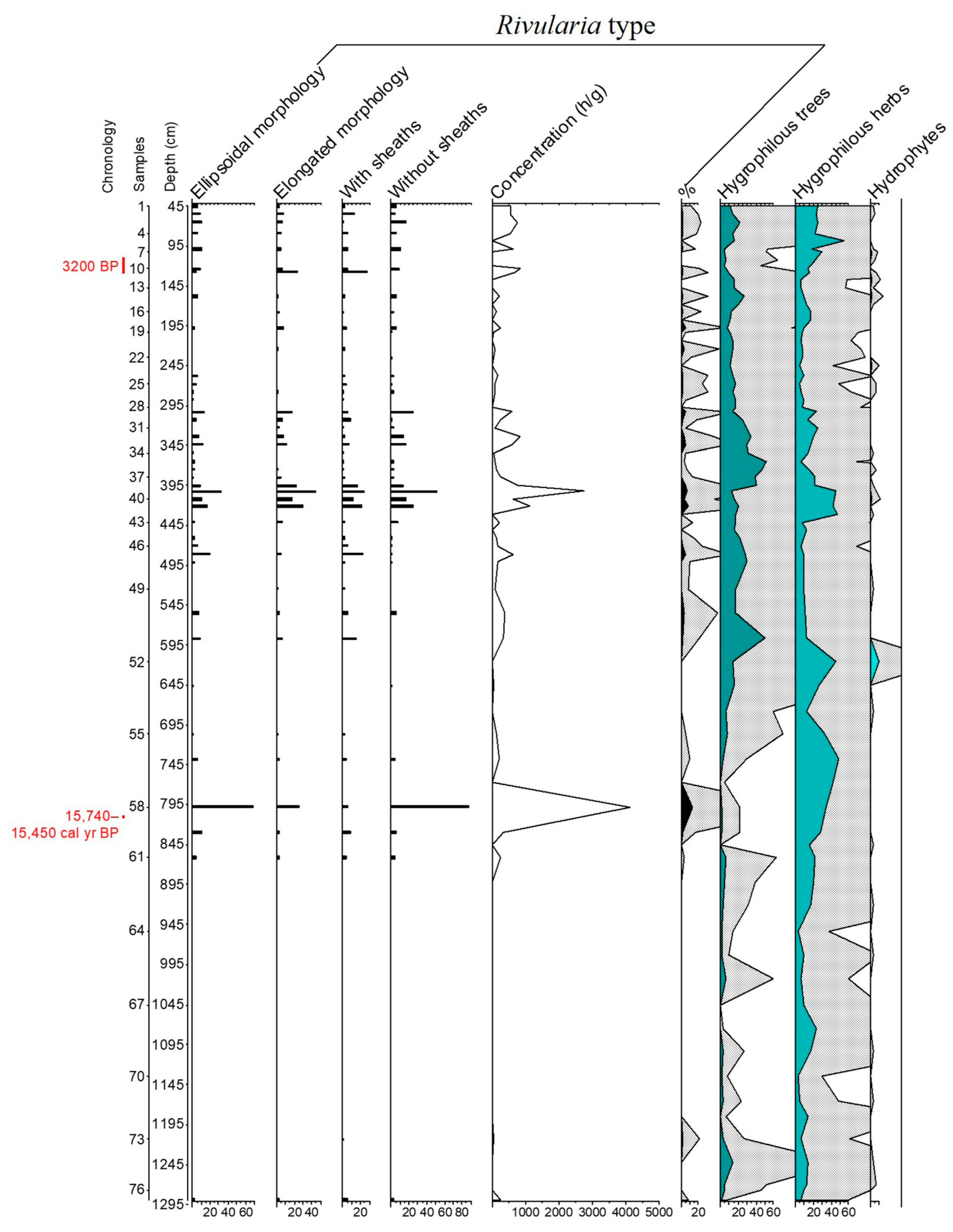
3.1. The Rivularia Type in the N-S3 Core
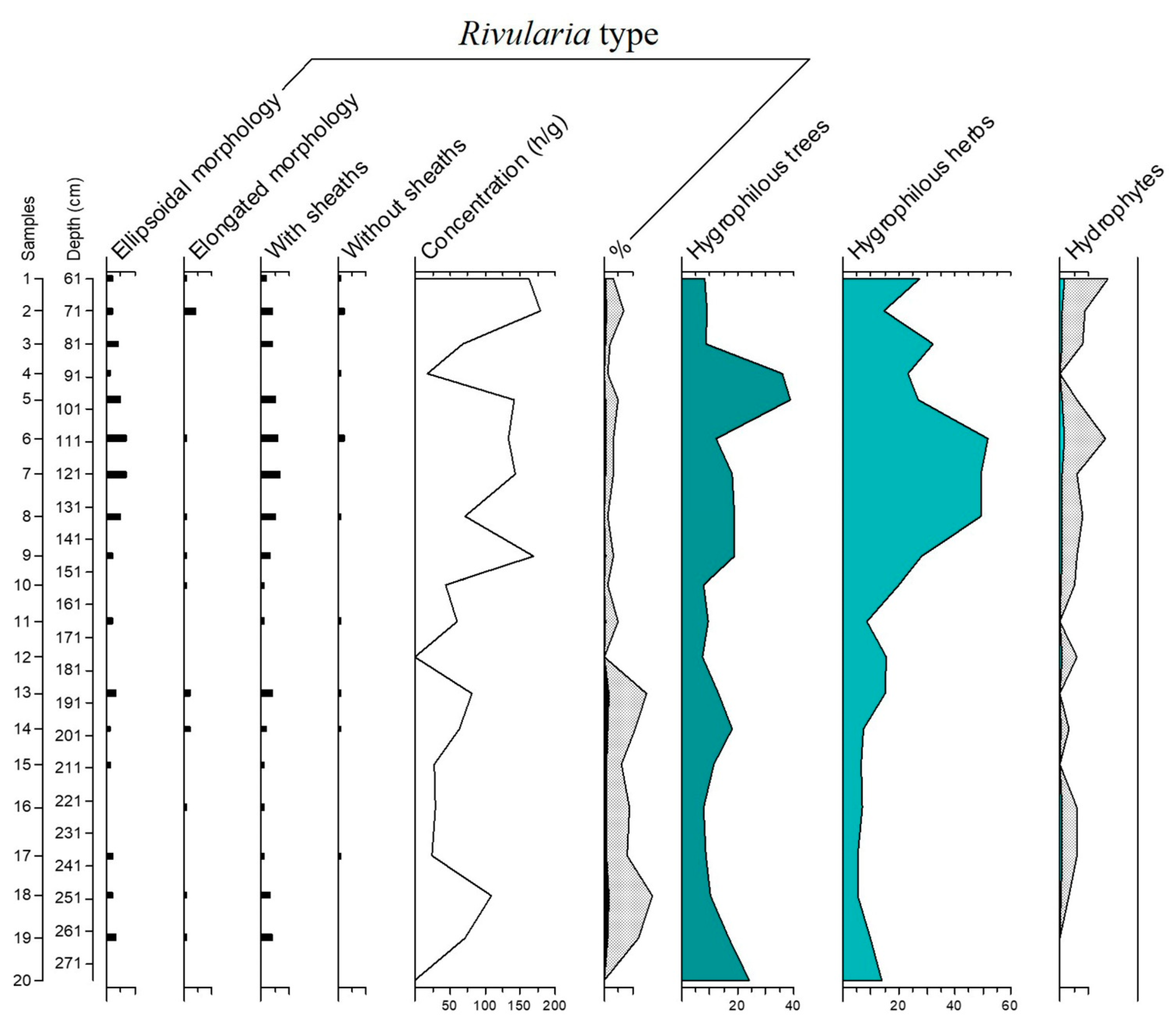
3.2. The Rivularia Type in the C-S1 Core
4. Discussion
4.1. Trend of the Rivularia Type in the Cores
4.2. Comparison with Pollen Curves
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miola, A. Tools for Non-Pollen Palynomorphs (NPPs) analysis: A list of Quaternary NPP types and reference literature in English language (1972–2011). Rev. Palaeobot. Palynol. 2012, 186, 142–161. [Google Scholar] [CrossRef]
- Shumilovskikh, L.S.; O’Keefe, J.M.K.; Marret, F. An overview of the taxonomic groups of non-pollen palynomorphs. Geol. Soc. Spec. Publ. 2021, 511, 13–61. [Google Scholar] [CrossRef]
- Shumilovskikh, L.S.; Shumilovskikh, E.S.; Schlütz, F.; van Geel, B. NPP-ID: Non-Pollen Palynomorph Image Database as a research and educational platform. Veg. Hist. Archaeobot. 2022, 31, 323–328. [Google Scholar] [CrossRef]
- van Geel, B. Application of fungal and algal remains and other microfossils in palynological analyses. In Handbook of Holocene Palaeoecology and Palaeohydrology; Berglund, B.E., Ed.; John Wiley & Sons, Inc.: Chichester, UK, 1986; pp. 497–505. [Google Scholar]
- van Geel, B. Non-pollen palynomorphs. In Tracking Environmental Change Using Lake Sediments; Smol, J.P., Birks, H.J.B., Last, W.M., Eds.; Volume 3: Terrestial, Algal and Silicaceaous Indicators; Springer: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Florenzano, A.; Mercuri, A.M.; Pederzoli, A.; Torri, P.; Bosi, G.; Olmi, L.; Rinaldi, R.; Bandini Mazzanti, M. The significance of intestinal parasite remains in pollen samples from Medieval pits in the Piazza Garibaldi of Parma, Emilia Romagna, Northern Italy. Geoarchaeology 2012, 27, 34–47. [Google Scholar] [CrossRef]
- Shumilovskikh, L.S.; van Geel, B. Non-pollen Palynomorphs. In Handbook for the Analysis of Micro-Particles in Archaeological Samples; Henry, A.G., Ed.; Springer: Berlin, Germany, 2020; pp. 65–94. [Google Scholar] [CrossRef]
- van Geel, B. ‘Quaternary non-pollen palynomorphs’ deserve our attention! Rev. Palaeobot. Palynol. 2006, 141, 7–8. [Google Scholar] [CrossRef]
- Gelorini, V.; Verbeken, A.; van Geel, B.; Verschuren, D. Modern non-pollen palynomorphs from East African lake sediments. Rev. Palaeobot. Palynol. 2011, 164, 143–173. [Google Scholar] [CrossRef]
- Revelles, J.; Burjachs, F.; van Geel, B. Pollen and Non-pollen Palynomorphs from the Early Neolithic settlement of La Draga (Girona, Spain). Rev. Palaeobot. Palynol. 2016, 225, 1–20. [Google Scholar] [CrossRef]
- Gill, J.L.; Williams, J.W.; Jackson, S.T.; Lininger, K.B.; Robinson, G.S. Pleistocene megafaunal collapse, novel plant communities, and enhanced fire regimes in North America. Science 2009, 326, 1100–1103. [Google Scholar] [CrossRef] [Green Version]
- Cugny, C.; Mazier, F.; Galop, D. Modern and fossil non-pollen palynomorphs from the Basque mountains (western Pyrenees, France): The use of coprophilous fungi to reconstruct pastoral activity. Veg. Hist. Archaeobot. 2010, 19, 391–408. [Google Scholar] [CrossRef]
- Perrotti, A.G.; van Asperen, E. Dung fungi as a proxy for megaherbivores: Opportunities and limitations for archaeological applications. Veg. Hist. Archaeobot 2019, 28, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.M.; van Geel, B.; Gosling, W.D. On the use of spores of coprophilous fungi preserved in sediments to indicate past herbivore presence. Quaternary 2022, 5, 30. [Google Scholar] [CrossRef]
- Stivrins, N.; Aakala, T.; Ilvonen, L.; Pasanen, L.; Kuuluvainen, T.; Vasander, H.; Gałka, M.; Disbrey, H.R.; Liepins, J.; Hölmström, L.; et al. Integrating fire-scar, charcoal and fungal spore data to study fire events in the boreal forest of northern Europe. Holocene 2019, 29, 1480–1490. [Google Scholar] [CrossRef]
- Kołaczek, P.; Zubek, S.; Błaszkowski, J.; Mleczko, P.; Margielewski, W. Erosion or plant succession—How to interpret the presence of arbuscular mycorrhizal fungi (Glomeromycota) spores in pollen profiles collected from mires. Rev. Palaeobot. Palynol. 2013, 189, 29–37. [Google Scholar] [CrossRef]
- McCarthy, F.M.G.; Riddick, N.L.; Volik, O.; Danesh, D.C.; Krueger, A.M. Algal palynomorphs as proxies of human impact on freshwater resources in the Great Lake region. Anthropocene 2018, 21, 16–31. [Google Scholar] [CrossRef]
- McCarthy, F.M.G.; Pilkington, P.M.; Volik, O.; Heyde, A.; Cocker, S.L. Non-pollen palynomorphs in freshwater sediments and their palaeolimnological potential and selected applications. Geol. Soc. Spec. Publ. 2021, 511, 121–150. [Google Scholar] [CrossRef]
- Limaye, R.B.; Kumaran, K.P.N.; Nair, K.M.; Padmalal, D. Non-Pollen Palynomorphs (NPP) as potential palaeoenvironmental indicators in the Late Quaternary sediments of west coast of India. Curr. Sci. 2007, 92, 1370–1382. [Google Scholar]
- Limaye, R.B.; Kumaran, K.P.N.; Nair, K.M.; Padmalal, D. Cyanobacteria as potential biomarkers of hydrological changes in the late Quaternary sediments of South Kerala Sedimentary Basin, India. Quat. Int. 2010, 213, 79–90. [Google Scholar] [CrossRef]
- Limaye, R.B.; Padmalal, D.; Kumaran, K.P.N. Cyanobacteria and testate amoeba as potential proxies for Holocene hydrological changes and climate variability: Evidence from tropical coastal lowlands of SW India. Quat. Int. 2017, 443, 99–114. [Google Scholar] [CrossRef]
- Mehdizadeh Allaf, M.; Peerhossaini, H. Cyanobacteria: Model Microorganisms and Beyond. Microorganisms 2022, 10, 696. [Google Scholar] [CrossRef]
- Hieronymus, J.; Eilola, K.; Olofsson, M.; Hence, I.; Meier, H.E.M.; Almroth-Rosell, E. Modeling cyanobacteria life cycle dynamics and historical nitrogen fixation in the Baltic Proper. Biogeosciences 2021, 18, 6213–6227. [Google Scholar] [CrossRef]
- Sukenik, A.; Quesada, A.; Salmaso, N. Global expansion of toxic and non-toxic cyanobacteria: Effect on ecosystem functioning. Biodivers. Conserv. 2015, 24, 889–908. [Google Scholar] [CrossRef]
- Hense, I.; Beckmann, A. Towards a model of cyanobacteria life cycle—Effects of growing and resting stages on bloom formation of N2-fixing species. Ecol. Modell. 2006, 195, 205–218. [Google Scholar] [CrossRef]
- Wan, L.; Chen, X.; Deng, Q.; Yang, L.; Li, X.; Zhang, J.; Song, C.; Zhou, Y.; Cao, X. Phosphorus strategy in bloom-forming cyanobacteria (Dolichospermum and Microcystis) and its role in their succession. Harmful Algae 2019, 84, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Karl, D.; Letelier, R.; Tupas, L.; Dore, J.; Christian, J.; Hebel, D. The role of nitrogen fixation in biogeochemical cycling in the subtropical North Pacific Ocean. Nature 1997, 388, 533–538. [Google Scholar] [CrossRef]
- Larsson, U.; Hajdu, S.; Walve, J.; Elmgren, R. Baltic Sea nitrogen fixation estimated from the summer increase in upper mixed layer total nitrogen. Limnol. Oceanogr. 2001, 46, 811–820. [Google Scholar] [CrossRef]
- Meeks, J.C.; Elhai, J. Regulation of cellular differentiation in filamentous cyanobacteria in free-living and plant-associated symbiotic growth states. Microbiol. Mol. Biol. Rev. 2002, 66, 94–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolk, C.P.; Ernst, A.; Elhai, J. Heterocyst metabolism and development. In The Molecular Biology of Cyanobacteria; Bryant, D.A., Ed.; Kluwer Academic Publishers: Boston, MA, USA, 1994; pp. 769–823. [Google Scholar]
- Whitton, B.A.; Mateo, P. Rivulariaceae. In Ecology of Cyanobacteria II. Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: London, UK, 2012; pp. 561–591. [Google Scholar]
- Sinclair, C.; Whitton, B.A. Influence of nutrient deficiency on hair formation in the Rivulariaceae. Br. Phycol. J. 1977, 12, 297–313. [Google Scholar] [CrossRef]
- Whitton, B.A. The biology of Rivulariaceae. In The Cyanobacteria—A Comprehensive Review; Fay, P., Van Baalen, C., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 513–534. [Google Scholar]
- Kumar, K.; Mella-Herrera, R.A.; Golden, J.W. Cyanobacterial Heterocysts. Cold Spring Harb. Perspect. Biol. 2010, 14, a000315. [Google Scholar] [CrossRef] [Green Version]
- Carrión, J.S.; Navarro, C. Cryptogam spores and other non-pollen microfossils as sources of palaeoecological information: Case studies from Spain. Ann. Bot. Fenn. 2002, 39, 1–14. [Google Scholar]
- van Geel, B. The paleo-environmental indicator value of non-pollen palynomorphs in lake sediments, peat deposits, and archaeological sites. Pollen 2004, 14, 276–277. [Google Scholar]
- van Geel, B.; Hallewas, D.P.; Pals, J.P. A late Holocene deposit under the Westfriese Zeedijk near Enkhuiden (Prov. of Noord-Holland, The Netherlands): Palaeoecological and archaeological aspects. Rev. Palaeobot. Palynol. 1983, 38, 269–335. [Google Scholar] [CrossRef]
- van Geel, B.; van der Hammen, T. Zygnemataceae in Quaternary Columbian sediments. Rev. Palaeobot. Palynol. 1978, 25, 377–391. [Google Scholar] [CrossRef]
- Herrero, A.; Stavans, J.; Flores, E. The multicellular nature of filamentous heterocyst-forming cyanobacteria. FEMS Microbiol. Rev. 2016, 40, 831–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Baldassarre, G.; Kooy, M.; Kemerink, J.S.; Brandimarte, L. Towards understanding the dynamic behaviour of floodplains as human-water systems. Hydrol. Earth Syst. Sci. 2013, 17, 3235–3244. [Google Scholar] [CrossRef] [Green Version]
- Clò, E. Climate change and human impact in a long-term perspective: Palynology of Central Po Plain deposits since the Lateglacial. Ph.D. Thesis, Università degli Studi di Modena e Reggio Emilia, Modena, Italy, 2022. [Google Scholar]
- Cremaschi, M.; Mercuri, A.M.; Benatti, A.; Bosi, G.; Brandolini, F.; Clò, E.; Florenzano, A.; Furia, E.; Mariani, G.S.; Mazzanti, M.; et al. The SUCCESSO-TERRA Project: A lesson of sustainability from the Terramare culture, the Middle Bronze Age of the Po Plain (Northern Italy). IANSA 2018, 9, 59–67. [Google Scholar] [CrossRef]
- Bernabò Brea, M.; Cremaschi, M. Il Villaggio Piccolo della Terramara di S. Rosa a Poviglio. Scavi 1987/1992; Istituto Italiano Preistoria e Protostoria: Firenze, Italy, 2004. [Google Scholar]
- Cremaschi, M. La terramara di Santa Rosa ed il suo territorio: Aspetti geomorfologici. In Il Villaggio Piccolo della Terramara di S. Rosa a Poviglio. Scavi 1987/1992; Bernabò Brea, M., Cremaschi, M., Eds.; Istituto Italiano Preistoria e Protostoria: Firenze, Italy, 2004; pp. 21–37. [Google Scholar]
- Mele, M.; Cremaschi, M.; Giudici, M.; Lozej, A.; Pizzi, C.; Bassi, A. The terramare and the surrounding hydraulic structures: A geophysical survey of the Santa Rosa site at Poviglio (Bronze Age; Northern Italy). J. Archaeol. Sci. 2013, 40, 4648–4662. [Google Scholar] [CrossRef]
- Cremaschi, M.; Pizzi, C. Terramara Santa Rosa di Poviglio—Le Strutture Idrauliche al Margine del Villaggio Grande (Scavi 1998–2011); Istituto Italiano di Preistoria e Protostoria: Firenze, Italy, 2021; Origines 35. [Google Scholar]
- Ravazzi, C.; Cremaschi, M.; Forlani, L. Ricostruzione della storia della vegetazione padana tra l’Età del Bronzo e l’Alto Medioevo in relazione all’ intervento antropico. La successione pollinica del fossato della Terramara di Poviglio (RE). Arch. Bot. Ital. 1992, 6, 198–220. [Google Scholar]
- Ravazzi, C.; Cremaschi, M.; Forlani, L. Studio archeopalinologico della terramara S. Rosa. Nuovi dati, analisi floristica e sintassonomica della vegetazione nell’età del Bronzo. In Il Villaggio Piccolo della Terramara di S. Rosa a Poviglio. Scavi 1987/1992; Bernabò Brea, M., Cremaschi, M., Eds.; Istituto Italiano Preistoria e Protostoria: Firenze, Italy, 2004; pp. 703–736. [Google Scholar]
- Cremaschi, M.; Mercuri, A.M.; Torri, P.; Florenzano, A.; Pizzi, C.; Marchesini, M.; Zerboni, A. Climate change versus land management in the Po Plain (Northern Italy) during the Bronze Age: New insights from the VP/VG sequence of the Terramara Santa Rosa di Poviglio. Quat. Sci. Rev. 2016, 136, 153–172. [Google Scholar] [CrossRef]
- Florenzano, A.; Clò, E.; Zappa, J.; Montecchi, M.C.; Furia, E.; Torri, P.; Mercuri, A.M. Paesaggio vegetale sulla base delle analisi del riempimento dei pozzi al margine del Villaggio Grande della Terramara Santa Rosa di Poviglio. In Terramara Santa Rosa di Poviglio—Le Strutture Idrauliche al Margine del Villaggio Grande (Scavi 1998–2011); Cremaschi, M., Pizzi, C., Eds.; Istituto Italiano di Preistoria e Protostoria: Firenze, Italy, 2021; Origines 35; pp. 373–387. [Google Scholar]
- Mercuri, A.M.; Torri, P.; Florenzano, A.; Clò, E.; Mariotti Lippi, M.; Sgarbi, E.; Bignami, C. Sharing the Agrarian Knowledge with Archaeology: First Evidence of the Dimorphism of Vitis Pollen from the Middle Bronze Age of N Italy (Terramara Santa Rosa di Poviglio). Sustainability 2021, 13, 2287. [Google Scholar] [CrossRef]
- Chiaffarelli, G.; Indipolage Kariyawasam, T.; Proserpio, B.; Rottoli, M. Indipolage Kariyawasam, T.; Proserpio, B.; Rottoli, M. I macroresti vegetali nelle strutture al margine del Villaggio Grande della Terramara Santa Rosa di Poviglio. In Terramara Santa Rosa di Poviglio—Le strutture idrauliche al margine del Villaggio Grande (Scavi 1998–2011); Cremaschi, M., Pizzi, C., Eds.; Istituto Italiano di Preistoria e Protostoria: Firenze, Italy, 2021; Origines 35; pp. 389–401. [Google Scholar]
- Rottoli, M.; Motella, S. I resti antracologici e lignei della terramara Santa Rosa di Poviglio. In Il Villaggio Piccolo della Terramara di S. Rosa a Poviglio. Scavi 1987/1992; Bernabò Brea, M., Cremaschi, M., Eds.; Istituto Italiano Preistoria e Protostoria: Firenze, Italy, 2004; pp. 737–742. [Google Scholar]
- Giudici, M.; Mele, M.; Cremaschi, M.; Zerboni, A. La prospezione geofisica della Terramara Santa Rosa di Poviglio e dintorni: Forma del sito, strutture idrauliche e contesto geomorfologico. In Terramara Santa Rosa di Poviglio—Le Strutture Idrauliche al Margine del Villaggio Grande (Scavi 1998–2011); Cremaschi, M., Pizzi, C., Eds.; Istituto Italiano di Preistoria e Protostoria: Firenze, Italy, 2021; Origines 35; pp. 17–24. [Google Scholar]
- Zerboni, A.; Cremaschi, M. I carotaggi. In Poviglio S. Rosa, Campagna di Scavo 2018 tra Villaggio Piccolo e Villaggio Grande. Relazione Preliminare; Cremaschi, M., Brandolini, F., Mariani, G.S., Eds.; Dipartimento di Scienze della Terra “A. Desio”: Milano, Italy, 2018. [Google Scholar]
- Lowe, J.J.; Accorsi, C.A.; Bandini Mazzanti, M.; Bishop, A.; Forlani, L.; Van der Kaars, S.; Mercuri, A.M.; Rivalenti, C.; Torri, P.; Watson, C. Pollen stratigraphy of sediment sequences from crater lakes (Lago Albano and Lago Nemi) and the Central Adriatic spanning the interval from Oxygen isotope Stage 2 to present day. Mem. Ist. Ital. Idrobiol. 1996, 55, 71–98. [Google Scholar]
- van der Kaars, S.; Penny, D.; Tibby, J.; Fluin, J.; Dam, R.; Suparan, P. Late Quaternary palaeoecology, palynology and palaeolimnology of a tropical lowland swamp: Rawa Danau, West Java, Indonesia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2001, 171, 185–212. [Google Scholar] [CrossRef]
- Berglund, B.E. Handbook of Holocene Palaeoecology and Palaeohydrology; John Wiley & Sons, Inc.: Chichester, UK, 1986. [Google Scholar]
- Zini, V.; Taglini, F.; Torri, P.; Florenzano, A.; Mercuri, A.M.; Clò, E. Rivularia heterocystis as indicator of long–term changes of moisture and nutrients in soils: A quali–quantitative study at the Terramara S. Rosa di Poviglio (Reggio Emilia, Italy). In Abstracts Book of the Mediterranean Palynology Societies Symposium (Sep 06–08, Modena); Florenzano, A., Clò, E., Eds.; Università degli Studi di Modena e Reggio Emilia: Modena, Italy, 2021; pp. 129–130. [Google Scholar]
- Grimm, E.C. TILIA and TGView; Illinois State Museum: Springfield, IL, USA, 2004. [Google Scholar]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Breed, M. The effect of eutrophication and global change on heterocystous cyanobacteria in freshwater lakes [Data set]. Zenodo 2019. [Google Scholar] [CrossRef]
- González, E.J.; Roldán, G. Eutrophication and Phytoplankton: Some Generalities from Lakes and Reservoirs of the Americas. In Microalgae—From Physiology to Application; Vítová, M., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Herrero, A.; Picossi, S.; Flores, E. Gene expression during heterocyst differentiation. Adv. Bot. Res. 2013, 65, 281–329. [Google Scholar] [CrossRef]
- Bauersachs, T.; Speelman, E.N.; Hopmans, E.C.; Reichart, G.-J.; Schouten, S.; Sinninghe Damsté, J.S. Fossilized glycolipids reveal past oceanic N2 fixation by heterocystous cyanobacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 19190–19194. [Google Scholar] [CrossRef] [Green Version]
- Stivrins, N.; Soininen, J.; Tonno, I.; Freiberg, R.; Veski, S.; Kisand, V. Towards understanding the abundance of non-pollen palynomorphs: A comparison of fossil algae, algal pigments and sedaDNA from temperate lake sediments. Rev. Palaeobot. Palynol. 2018, 249, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Stivrins, N.; Soininen, J.; Amon, L.; Fontana, S.L.; Gryguc, G.; Heikkilä, M.; Heiri, O.; Kisielienė, D.; Reitalu, T.; Stančikaitė, M.; et al. 2016. Biotic turnover rates during the Pleistocene-Holocene transition. Quat. Sci. Rev. 2016, 151, 100–110. [Google Scholar] [CrossRef]
- Tappan, H. The Paleobiology of Plant Protists; Freeman: San Francisco, CA, USA, 1980. [Google Scholar]
- Whitton, B.A.; Potts, M. The Ecology of Cyanobacteria: Their Diversity in Time and Space; Springer: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Kuzovkina, Y.A. Willows beyond wetlands: Uses of Salix L. species for environmental projects. Water Air Soil Pollut. 2005, 162, 183–204. [Google Scholar] [CrossRef]
- Houston Durrant, T.; de Rigo, D.; Caudullo, G. Alnus glutinosa in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-MiguelAyanz, J., de Rigo, D., Caudullo, G., Houstoun Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016; pp. 64–65. [Google Scholar]
- Blasi, C. La Vegetazione d’Italia, Carta della Vegetazione, Scala 1:500000; Palombi & Partner, S.r.l.: Roma, Italy, 2010. [Google Scholar]
- Stancheva, R.; Sheath, R.G.; Read, B.A.; Mcarthur, K.D.; Schroepfer, C.L.; Kociolek, J.P.; Fetscher, A.E. Nitrogen-fixing cyanobacteria (free-living and diatom endosymbionts): Their use in southern California stream bioassessment. Hydrobiologia 2013, 720, 111–127. [Google Scholar] [CrossRef]
- El-Adl, M.F.; Bream, A.S. First record of the alien macroalgae, Rivularia atra and Polysiphonia opaca, on the Libyan coastline with special reference to their bionomics. Appl. Ecol. Environ. Res. 2016, 14, 249–263. [Google Scholar] [CrossRef]
- Legrand, B.; Miras, Y.; Beauger, A.; Dussauze, M.; Latour, D. Akinetes and ancient DNA reveal toxic cyanobacterial recurrences and their potential for resurrection in a 6700-year-old core from a eutrophic lake. Sci. Total Environ. 2019, 687, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
| CeDaD Laboratory Code | Uncal14C Age (Years BP) | δ 13C‰ | Site/Depth | Dated Material |
|---|---|---|---|---|
| LTL21118 | 4503 ± 45 | −22.1 ± 0.5 | PVG C-S1 −407/412 cm | Organic soil |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clò, E.; Florenzano, A. Heterocysts of Rivularia Type for Interpreting a Palaeoenvironmental Context of the Late Quaternary in Northern Italy. Sustainability 2022, 14, 15332. https://doi.org/10.3390/su142215332
Clò E, Florenzano A. Heterocysts of Rivularia Type for Interpreting a Palaeoenvironmental Context of the Late Quaternary in Northern Italy. Sustainability. 2022; 14(22):15332. https://doi.org/10.3390/su142215332
Chicago/Turabian StyleClò, Eleonora, and Assunta Florenzano. 2022. "Heterocysts of Rivularia Type for Interpreting a Palaeoenvironmental Context of the Late Quaternary in Northern Italy" Sustainability 14, no. 22: 15332. https://doi.org/10.3390/su142215332







