Opportunities and Challenges from Symbiosis of Agro-Industrial Residue Anaerobic Digestion with Microalgae Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Digestate and Microalgal Culture
2.2. Experimental Setup
- bicarbonate, added to provide the optimum carbon to nitrogen ratio (C/N) of 100:20;
- bicarbonate, to ensure a carbon-excess condition (C/N = 300:20);
- CO2 from air, via the free surface;
- CO2 from air, via air flushing at 300 mLair min−1
2.3. Analytical Methods
3. Results
3.1. Bicarbonate as Carbon Source
3.2. CO2 as Carbon Source
3.3. Nitrogen Source: Ammonium vs. Nitrates
3.4. Liquid Fraction of the Digestate as Nitrogen Source
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Policastro, G.; Giugliano, M.; Luongo, V.; Napolitano, R.; Fabbricino, M. Carbon Catabolite Repression Occurrence in Photo Fermentation of Ethanol-Rich Substrates. J. Environ. Manag. 2021, 297, 113371. [Google Scholar] [CrossRef] [PubMed]
- Nishio, N.; Nakashimada, Y. Recent Development of Anaerobic Digestion Processes for Energy Recovery from Wastes. J. Biosci. Bioeng. 2007, 103, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Xia, A.; Murphy, J.D. Microalgal Cultivation in Treating Liquid Digestate from Biogas Systems. Trends Biotechnol. 2016, 34, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrère, H. New Opportunities for Agricultural Digestate Valorization: Current Situation and Perspectives. Energy Environ. Sci. 2015, 8, 2600–2621. [Google Scholar] [CrossRef]
- Stiles, W.A.V.; Styles, D.; Chapman, S.P.; Esteves, S.; Bywater, A.; Melville, L.; Silkina, A.; Lupatsch, I.; Fuentes Grünewald, C.; Lovitt, R.; et al. Using Microalgae in the Circular Economy to Valorise Anaerobic Digestate: Challenges and Opportunities. Bioresour. Technol. 2018, 267, 732–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, R.B.; Costanza-Robinson, M.S.; Spatafora, G.A. Neochloris Oleoabundans Grown on Anaerobically Digested Dairy Manure for Concomitant Nutrient Removal and Biodiesel Feedstock Production. Biomass Bioenerg. 2011, 35, 40–49. [Google Scholar] [CrossRef]
- Rawat, I.; Ranjith Kumar, R.; Mutanda, T.; Bux, F. Biodiesel from Microalgae: A Critical Evaluation from Laboratory to Large Scale Production. Appl. Energy 2013, 103, 444–467. [Google Scholar] [CrossRef]
- Bauer, L.; Ranglová, K.; Masojídek, J.; Drosg, B.; Meixner, K. Digestate as Sustainable Nutrient Source for Microalgae—Challenges and Prospects. Appl. Sci. 2021, 11, 1056. [Google Scholar] [CrossRef]
- Li, G.; Hu, R.; Wang, N.; Yang, T.; Xu, F.; Li, J.; Wu, J.; Huang, Z.; Pan, M.; Lyu, T. Cultivation of Microalgae in Adjusted Wastewater to Enhance Biofuel Production and Reduce Environmental Impact: Pyrolysis Performances and Life Cycle Assessment. J. Clean. Prod. 2022, 355, 131768. [Google Scholar] [CrossRef]
- Li, G.; Hao, Y.; Yang, T.; Xiao, W.; Pan, M.; Huo, S.; Lyu, T. Enhancing Bioenergy Production from the Raw and Defatted Microalgal Biomass Using Wastewater as the Cultivation Medium. Bioengineering 2022, 9, 637. [Google Scholar] [CrossRef]
- Yang, L.; Tan, X.; Li, D.; Chu, H.; Zhou, X.; Zhang, Y.; Yu, H. Nutrients Removal and Lipids Production by Chlorella Pyrenoidosa Cultivation Using Anaerobic Digested Starch Wastewater and Alcohol Wastewater. Bioresour. Technol. 2015, 181, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Chu, H.; Zhang, Y.; Yang, L.; Zhao, F.; Zhou, X. Chlorella Pyrenoidosa Cultivation Using Anaerobic Digested Starch Processing Wastewater in an Airlift Circulation Photobioreactor. Bioresour. Technol. 2014, 170, 538–548. [Google Scholar] [CrossRef]
- Chawla, P.; Malik, A.; Sreekrishnan, T.R.; Dalvi, V.; Gola, D. Selection of Optimum Combination via Comprehensive Comparison of Multiple Algal Cultures for Treatment of Diverse Wastewaters. Environ. Technol. Innov. 2020, 18, 100758. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Chen, P.; Ruan, R. Semi-Continuous Cultivation of Chlorella Vulgaris for Treating Undigested and Digested Dairy Manures. Appl. Biochem. Biotechnol. 2010, 162, 2324–2332. [Google Scholar] [CrossRef]
- Gao, F.; Yang, Z.H.; Li, C.; Wang, Y.J.; Jin, W.H.; Deng, Y.B. Concentrated Microalgae Cultivation in Treated Sewage by Membrane Photobioreactor Operated in Batch Flow Mode. Bioresour. Technol. 2014, 167, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, P.; Prajapati, S.K.; Kumar, P.; Malik, A.; Pant, K.K. Development and Performance Evaluation of an Algal Biofilm Reactor for Treatment of Multiple Wastewaters and Characterization of Biomass for Diverse Applications. Bioresour. Technol. 2017, 224, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, S.; Hu, C.; Zhang, H.; Xu, J.; Ping, L. Performance of Three Microalgal Strains in Biogas Slurry Purification and Biogas Upgrade in Response to Various Mixed Light-Emitting Diode Light Wavelengths. Bioresour. Technol. 2015, 187, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Chuka-ogwude, D.; Ogbonna, J.; Moheimani, N.R. A Review on Microalgal Culture to Treat Anaerobic Digestate Food Waste Effluent. Algal Res. 2020, 47, 101841. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. Handbook of Microalgal Culture; Blackwell: Hoboken, NJ, USA, 2004; pp. 97–114. [Google Scholar]
- Tuantet, K.; Temmink, H.; Zeeman, G.; Janssen, M.; Wijffels, R.H.; Buisman, C.J.N. Nutrient Removal and Microalgal Biomass Production on Urine in a Short Light-Path Photobioreactor. Water Res. 2014, 55, 162–174. [Google Scholar] [CrossRef]
- Policastro, G.; Cesaro, A.; Fabbricino, M. Photo-Fermentative Hydrogen Production from Cheese Whey: Engineering of a Mixed Culture Process in a Semi-Continuous, Tubular Photo-Bioreactor. Int. J. Hydrogen Energy 2022. in print. [Google Scholar] [CrossRef]
- Policastro, G.; Luongo, V.; Frunzo, L.; Fabbricino, M. A Comprehensive Review of Mathematical Models of Photo Fermentation. Crit. Rev. Biotechnol. 2021, 41, 628–648. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA: Washington, DC, USA, 2005; ISBN 0875532357. [Google Scholar]
- Abedini Najafabadi, H.; Malekzadeh, M.; Jalilian, F.; Vossoughi, M.; Pazuki, G. Effect of Various Carbon Sources on Biomass and Lipid Production of Chlorella Vulgaris during Nutrient Sufficient and Nitrogen Starvation Conditions. Bioresour. Technol. 2015, 180, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Ermis, H.; Altinbas, M. Effect of Salinity on Mixed Microalgae Grown in Anaerobic Liquid Digestate. Water Environ. J. 2020, 34, 820–830. [Google Scholar] [CrossRef]
- Mondal, M.; Goswami, S.; Ghosh, A.; Oinam, G.; Tiwari, O.N.; Das, P.; Gayen, K.; Mandal, M.K.; Halder, G.N. Production of Biodiesel from Microalgae through Biological Carbon Capture: A Review. 3 Biotech 2017, 7, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.Y.; Heo, J.; Kim, K.; Chung, J.; Han, J.I. Electrochemical PH Control and Carbon Supply for Microalgae Cultivation. Chem. Eng. J. 2021, 426, 131796. [Google Scholar] [CrossRef]
- Kong, Q.X.; Li, L.; Martinez, B.; Chen, P.; Ruan, R. Culture of Microalgae Chlamydomonas Reinhardtii in Wastewater for Biomass Feedstock Production. Appl. Biochem. Biotechnol. 2010, 160, 9–18. [Google Scholar] [CrossRef]
- Zhu, B.; Chen, G.; Cao, X.; Wei, D. Molecular Characterization of CO2 Sequestration and Assimilation in Microalgae and Its Biotechnological Applications. Bioresour. Technol. 2017, 244, 1207–1215. [Google Scholar] [CrossRef]
- Thakur, I.S.; Kumar, M.; Varjani, S.J.; Wu, Y.; Gnansounou, E.; Ravindran, S. Sequestration and Utilization of Carbon Dioxide by Chemical and Biological Methods for Biofuels and Biomaterials by Chemoautotrophs: Opportunities and Challenges. Bioresour. Technol. 2018, 256, 478–490. [Google Scholar] [CrossRef]
- Ma, X.; Mi, Y.; Zhao, C.; Wei, Q. A Comprehensive Review on Carbon Source Effect of Microalgae Lipid Accumulation for Biofuel Production. Sci. Total Environ. 2022, 806, 151387. [Google Scholar] [CrossRef]
- Zhu, L.; Li, S.; Hu, T.; Nugroho, Y.K.; Yin, Z.; Hu, D.; Chu, R.; Mo, F.; Liu, C.; Hiltunen, E. Effects of Nitrogen Source Heterogeneity on Nutrient Removal and Biodiesel Production of Mono- and Mix-Cultured Microalgae. Energy Convers. Manag. 2019, 201, 112144. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Glass, A.D.M.; Siddiqi, M.Y. Inhibition of Nitrate Uptake by Ammonium in Barley. Analysis of Component Fluxes. Plant Physiol. 1999, 120, 283–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheeler, P.A.; Kokkinakis, S.A. Ammonium Recycling Limits Nitrate Use in the Oceanic Subarctic Pacific. Limnol. Oceanogr. 1990, 35, 1267–1278. [Google Scholar] [CrossRef]
- González-Fernández, C.; Molinuevo-Salces, B.; García-González, M.C. Nitrogen Transformations under Different Conditions in Open Ponds by Means of Microalgae-Bacteria Consortium Treating Pig Slurry. Bioresour. Technol. 2011, 102, 960–966. [Google Scholar] [CrossRef]
- Mooij, P.R. On the Use of Selective Environments in Microalgal Cultivation; Delft University of Technology: Delft, The Netherlands, 2016; ISBN 9789461865854. [Google Scholar]
- Chan, S.S.; Khoo, K.S.; Chew, K.W.; Ling, T.C.; Show, P.L. Recent Advances Biodegradation and Biosorption of Organic Compounds from Wastewater: Microalgae-Bacteria Consortium—A Review. Bioresour. Technol. 2022, 344, 126159. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.; Eraky, M.; Alhajeri, N.S.; Osman, A.I.; Rooney, D.W. Cultivation of Microalgae on Liquid Anaerobic Digestate for Depollution, Biofuels and Cosmetics: A Review. Environ. Chem. Lett. 2022. in print. [Google Scholar] [CrossRef]
- Ermis, H.; Guven-Gulhan, U.; Cakir, T.; Altinbas, M. Effect of Iron and Magnesium Addition on Population Dynamics and High Value Product of Microalgae Grown in Anaerobic Liquid Digestate. Sci. Rep. 2020, 10, 3510. [Google Scholar] [CrossRef]

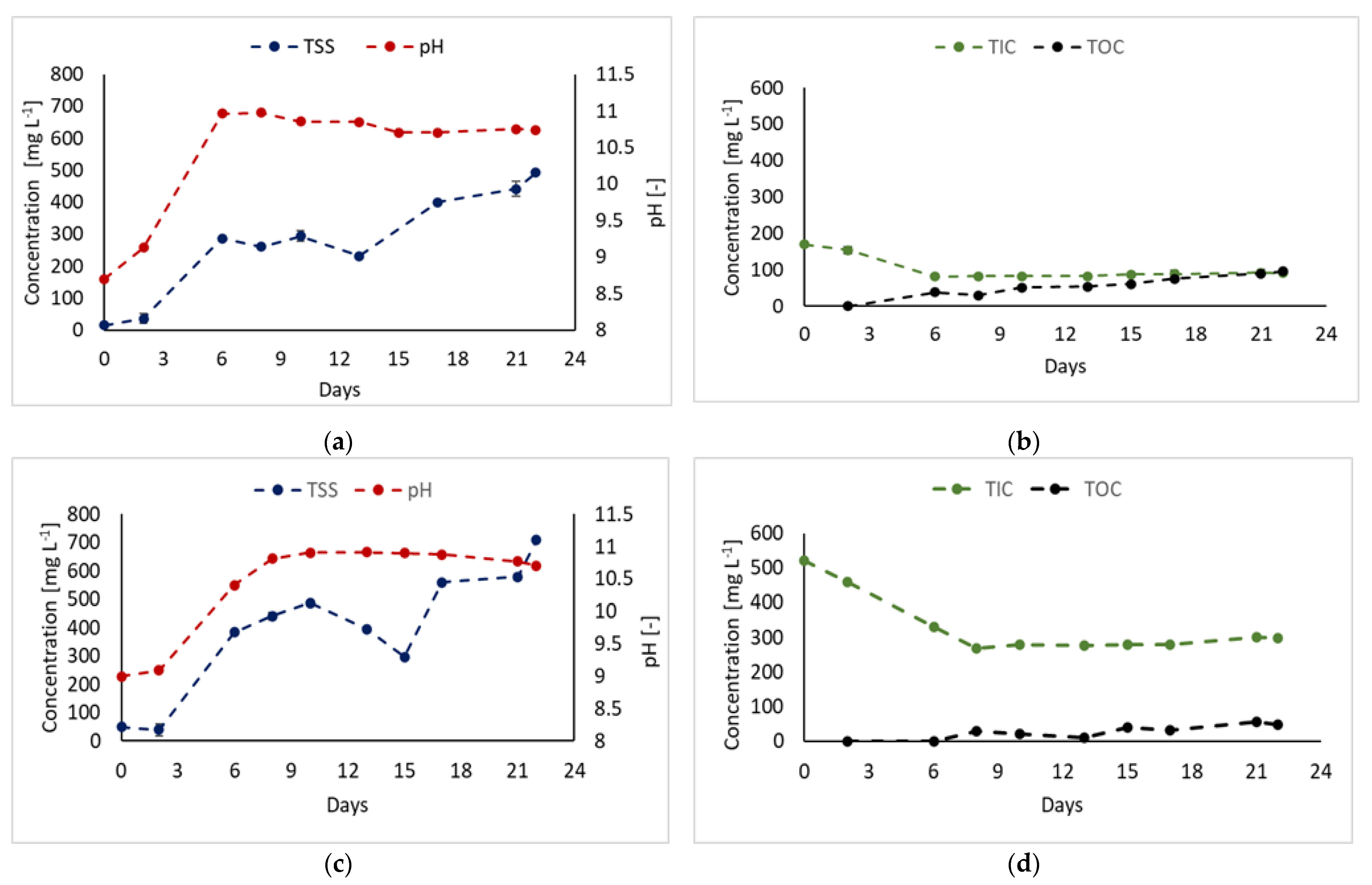
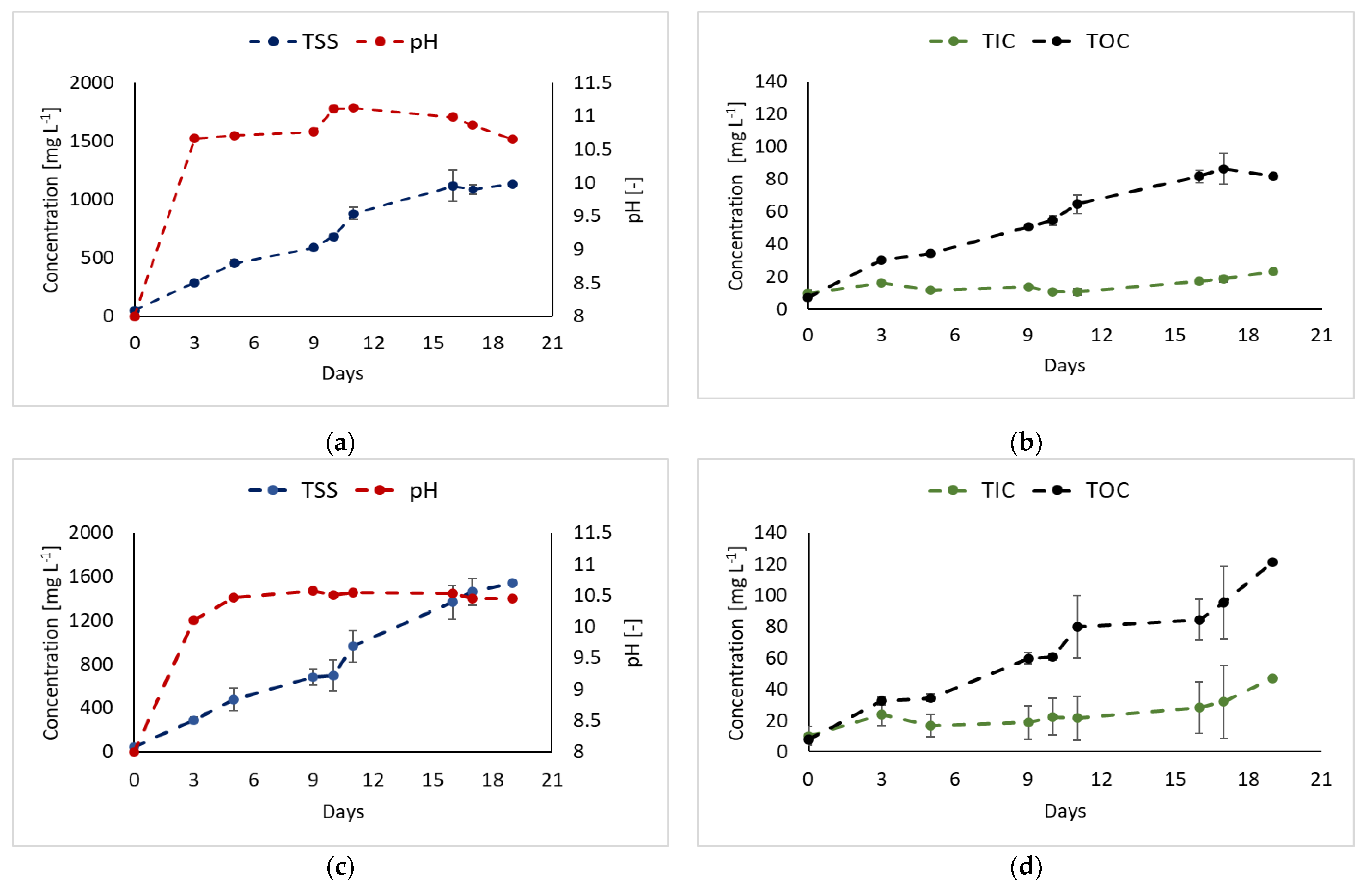
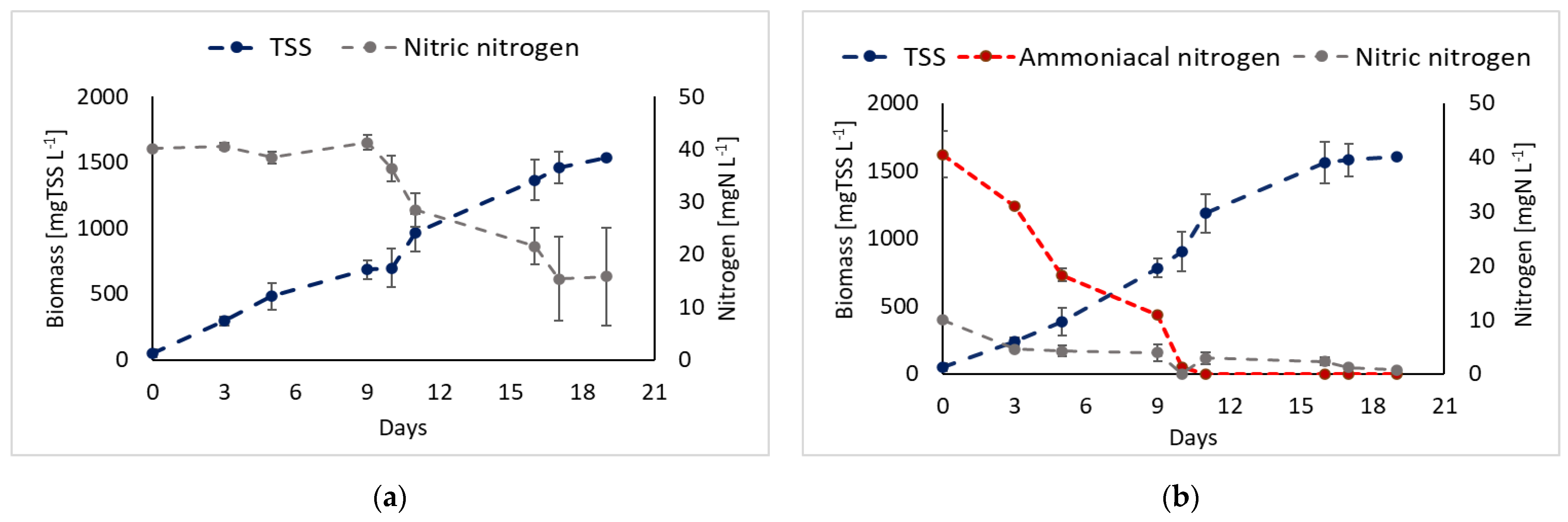
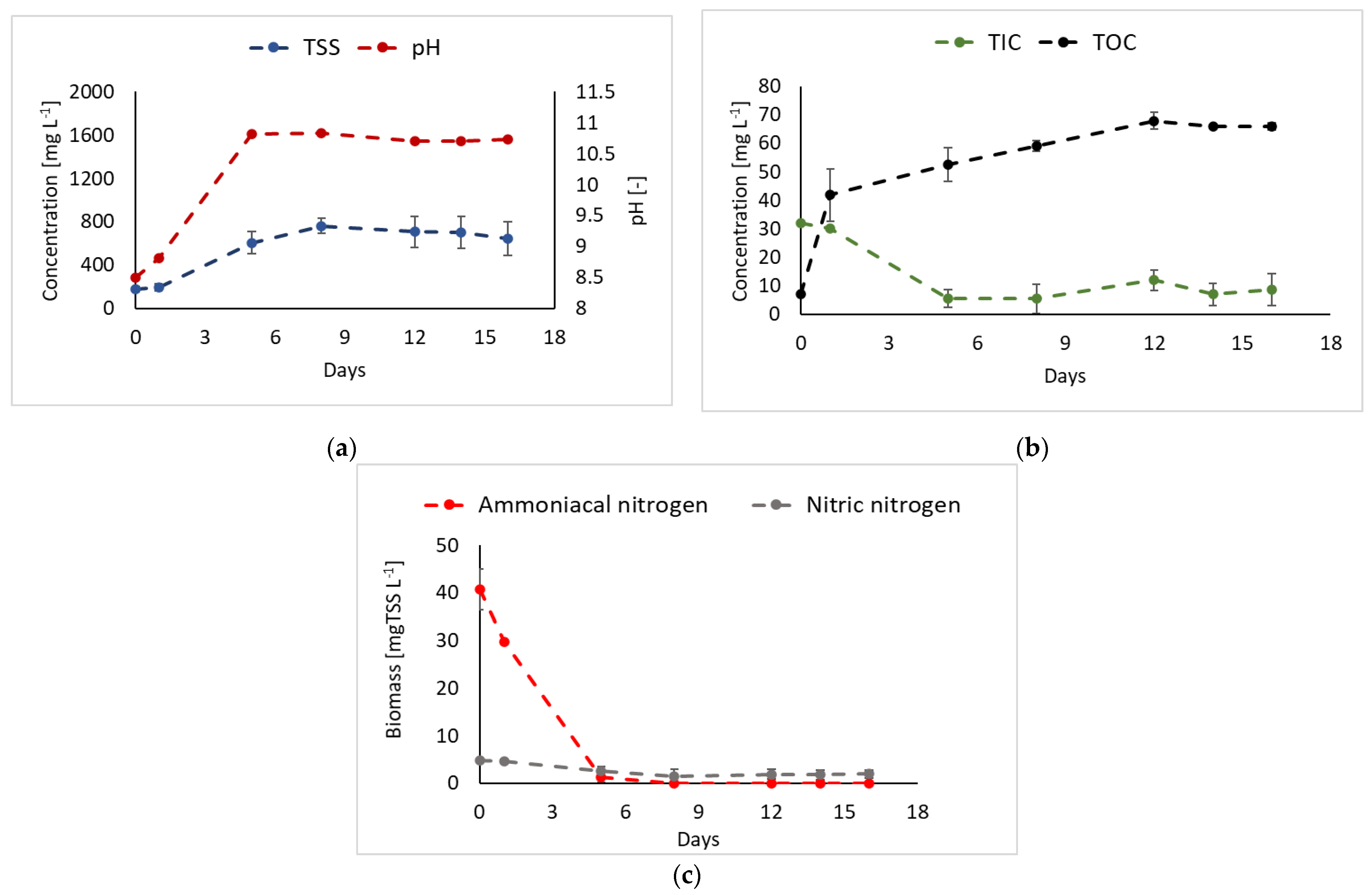
| Strain | Digestate Source | Pretreatment | Feeding Mode | Biomass Production | Nitrogen Removal Efficiency | Refs. |
|---|---|---|---|---|---|---|
| Chlorella pyrenoidosa | Starch wastewater | Filtration Sterilization Dilution | Batch | 3.01 g/L | TN 91.6% | [11] |
| Chlorella pyrenoidosa | Starch processing wastewater | Precipitation Filtration | Batch | 2.05 g/L | TN 83.1% | [12] |
| Chlorella pyrenoidosa | Municipal wastewater Dairy wastewater | Centrifugation | Batch | 0.6–0.7 g/L | NO3− 72–89% NH4+ 90–91% | [13] |
| Desmodemus sp. | Pig manure | Filtration Dilution | Batch | 0.412 g/L | TN: 100% | [6] |
| Chlorella vulgaris | Dairy manure | Dilution | Semicontinuous | 0.760 g L−1 | TN: 93.6% NH4+: 100% | [14] |
| Chlorella vulgaris | Municipal wastewater | NP | Batch (membrane reactor) | 39 mg L−1d−1 | TN: 56% | [15] |
| Chlorella Phormidium sp. | Dairy wastewater | NR | Batch (biofilm reactor) | 3.1 g m−2d−1 | TN: 94% | [16] |
| C. vulgaris, Scenedesmus obliquus, Neochloris oleoabundans | NR | Sterilization; Filtration | Batch | 0.01–0.06 gL−1d−1 | TN: 76.0% | [17] |
| Test Code | Carbon Source | Nitrogen Source | C/N Ratio | Nitrogen Concentration |
|---|---|---|---|---|
| C1 | NaHCO3 | NaNO3 | 100:20 | 250 mg NaNO3 L−1 41 mgN L−1 |
| C2 | NaHCO3 | NaNO3 | 300:20 | 250 mg NaNO3 L−1 41 mgN L−1 |
| C3 | Air (CO2), free surface | NaNO3 | - | 250 mg NaNO3 L−1 41 mgN L−1 |
| C4 | Air (CO2), flushing | NaNO3 | - | 250 mg NaNO3 L−1 41 mgN L−1 |
| N | Air (CO2), flushing | NH4Cl | 146 mg NH4Cl L−1 41 mgN L−1 | |
| D | Air (CO2), flushing | Digestate | 15 mL digestate 41 mgN L−1 |
| Test Code (N-Source) | Total Nitrogen Removal [%] | Ammoniacal Nitrogen Removal [%] | Nitric Nitrogen Removal [%] | Biomass Production [gTSS L−1] |
|---|---|---|---|---|
| C4 (NaNO3) | 62 ± 20 | - | 62 ± 20 | 1.53 ± 0.01 |
| N (NH4Cl) | 96 ± 1 | 100 ± 0.00 | 60 ± 1 | 1.61 ± 0.01 |
| D (Diegstate) | 98.5 ± 1 | 100 ± 0.00 | 93 ± 1 | 0.65 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Policastro, G.; Cesaro, A.; Fabbricino, M.; Pirozzi, F. Opportunities and Challenges from Symbiosis of Agro-Industrial Residue Anaerobic Digestion with Microalgae Cultivation. Sustainability 2022, 14, 15607. https://doi.org/10.3390/su142315607
Policastro G, Cesaro A, Fabbricino M, Pirozzi F. Opportunities and Challenges from Symbiosis of Agro-Industrial Residue Anaerobic Digestion with Microalgae Cultivation. Sustainability. 2022; 14(23):15607. https://doi.org/10.3390/su142315607
Chicago/Turabian StylePolicastro, Grazia, Alessandra Cesaro, Massimiliano Fabbricino, and Francesco Pirozzi. 2022. "Opportunities and Challenges from Symbiosis of Agro-Industrial Residue Anaerobic Digestion with Microalgae Cultivation" Sustainability 14, no. 23: 15607. https://doi.org/10.3390/su142315607
APA StylePolicastro, G., Cesaro, A., Fabbricino, M., & Pirozzi, F. (2022). Opportunities and Challenges from Symbiosis of Agro-Industrial Residue Anaerobic Digestion with Microalgae Cultivation. Sustainability, 14(23), 15607. https://doi.org/10.3390/su142315607












