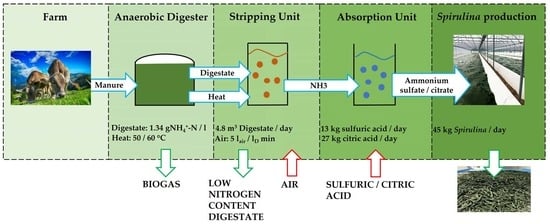
Efficient Nitrogen Recovery from Agro-Energy Effluents for Cyanobacteria Cultivation (Spirulina)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Digestate
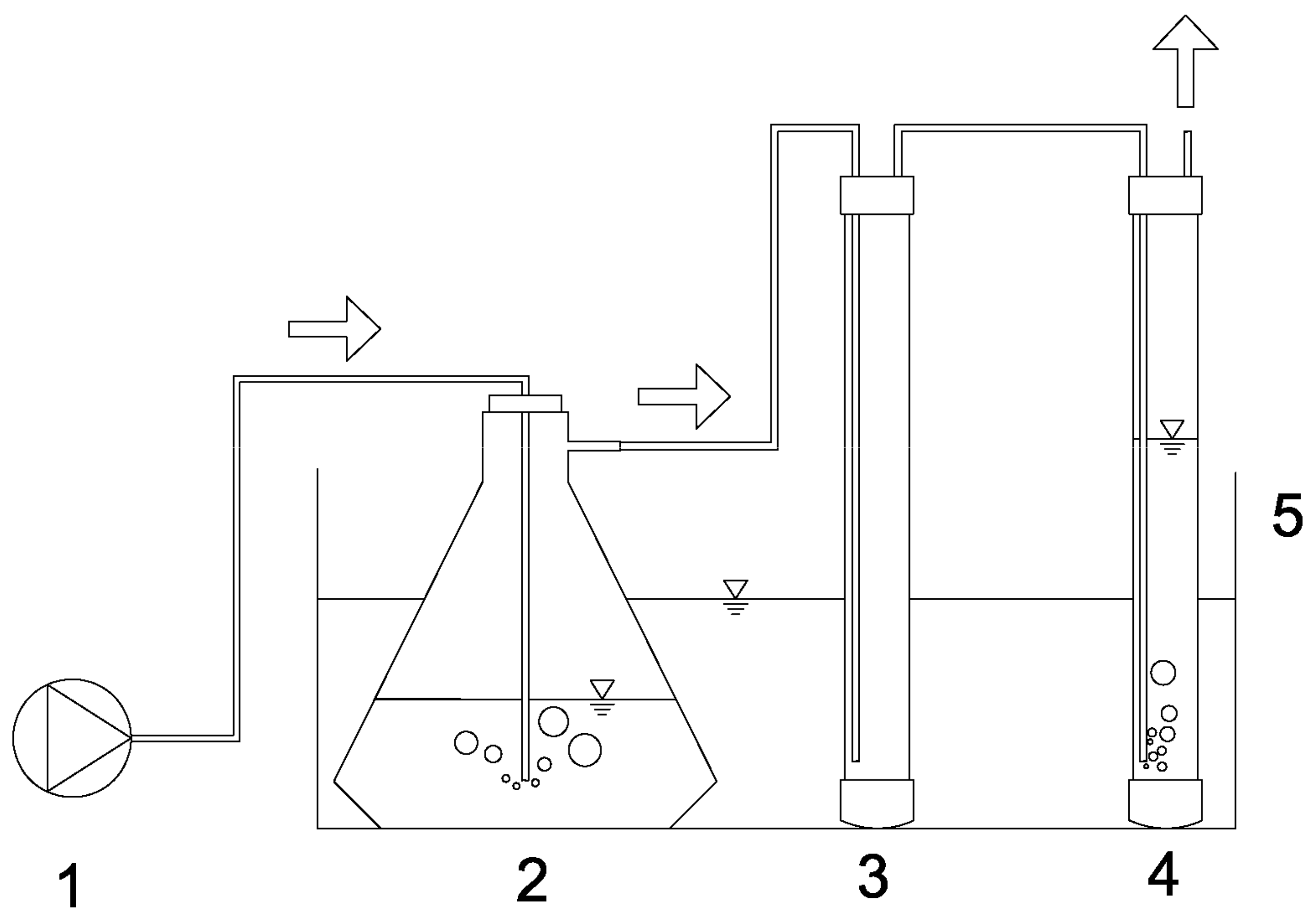
2.2. Ammonia Recovery Batch Tests
2.3. Setup for the Cultivation of Spirulina
2.4. Analytical Methods
2.5. Calculations
3. Results and Discussion
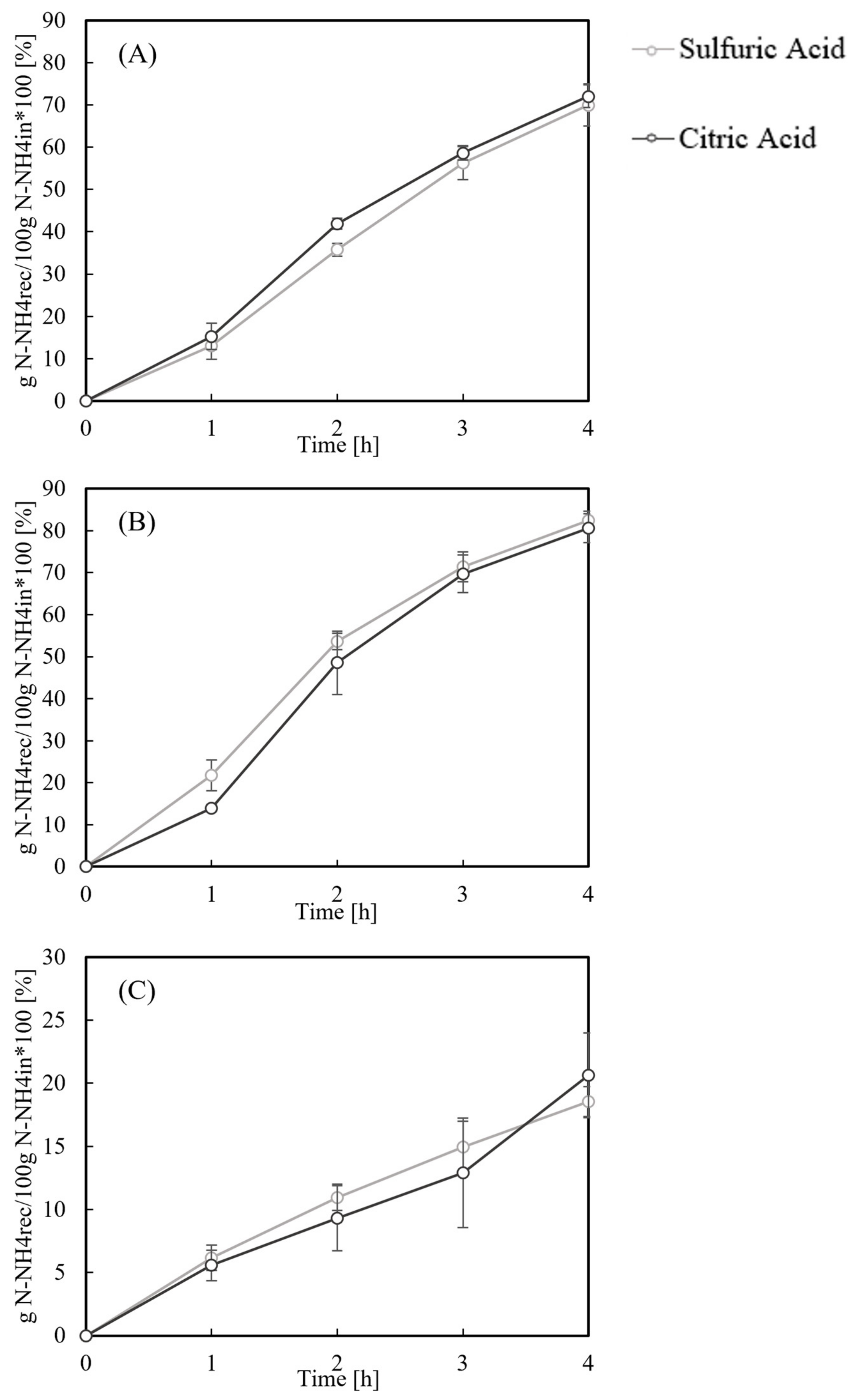
3.1. Nitrogen Recovery
Foam Formation
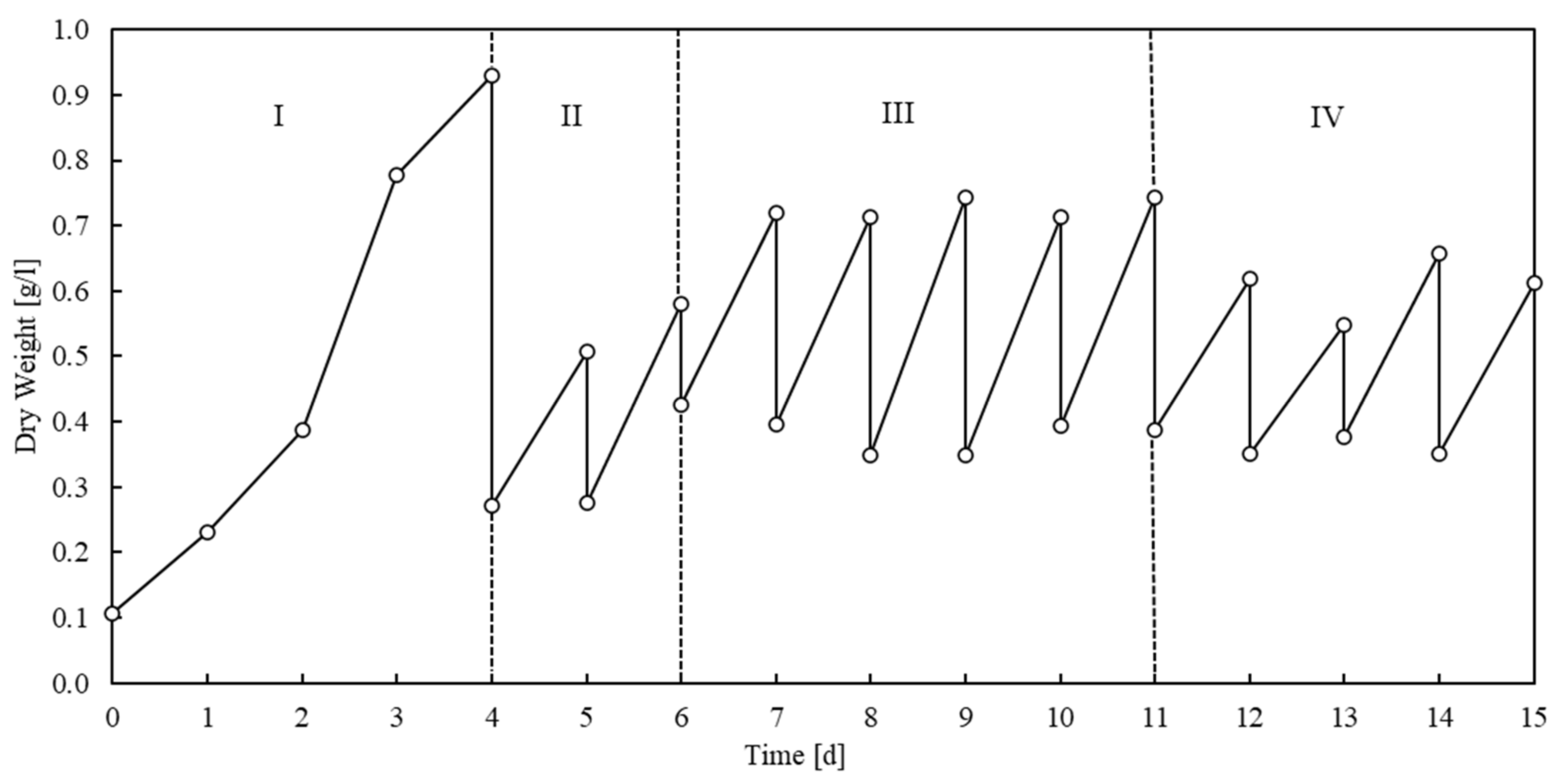
3.2. Nitrogen Utilization by Spirulina
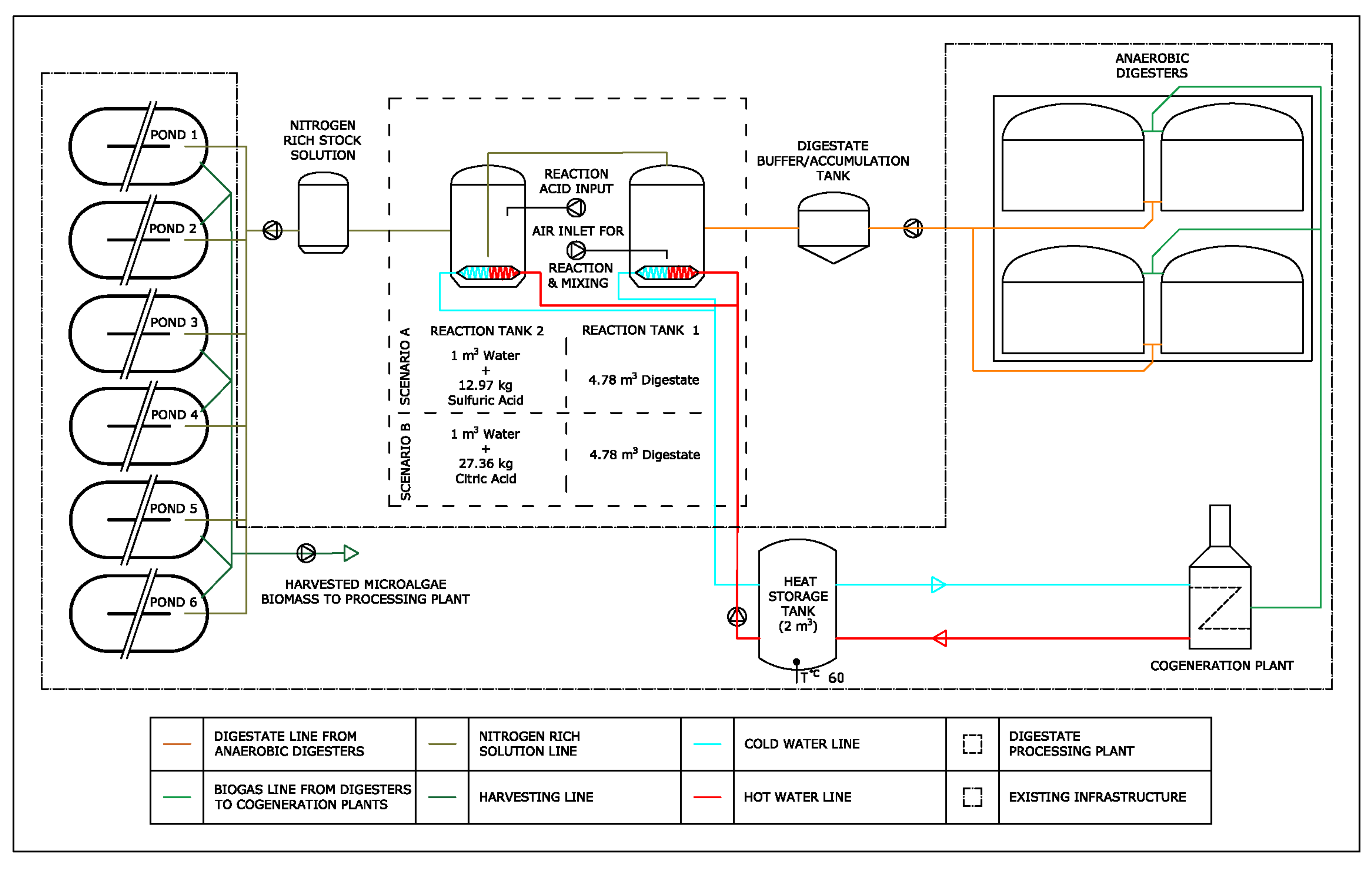
3.3. Preliminary Considerations on the Implementation of a Plant for Combined Nitrogen Recovery and Spirulina Growth
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission-Press Release Reducing Greenhouse Gas Emissions: Commission Adopts EU Methane Strategy as Part of European Green Deal: Brussels, Belgium. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1603122077630&uri=CELEX:52020DC0663 (accessed on 10 May 2022).
- Feng, L.; Ward, A.J.; Moset, V.; Møller, H.B. Methane Emission during On-Site Pre-Storage of Animal Manure Prior to Anaerobic Digestion at Biogas Plant: Effect of Storage Temperature and Addition of Food Waste. J. Environ. Manag. 2018, 225, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Laureni, M.; Palatsi, J.; Llovera, M.; Bonmatí, A. Influence of Pig Slurry Characteristics on Ammonia Stripping Efficiencies and Quality of the Recovered Ammonium-Sulfate Solution. J. Chem. Technol. Biotechnol. 2013, 88, 1654–1662. [Google Scholar] [CrossRef]
- Lei, X.; Sugiura, N.; Feng, C.; Maekawa, T. Pretreatment of Anaerobic Digestion Effluent with Ammonia Stripping and Biogas Purification. J. Hazard. Mater. 2007, 145, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Limoli, A.; Langone, M.; Andreottola, G. Ammonia Removal from Raw Manure Digestate by Means of a Turbulent Mixing Stripping Process. J. Environ. Manag. 2016, 176, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Q.; Jia, Z.; Wang, C.; Ye, X.; Du, J.; Kong, X.; Xi, Y. Relieving Ammonia Nitrogen Inhibition in High Concentration Anaerobic Digestion of Rural Organic Household Waste by Prussian Blue Analogue Nanoparticles Addition. Bioresour. Technol. 2021, 330, 124979. [Google Scholar] [CrossRef] [PubMed]
- Nkoa, R. Agricultural Benefits and Environmental Risks of Soil Fertilization with Anaerobic Digestates: A Review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef] [Green Version]
- Palakodeti, A.; Azman, S.; Rossi, B.; Dewil, R.; Appels, L. A Critical Review of Ammonia Recovery from Anaerobic Digestate of Organic Wastes via Stripping. Renew. Sustain. Energy Rev. 2021, 143, 110903. [Google Scholar] [CrossRef]
- Jamaludin, Z.; Rollings-Scattergood, S.; Lutes, K.; Vaneeckhaute, C. Evaluation of Sustainable Scrubbing Agents for Ammonia Recovery from Anaerobic Digestate. Bioresour. Technol. 2018, 270, 596–602. [Google Scholar] [CrossRef]
- Costamagna, P.; Delucchi, M.; Busca, G.; Giordano, A. System for Ammonia Removal from Anaerobic Digestion and Associated Ammonium Sulfate Production: Simulation and Design Considerations. Process Saf. Environ. Prot. 2020, 144, 133–142. [Google Scholar] [CrossRef]
- Zhao, Q.B.; Ma, J.; Zeb, I.; Yu, L.; Chen, S.; Zheng, Y.M.; Frear, C. Ammonia Recovery from Anaerobic Digester Effluent through Direct Aeration. Chem. Eng. J. 2015, 279, 31–37. [Google Scholar] [CrossRef]
- Bonmatí, A.; Flotats, X. Air Stripping of Ammonia from Pig Slurry: Characterisation and Feasibility as a Pre- or Post-Treatment to Mesophilic Anaerobic Digestion. Waste Manag. 2003, 23, 261–272. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Lebuf, V.; Michels, E.; Belia, E.; Vanrolleghem, P.A.; Tack, F.M.G.; Meers, E. Nutrient Recovery from Digestate: Systematic Technology Review and Product Classification. Waste Biomass Valorization 2017, 8, 21–40. [Google Scholar] [CrossRef] [Green Version]
- Baldi, M.; Collivignarelli, M.C.; Abbà, A.; Benigna, I. The Valorization of Ammonia in Manure Digestate by Means of Alternative Stripping Reactors. Sustainability 2018, 10, 3073. [Google Scholar] [CrossRef] [Green Version]
- Sobhi, M.; Han, T.; Stinner, W.; Cui, X.; Sun, H.; Li, B.; Guo, J.; Dong, R. Hybrid Technology for Nutrients Recovery as Microbial Biomass and Ammonium Sulfate from Un-Diluted Biogas Liquid Digestate Using a Modified Airlift Reactor. J. Clean. Prod. 2020, 267, 121976. [Google Scholar] [CrossRef]
- Brienza, C.; Sigurnjak, I.; Michels, E.; Meers, E. Ammonia Stripping and Scrubbing for Mineral Nitrogen Recovery. In Biorefinery of Inorganics; Wiley: New York, NY, USA, 2020; pp. 95–106. [Google Scholar]
- Hang, Y.D.; Woodams, E.E. Solid State Fermentation of Apple Pomace for Citric Acid Production. World J. Appl. Microbiol. Biotechnol. 1986, 2, 283–287. [Google Scholar]
- Sawant, O.; Mahale, S.; Ramchandran, V.; Nagaraj, G.; Bankar, A. Fungal Citric Acid Production Using Waste Materials: A Mini-Review. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 821–828. [Google Scholar] [CrossRef]
- Soccol, C.R.; Vandenberghe, L.P.S.; Rodrigues, C.; Pandey, A. New Perspectives for Citric Acid Production and Application. Food Technol. Biotechnol. 2006, 44, 141–149. [Google Scholar]
- Masojídek, J.; Torzillo, G. Mass Cultivation of Freshwater Microalgae. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Thevarajah, B.; Nishshanka, G.K.S.H.; Premaratne, M.; Nimarshana, P.H.V.; Nagarajan, D.; Chang, J.-S.; Ariyadasa, T.U. Large-Scale Production of Spirulina-Based Proteins and c-Phycocyanin: A Biorefinery Approach. Biochem. Eng. J. 2022, 185, 108541. [Google Scholar] [CrossRef]
- Soletto, D.; Binaghi, L.; Lodi, A.; Carvalho, J.C.M.; Converti, A. Batch and Fed-Batch Cultivations of Spirulina Platensis Using Ammonium Sulphate and Urea as Nitrogen Sources. Aquaculture 2005, 243, 217–224. [Google Scholar] [CrossRef]
- Carvalho, J.C.M.; Francisco, F.R.; Almeida, K.A.; Sato, S.; Converti, A. Cultivation of Arthrospira (Spirulina) Platensis (Cyanophyceae) by Fed-Batch Addition of Ammonium Chloride at Exponentially Increasing Feeding Rates. J. Phycol. 2004, 40, 589–597. [Google Scholar] [CrossRef]
- Alengebawy, A.; Mohamed, B.A.; Jin, K.; Liu, T.; Ghimire, N.; Samer, M.; Ai, P. A Comparative Life Cycle Assessment of Biofertilizer Production towards Sustainable Utilization of Anaerobic Digestate. Sustain. Prod. Consum. 2022, 33, 875–889. [Google Scholar] [CrossRef]
- Malhotra, M.; Aboudi, K.; Pisharody, L.; Singh, A.; Banu, J.R.; Bhatia, S.K.; Varjani, S.; Kumar, S.; González-Fernández, C.; Kumar, S.; et al. Biorefinery of Anaerobic Digestate in a Circular Bioeconomy: Opportunities, Challenges and Perspectives. Renew. Sustain. Energy Rev. 2022, 166, 112642. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-Based Fertilizers: A Practical Approach towards Circular Economy. Bioresour. Technol. 2020, 295, 122223. [Google Scholar] [CrossRef] [PubMed]
- Orner, K.D.; Smith, S.J.; Breunig, H.M.; Scown, C.D.; Nelson, K.L. Fertilizer Demand and Potential Supply through Nutrient Recovery from Organic Waste Digestate in California. Water Res 2021, 206, 117717. [Google Scholar] [CrossRef] [PubMed]
- Transforming Our World: The 2030 Agenda for Sustainable Development. In A New Era in Global Health; Springer Publishing Company: Berlin/Heidelberg, Germany, 2018.
- Matassa, S.; Pelagalli, V.; Papirio, S.; Zamalloa, C.; Verstraete, W.; Esposito, G.; Pirozzi, F. Direct Nitrogen Stripping and Upcycling from Anaerobic Digestate during Conversion of Cheese Whey into Single Cell Protein. Bioresour. Technol. 2022, 358, 127308. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Ukwuani, A.T. Coupling Thermal Stripping and Acid Absorption for Ammonia Recovery from Dairy Manure: Ammonia Volatilization Kinetics and Effects of Temperature, PH and Dissolved Solids Content. Chem. Eng. J. 2015, 280, 188–196. [Google Scholar] [CrossRef]
- Zarrouk, C. Contribution à l’étude d’une Cyanophycée. Influence de Divers Facteurs Physiques et Chimiques Sur La Croissance et La Photosynthèse de Spirulina Maxima. Ph.D. Thesis, Université de Paris, Pairs, France, 1966. [Google Scholar]
- Da Rosa, G.M.; Moraes, L.; de Souza, M.d.R.A.Z.; Costa, J.A.V. Spirulina Cultivation with a CO2 Absorbent: Influence on Growth Parameters and Macromolecule Production. Bioresour. Technol. 2016, 200, 528–534. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater American Public Health Association, American Water Works Association and Water Environmental Federation, 20th ed.; APHA: Washington, DC, USA, 1998. [Google Scholar]
- APAT-CNR-IRSA. Metodi Analitici per Le Acque, Manuali e Linee Guida; APAT: Rome, Italy, 2003. [Google Scholar]
- Kim, E.J.; Kim, H.; Lee, E. Influence of Ammonia Stripping Parameters on the Efficiency and Mass Transfer Rate of Ammonia Removal. Appl. Sci. 2021, 11, 441. [Google Scholar] [CrossRef]
- Folino, A.; Zema, D.A.; Calabrò, P.S. Organic Matter Removal and Ammonia Recovery by Optimised Treatments of Swine Wastewater. J. Environ. Manag. 2020, 270, 110692. [Google Scholar] [CrossRef]
- Campos, J.C.; Moura, D.; Costa, A.P.; Yokoyama, L.; Araujo, F.V.D.F.; Cammarota, M.C.; Cardillo, L. Evaluation of PH, Alkalinity and Temperature during Air Stripping Process for Ammonia Removal from Landfill Leachate. J. Env. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2013, 48, 1105–1113. [Google Scholar] [CrossRef]
- Jiang, A.; Zhang, T.; Zhao, Q.B.; Li, X.; Chen, S.; Frear, C.S. Evaluation of an Integrated Ammonia Stripping, Recovery, and Biogas Scrubbing System for Use with Anaerobically Digested Dairy Manure. Biosyst. Eng. 2014, 119, 117–126. [Google Scholar] [CrossRef]
- Wu, H.; Dong, R.; Wu, S. Exploring Low-Cost Practical Antifoaming Strategies in the Ammonia Stripping Process of Anaerobic Digested Slurry. Chem. Eng. J. 2018, 344, 228–235. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Ferreira, L.S.; Converti, A.; Sato, S.; de Carvalho, J.C.M. Influence of Ammonium Sulphate Feeding Time on Fed-Batch Arthrospira (Spirulina) Platensis Cultivation and Biomass Composition with and without PH Control. Bioresour. Technol. 2011, 102, 6587–6592. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, R.P.; Montoya, E.Y.O.; Sato, S.; Perego, P.; de Carvalho, J.C.M.; Converti, A. Effects of Light Intensity and Dilution Rate on the Semicontinuous Cultivation of Arthrospira (Spirulina) Platensis. A Kinetic Monod-Type Approach. Bioresour. Technol. 2011, 102, 3215–3219. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Unit | Value |
|---|---|---|
| pH | - | 7.6 ± 0.1 |
| Total solids | % | 6.6 ± 0.1 |
| Volatile solids | % TS | 68 ± 2 |
| Ammonia nitrogen | g L−1 | 1.34 ± 0.16 |
| Chemical oxygen demand | g L−1 | 45.9 ± 0.5 |
| Test | Acid | NH4+-Nrecovered [%] | NH4+-Nstripped [%] | Balance (N) [%] | Initial/Final pH | fNH3 | Digestate Volume Decrease [%] |
|---|---|---|---|---|---|---|---|
| A (50 °C) | Sulfuric | 70.0 ± 2.5 | 76.1 ± 8.0 | 93.9 ± 6.0 | 8.22 ± 0.21/9.44 ± 0.06 | 0.36/0.90 | 12 |
| Citric | 72.1 ± 2.7 | 74.6 ± 3.3 | 97.5 ± 5.8 | ||||
| B (70 °C) | Sulfuric | 82.7 ± 2.1 | 89.7 ± 4.5 | 93.9 ± 5.6 | 8.22 ± 0.03/9.87 ± 0.19 | 0.63/0.98 | 23 |
| Citric | 79.5 ± 1.6 | 86.1 ± 6.6 | 94.0 ± 9.1 | ||||
| C (25 °C, pH = 10) | Sulfuric | 18.6 ± 0.9 | 25.5 ± 5.6 | 93.0 ± 4.9 | 10.0 ± 0.1/9.91 ± 0.04 | 0.87 | 2.5 |
| Citric | 20.6 ± 4.9 | 25.6 ± 5.5 | 94.8 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attene, L.; Deiana, A.; Carucci, A.; De Gioannis, G.; Asunis, F.; Ledda, C. Efficient Nitrogen Recovery from Agro-Energy Effluents for Cyanobacteria Cultivation (Spirulina). Sustainability 2023, 15, 675. https://doi.org/10.3390/su15010675
Attene L, Deiana A, Carucci A, De Gioannis G, Asunis F, Ledda C. Efficient Nitrogen Recovery from Agro-Energy Effluents for Cyanobacteria Cultivation (Spirulina). Sustainability. 2023; 15(1):675. https://doi.org/10.3390/su15010675
Chicago/Turabian StyleAttene, Luca, Andrea Deiana, Alessandra Carucci, Giorgia De Gioannis, Fabiano Asunis, and Claudio Ledda. 2023. "Efficient Nitrogen Recovery from Agro-Energy Effluents for Cyanobacteria Cultivation (Spirulina)" Sustainability 15, no. 1: 675. https://doi.org/10.3390/su15010675
APA StyleAttene, L., Deiana, A., Carucci, A., De Gioannis, G., Asunis, F., & Ledda, C. (2023). Efficient Nitrogen Recovery from Agro-Energy Effluents for Cyanobacteria Cultivation (Spirulina). Sustainability, 15(1), 675. https://doi.org/10.3390/su15010675