Comprehensive Assessment of Soil Chemical Properties for Land Reclamation Purposes in the Toshka Area, EGYPT
Abstract
:1. Introduction
2. Materials and Methods
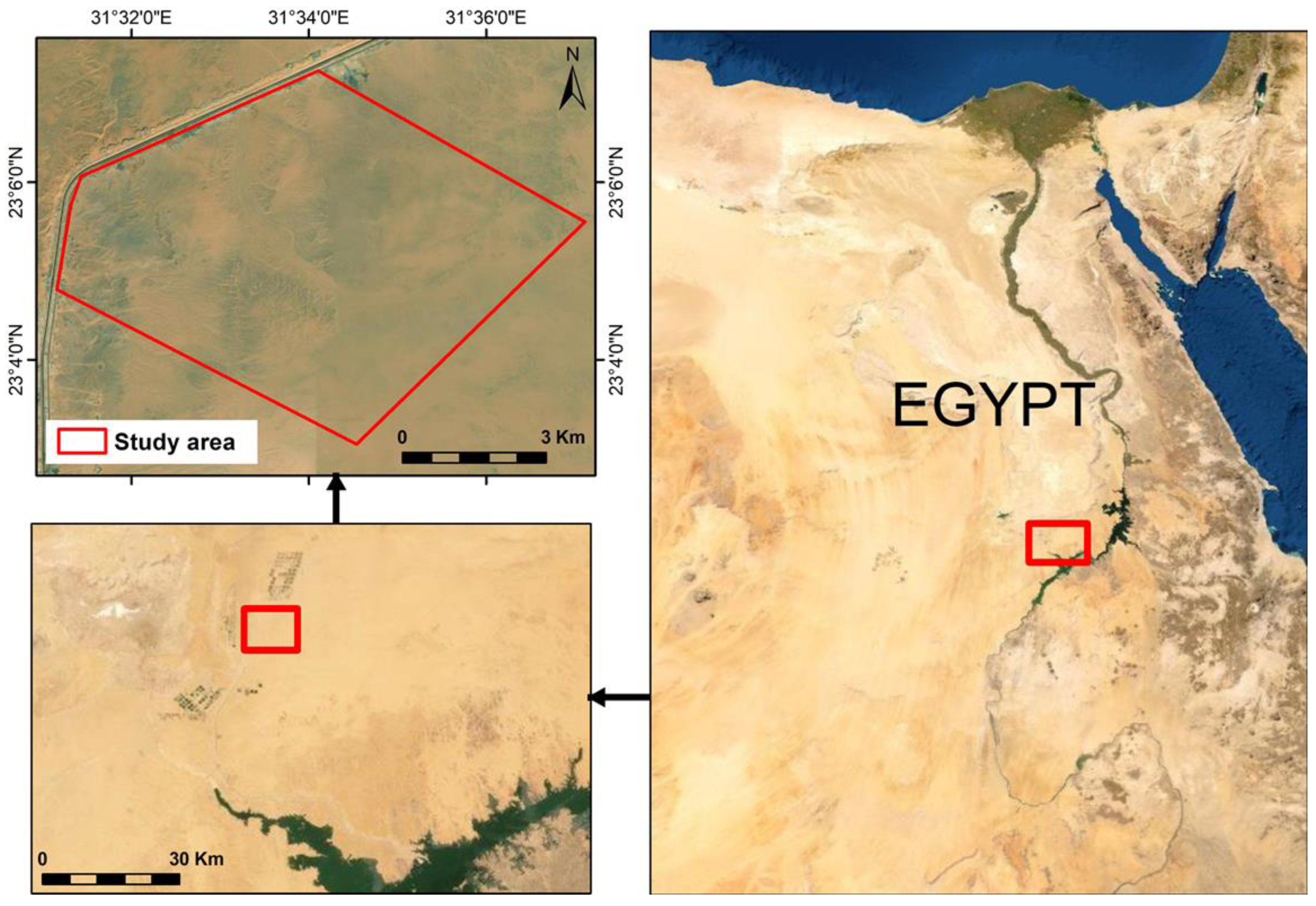
2.1. Description of the Study Area and Climatic Conditions
2.2. Soil Sample Collection and Preparation
2.3. Soil Chemical Properties Determination
3. Results
3.1. Particle Size Distribution and Soil Texture
3.2. Soil Chemical Properties
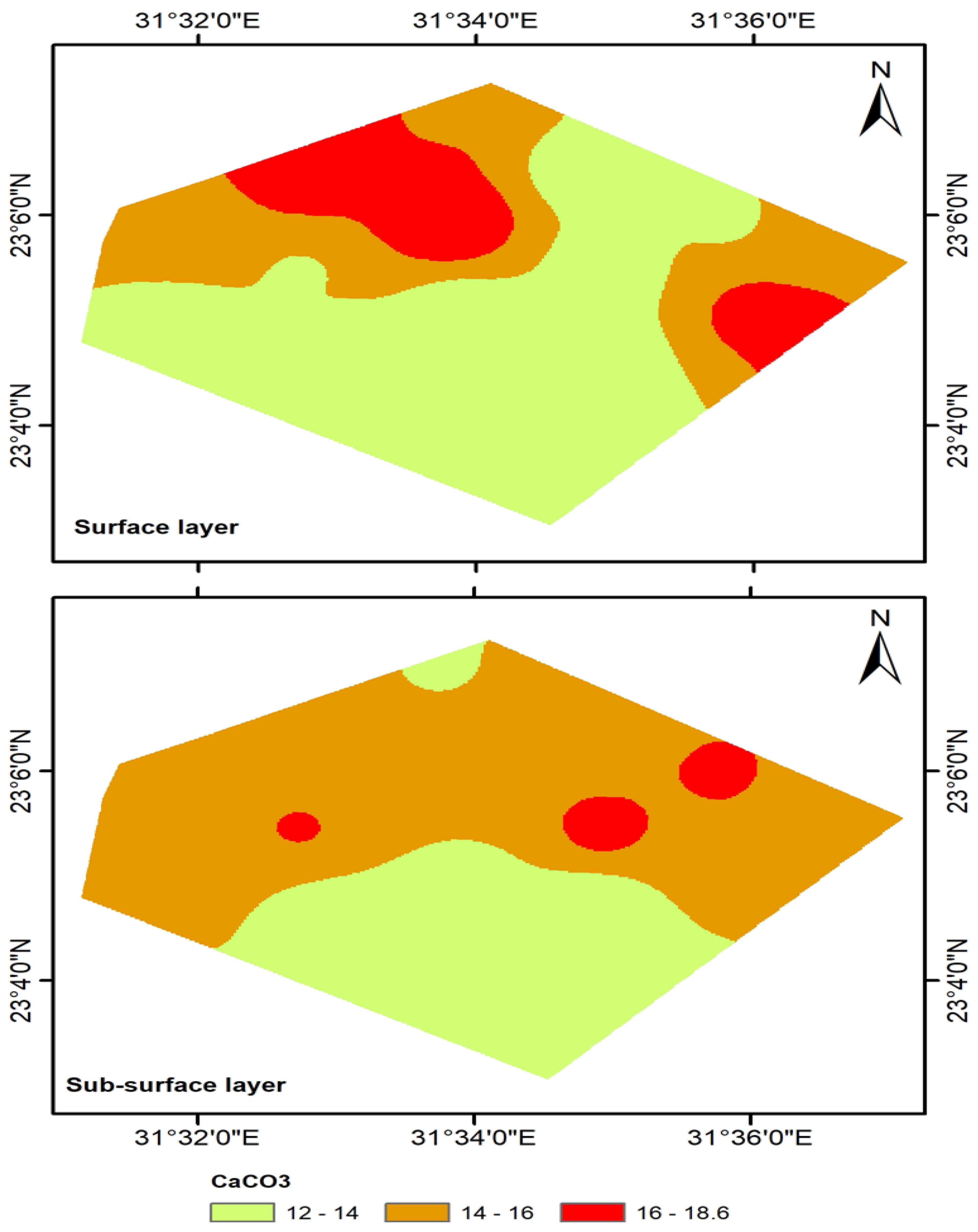
3.2.1. Soil pH, CaCO3 Content, Soil Organic Matter, and Cation Exchange Capacity
3.2.2. Soil Fertility Status
3.2.3. Correlation Coefficient between Soil Texture and Soil Chemical Properties
3.2.4. Stepwise Multi-Regression Analysis between Soluble Ions and ECe
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Declaration of the World Summit on Food Security; WSFS 2009/2; FAO: Rome, Italy, 2009. [Google Scholar]
- Von Grebmer, K.; Torero, M.; Olofinbiyi, T.; Fritschel, H.; Wiesmann, D.; Yohannes, Y. Global Hunger Index: The Challenge of Hunger: Taming Price Spikes and Excessive Food Price Volatility; International Food Policy Research Institute: Washington, DC, USA, 2011. [Google Scholar]
- Awad, A.A.M.; Rady, M.M.; Semida, W.M.; Belal, E.E.; Omran, W.M.; Al-Yasi, H.M.; Ali, E.F. Foliar Nourishment with Different Zinc-Containing Forms Effectively Sustains Carrot Performance in Zinc-Deficient Soil. Agronomy 2021, 11, 1853. [Google Scholar] [CrossRef]
- FAO. Evaluation of FAO’s Contribution to the Arab Republic of Egypt 2012–2017; FAO: Rome, Italy, 2018. [Google Scholar]
- Sultana, M.S.; Rahman, M.H.; Rahman, M.S.; Sultana, T.; Paul, A.K. Postharvest soil fertility status of rice (Hybrid Dhan Hira 2) as influenced by vermicompost, pressmud and urea. Int. J. Sci. Res. Publ. 2016, 6, 335–340, ISSN 2250-3153. [Google Scholar]
- Abd El-Hady, A.M.; Abdelaty, E.F. GIS-comprehensive analytical approach for soil use by linking crop soil suitability to soil management and reclamation. Alex. Sci. Exch. J. 2019, 40, 60–81. [Google Scholar] [CrossRef] [Green Version]
- Abdelazem, A.H.; Khozyem, H.M.; Gamee, M.A.; Awad, A.A.M.; Mohamed, A.G. Assessment of some Physical and Chemical Properties of Soils in West Edfu Area, Aswan Governorate, Egypt. Assiut J. Agric. Sci. 2020, 51, 150–170. [Google Scholar]
- Awad, A.A.M.; El-Taib, A.B.A.; Sweed, A.A.A.; Omran, A.A.M. Nutrient contents and productivity of Triticum aestivum plants grown in clay loam soil depending on humic substances and varieties and their interactions. Agronomy 2022, 12, 705. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Elgharably, G.A.; Rabie, M.H. Evaluation of Soil Fertility Status in Toshka, Egypt: Available Micronutrients. World J. Agric. Sci 2019, 15, 1–6. [Google Scholar]
- Mamun, M.A.A.; Haque, M.M.; Khaliq, Q.A.; Karim, A.J.M.S.; Mridha, A.J.; Saleque, M.A. Assessment of soil chemical properties and rice yield in tidal submergence ecosystem. Bangladesh Agron. J. 2015, 18, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Shi, R.Y.; Li, J.; Ni, N.; Mehmood, K.; Xu, R.; Qian, W. Effects of biomass ash, bone meal, and alkaline slag applied alone and combined on soil acidity and wheat growth. J. Soils Sediments 2017, 17, 2116–2126. [Google Scholar] [CrossRef]
- Fadl, M.E.; Abuzaid, A.S. Assessment of land suitability and water requirements for different crops in Dahrla Oasis, Western desert, Egypt. Int. J. Plant Soil Sci. 2017, 16, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Aziz, S.H. Soil capability and suitability assessment of Tushka area, Egypt by using different programs (ASLE, Microleis and modified STORIE index). Malays. J. Sustain. Agric. 2018, 2, 9–15. [Google Scholar] [CrossRef]
- Awad, A.A.M.; Sweed, A.A.A. Influence of organic manures on soil characteristics and yield of Jerusalem artichoke. Commun. Soil Sci. Plant Anal. 2020, 51, 1101–1113. [Google Scholar] [CrossRef]
- Jin, J.; Xu, Y.; Ye, H.; Shen, C.; Huang, Y. Effect of land use and soil management practices on soil fertility quality in North China Cities’ urban fringe. Afr. J. Agric. Res. 2011, 6, 2059–2065. [Google Scholar]
- Salem, M.Z.; Ageeb, G.W.; Rahim, I.S. Land suitability for agricultural of certain crops in Albostan area, Egypt. Res. J. Agric. Biol. Sci. 2008, 4, 485–499. [Google Scholar]
- NajafiGhiri, M.; Abtahi, A.; Jaberian, F.; Owliaie, H.R. Relationship between soil potassium forms and mineralogy in highly calcareous soils of southern Iran. Aust. J. Basic Appl. Sci. 2010, 4, 434–441. [Google Scholar]
- Pieri, C.; Dumanski, J.; Hamblin, A.; Young, A. Land Quality Indicators; World Bank Discussion Papers, 315; World Bank: Washington, DC, USA, 1996. [Google Scholar]
- Toth, G.; Stolbovoy, V.; Montanarella, L. Soil Quality and Sustainability Evaluation—An Integrated Approach to Support Soil-Related Policies of the EUROPEAN Union; Office for Official Publications of the European Communities: Luxembourg, 2007; 40p. [Google Scholar]
- Williams, H.; Colombi, T.; Keller, T. The Influence of Soil Management on Soil Health: An On-Farm Study in Southern Sweden. Geoderma 2020, 360, 114010. [Google Scholar] [CrossRef]
- Kinoshita, R.; Moebius-Clune, B.N.; Vanes, H.M.; Hively, W.D.; Bilgilis, A.V. Strategies for soil quality assessment using visible and near-infrared reflectance spectroscopy in a Western Kenya Chronosequence. Soil Sci. Soc. Am. J. 2012, 76, 1776–1788. [Google Scholar] [CrossRef]
- Li, N.; Chen, G.; Zeng, G.; Ye, J. The study of soil fertility spatial variation feature based on GIS and data mining. In International Conference on Computer and Computing Technologies in Agriculture; Springer: Berlin/Heidelberg, Germany, 2012; pp. 211–220. [Google Scholar]
- Zornoza, R.; Acosta, J.A.; Bastida, F.; Domínguez, S.G.; Toledo, D.M.; Faz, A. Identification of sensitive indicators to assess the interrelationship between soil quality, management practices and human health. Soil J. 2015, 1, 173–185. [Google Scholar] [CrossRef] [Green Version]
- Carter, M.R. Soil quality for sustainable land management: Organic matter and aggregation interactions that maintain soil functions. Agron. J. 2002, 94, 38–47. [Google Scholar] [CrossRef]
- Ali, M.E.; El-Husseiny, O.H.M.; Rashed, H.S.A.; Mohamed, E.S.; Salama, O.H.E. Assessment of soil quality using remote sensing and GIS techniques in some areas of North-East Nile delta, Egypt. Egypt. J. Soil Sci. 2015, 55, 621–638. [Google Scholar]
- Elnaggar, A.A.; Mosa, A.A.; El-Shebiny, G.; El-Seedy, M.E.; El-Bakry, F.A. Evaluation of Soil Fertility by Using GIS Techniques for Some Soils of Dakahlia Governorate, Egypt. J. Soil Sci. Agric. Eng. Mansoura Univ. 2016, 7, 713–720. [Google Scholar] [CrossRef] [Green Version]
- Hamed, M.H.; Khalafallh, M.Y. Available nutrients and some soil properties of El-Qasr soils, El-Dakhla Oasis, Egypt. Int. J. Environ. Agric. Biotechnol. 2017, 2, 4243–4249. [Google Scholar] [CrossRef] [Green Version]
- Elwa, A.M.; Abou-Shady, A.M.; Sayed, A.; Showman, H. Impact of physical and chemical properties of soil on the growing plant in El Mounira-El Qattara New Valley. Egypt. J. Appl. Sci. 2021, 36, 148–175. [Google Scholar]
- Bouyoucos, C.J. Hydrometer method improved for making particle size analysis of soil. Soil Sci. Soc. Proc. 1981, 26, 446–465. [Google Scholar]
- McLean, E.O. Soil pH and lime requirement. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 199–224. [Google Scholar]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Method of Soil Analysis. Part 2. Chemical and Microbiological Methods; American Society of Agronomy: Madison, WI, USA, 1982; pp. 225–246. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis; International Science Publishers Inc.: New York, NY, USA, 1947. [Google Scholar]
- Walkey, A.; Black, C.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chronic acidification method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommeres, C.E. Total Carbon, Organic Carbon and Organic Matter. In Method of Soil Analysis. Part 2, 2nd ed.; Page, A.L., Ed.; Agronomy Monographs; Soil Science Society of America: Madison, WI, USA, 1982; pp. 539–571. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis, 1st ed.; Prentice Hall of India Pvt. Ltd.: New Delhi, India, 1973. [Google Scholar]
- Reeuwijk, L.P. Procedures for Soil Analysis, 6th ed.; Technical Paper 9; International Soil Reference and Information Centre & Food and Agricultural Organization of the United Nations: Wageningen, The Netherlands, 2002. [Google Scholar]
- Subbiah, B.; Asija, C.L. A rapid procedure for the estimation of available nitrogen in soils. Curr. Sci. 1956, 25, 256–260. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of DTPA soil test for zinc, iron, manganese and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Baird, R.B.; Eaton, E.D.; Rice, E.W. Standard Methods for the Examination of Water and Waste Water, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Bonfante, A.; Basile, A.; Bouma, J. Targeting the Soil Quality and Soil Health Concepts When Aiming for the United Nations Sustainable Development Goals and the EU Green Deal. Soil 2020, 6, 453–466. [Google Scholar] [CrossRef]
- Imran, M.; Khan, A.; Hassan, A.; Kanwal, F.; Liviu, M.; Amir, M.; Iqbal, M.A. Evaluation of physico-chemical characteristics of soil samples collected from Harrapa-Sahiwal (Pakistan). Asian J. Chem. 2010, 22, 4823–4830. [Google Scholar]
- Kiflu, A.; Beyene, S. Effects of different land use systems on selected soil properties in South Ethiopia. J. Soil Sci. Environ. Manag. 2013, 4, 100–107. [Google Scholar] [CrossRef]
- Mesfin, S.; Taye, G.; Desta, Y.; Sibhatu, B.; Muruts, H.; Mohammedbrhan, M. Short-term effects of bench terraces on selected soil physical and chemical properties: Landscape improvement for hillside farming in semi-arid areas of northern Ethiopia. Environ. Earth Sci. 2018, 77, 399. [Google Scholar] [CrossRef]
- Opeyemi, A.O.; Adewunmi, B.I.; Oluwas, A.I. Physical and chemical properties of soils in gambari forest reserve near ibadan, south western Nigeria. J. Bioresour. Manag. 2020, 7, 57–67. [Google Scholar] [CrossRef]
- Lema, B.; Mesfin, S.; Kebede, F.; Abraha, Z.; Fitiwy, I.; Haileselassie, H. Evaluation of soil physical properties of long-used cultivated lands as a deriving indicator of soil degradation, north Ethiopia. Phys. Geogr. 2019, 40, 323–338. [Google Scholar] [CrossRef]
- Thangasamy, A.; Naidu, M.V.S.; Ramavatharam, N.; Raghava, R.C. Characterization, classification and evaluation of soil resources in Sivagirimi crowatershed of Chittoor district in Andhra Pradesh for sustainable land use planning. J. Indian Soc. Soil Sci. 2005, 53, 11–21. [Google Scholar]
- Chivenge, P.P.; Murwira, H.K.; Giller, K.E.; Mapfumo, P.; Six, J. Long-term impact of reduced tillage and residue management on soil carbon stabilization: Implications for conservation agriculture on contrasting soils. Soil Tillage Res. 2007, 94, 328–337. [Google Scholar] [CrossRef]
- Fisher, R.F.; Binkley, D. Ecology and Management of Forest Soil; John Wiley & Soils Inc.: New York, NY, USA, 2000. [Google Scholar]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 13th ed.; Prentice-Hall Inc.: Hoboken, NJ, USA, 2002; p. 960. [Google Scholar]
- Faragallah, M.E.A. Relative Distribution of Certain Nutrients in Soils of the Nile Valley-Desert Interference Zone, East of Assiut city. Master’s Thesis, Faculty of Agriculture, Assiut University, Assiut, Egypt, 1995. [Google Scholar]
- Kusumandari, A.; Kusumawardani, F.; Subroto, S.A.; Wianti, K.F. Soil chemical and physical characteristics as a base for achieving sustainable forest land use in RPH Watugudel, KPH Ngawi, Jawa Timur. In Proceeding of the ICTA 2017 2nd International Conference on Tropical Agriculture, Yogyakarta, Indonesia, 16 November 2018. [Google Scholar]
- El-Sayed, M.A.; Abd El-Aziz, S.H.; El-Desoky, A.I.; Selmy, S.A.H. Pedomorphic features and soil classification of Gharb El-Mawhob area, ElDakhla Oasis, Western Desert, Egypt. Middle East J. Agric. Res. 2016, 5, 247–257. [Google Scholar]
- Richards, L.A. (Ed.) Diagnosis and Improvement of Saline and Alkaline Soils; Agriculture, 160, Handbook 60; US Department of Agriculture: Washington, DC, USA, 1954.
- Laekemariam, F.; Kibret, K.; Mamo, T.; Gebrekidan, H. Soil–plant nutrient status and their relations in maize-growing fields of Wolaita Zone, Southern Ethiopia. Commun. Soil Sci. Plant Anal. 2016, 47, 1343–1356. [Google Scholar] [CrossRef]
- Negasa, T.; Ketema, H.; Legesse, A.; Sisay, M.; Temesgen, H. Variation in soil properties under different land use types managed by smallholder farmers along the top sequence in southern Ethiopia. Geoderma 2017, 290, 40–50. [Google Scholar] [CrossRef]
- Vasu, D.; Singh, S.K.; Tiwary, P.; Chandran, P.; Ray, S.K.; Duraisami, V.P. Pedogenic processes and soil-land form relationships for identification of yield limiting properties. Soil Res. 2016, 55, 273–284. [Google Scholar] [CrossRef]
- Poggio, L.; Gimona, L. 3D mapping of soil texture in Scotland. Geoderma Reg. 2017, 9, 5–16. [Google Scholar] [CrossRef]
- Patil, P.L.; Kuligod, V.B.; Gundlur, S.S.; Katti, J.; Nagaral, I.N.; Shikrashetti, P.; Dasog, G.S. Soil fertility mapping in Dindur sub-watershed of Karnataka for site specific recommendations. J. Indian Soc. Soil Sci. 2016, 64, 381–390. [Google Scholar] [CrossRef]
- Dhotare, V.A.; Guldekar, V.D.; Bhoyar, S.M.; Ingle, S.N. Evaluation of soil nutrient index and their relation with soil chemical properties of Washim Road Farm of Dr.PDKV Akola, Maharashtra, India. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1773–1779. [Google Scholar] [CrossRef]
- Senjobi, B.A.; Ogunkunle, A.O. Effect of different land use types and their implications on land degradation and productivity in Ogun State, Nigeria. J. Agric. Biotechnol. Sustain. Dev. 2011, 3, 7–18. [Google Scholar]
- Kumar, R.; Tiku, A.K.; Mir, M.M. Effect of nitrogen, phosphorus and potassium on growth and yield of olive (Olea europea L.). Asian J. Hortic. 2009, 4, 52–54. [Google Scholar]
- Hazelton, P.; Murphy, B. Interpreting Soil Test Results: What Do All the Numbers Mean? CSIRO Publishing: Collingwood, VIC, Australia, 2007; 160p. [Google Scholar]
- Paz-Gonzalez, A.; Vieira, S.R.; Taboada Castro, M.T. The effect of cultivation on the spatial variability of selected properties of an umbric horizon. Geoderma 2000, 97, 273–292. [Google Scholar] [CrossRef]
- Maji, A.K.; Obi, R.G.P.; Thayalan, S.; Walke, N.J. Characteristics and classification of landforms and soils over basaltic terrain in sub-humid tropics of central India. J. Indian Soc. Soil Sci. 2005, 53, 154–162. [Google Scholar]
- Chude, V.O.; Malgwi, W.B.; Amapu, I.Y.; Ano, A.O. Manual on Soil Fertility Assessment; Federal Fertilizer Department, FAO and National program on Food Security: Abuja, Nigeria, 2011; p. 62. [Google Scholar]
- Wang, H.L.; Tian, C.Y.; Jiang, L.; Wang, L. Remediation of heavy metals contaminated saline soils: A halophyte choice? Environ. Sci. Technol. 2017, 48, 21–22. [Google Scholar] [CrossRef]
- Bharambe, P.R.; Kadam, S.G.; Shinde, S.D.; Shelke, D.K. Characterization of soils Majalgaon canal command area (Jayakwadi project stage II). J. Indian Soc. Soil Sci. 2001, 47, 749–754. [Google Scholar]
- Panday, D.; Maharjan, B.; Chalise, D.; Shrestha, R.K.; Twanabasu, B. Digital Soil Mapping in the Bara District of Nepal using Kriging Tool in ArcGIS. PLoS ONE 2018, 13, e0206350. [Google Scholar] [CrossRef]
- Kelepertzis, E. Accumulation of heavy metals in agricultural soils of Mediterranean; insights from Argolida basin, Peloponnese, Greece. Geoderma 2014, 221, 82–90. [Google Scholar] [CrossRef]
- Shukla, U.C.; Gupta, B.L. Response of Mn application and evaluation of chemical extractants to determine available Mn in some arid brown soils of Haryana. J. Indian Soc. Soil Sci. 1975, 23, 357–364. [Google Scholar]
- Sakal, R.; Singh, A.P.; Sinha, R.B. Effect of boron application on blackgram and chickpea production in calcareous soil. J. Indian Soc. Soil Sci. 1988, 36, 125–127. [Google Scholar]
- Wafaa, H.M. A yield quality and micronutrients uptake of carrot (Daucus carota L.) and some soil properties as affected by organic fertilizers and elemental sulphur application. Egypt. J. Soil Sci. 2013, 53, 537–554. [Google Scholar]
- Awad, A.A.M.; Ahmed, H.M.H. Response of Jerusalem artichoke plants grown under saline calcareous soil to application of different combined organic manures. Egypt J. Soil Sci. 2019, 59, 117–130. [Google Scholar] [CrossRef]
- Emam, S.M.; Awad, A.A.M. Impact of Plant Density and Humic Acid Application on Yield, Yield Components and Nutrient Uptakes of Sunflower (Helianthus annuus L.) Grown in a Newly Reclaimed Soil. J. Soil Sci. Agric. Eng. Mansoura Univ. 2017, 8, 635–642. [Google Scholar] [CrossRef]




| Month | Average Temperature | Average Humidity | Average Wind Speed | Average Precipitation | ||
|---|---|---|---|---|---|---|
| Max. | Min. | Mean | ||||
| January | 27.88 | 3.30 | 13.67 | 42.33 | 2.94 | 0.00 |
| February | 31.80 | 4.11 | 15.98 | 32.98 | 3.07 | 0.01 |
| March | 37.54 | 7.67 | 20.59 | 24.03 | 3.25 | 0.00 |
| April | 42.37 | 11.57 | 26.05 | 17.74 | 3.38 | 0.00 |
| May | 44.96 | 16.99 | 30.53 | 16.49 | 3.59 | 0.00 |
| June | 45.27 | 21.24 | 32.77 | 16.74 | 3.90 | 0.00 |
| July | 44.37 | 23.00 | 33.65 | 18.15 | 3.58 | 0.00 |
| August | 44.56 | 23.66 | 33.84 | 19.59 | 3.72 | 0.00 |
| September | 42.95 | 20.31 | 31.09 | 22.81 | 4.11 | 0.00 |
| October | 40.33 | 15.16 | 26.74 | 28.28 | 3.85 | 0.00 |
| November | 33.73 | 9.23 | 20.03 | 38.06 | 3.24 | 0.00 |
| December | 28.34 | 4.80 | 14.93 | 45.05 | 3.04 | 0.00 |
| Average | 46.24 | 2.72 | 25.03 | 26.84 | 3.47 | 0.00 |
| Position | Coordinates | Depth (cm) | ||||
|---|---|---|---|---|---|---|
| 0–30 | 30–60 | |||||
| Latitude N | Longitude E | Sample No. | Symbol | Sample No. | Symbol | |
| 1 | 23, 016366 | 31, 562427 | 1 | AA1 | 17 | aa1 |
| 2 | 23, 106825 | 31, 579489 | 2 | AA2 | 18 | aa2 |
| 3 | 23, 098874 | 31, 597729 | 3 | AA3 | 19 | aa3 |
| 4 | 23, 095585 | 31, 603224 | 4 | AA4 | 20 | aa4 |
| 5 | 23, 107061 | 31, 545507 | 5 | BB1 | 21 | bb1 |
| 6 | 23, 098458 | 31, 564112 | 6 | BB2 | 22 | bb2 |
| 7 | 23, 090853 | 31, 582186 | 7 | BB3 | 23 | bb3 |
| 8 | 23, 084447 | 31, 600490 | 8 | BB4 | 24 | bb4 |
| 9 | 23, 099278 | 31, 529625 | 9 | CC1 | 25 | cc1 |
| 10 | 23, 090910 | 31, 545520 | 10 | CC2 | 26 | cc2 |
| 11 | 23, 084391 | 31, 565033 | 11 | CC3 | 27 | cc3 |
| 12 | 23, 077438 | 31, 581355 | 12 | CC4 | 28 | cc4 |
| 13 | 23, 081814 | 31, 530447 | 13 | DD1 | 29 | dd1 |
| 14 | 23, 075473 | 31, 549800 | 14 | DD2 | 30 | dd2 |
| 15 | 23, 068928 | 31, 564684 | 15 | DD3 | 31 | dd3 |
| 16 | 23, 059622 | 31, 578320 | 16 | DD4 | 32 | dd4 |
| Depth (cm) | Sample No | Symbol | Particle Size Distribution | Texture | ||
|---|---|---|---|---|---|---|
| Sand | Silt | Clay | ||||
| (%) | ||||||
| 0–30 | 1 | AA1 | 53.6 | 27.1 | 19.3 | Sandy loam |
| 2 | AA2 | 70.4 | 20.3 | 9.3 | Sandy loam | |
| 3 | AA3 | 45.2 | 28.4 | 26.4 | Loam | |
| 4 | AA4 | 70.8 | 19.3 | 9.9 | Sandy loam | |
| 5 | BB1 | 72.5 | 18.6 | 8.9 | Sandy loam | |
| 6 | BB2 | 61.9 | 23.5 | 14.6 | Sandy loam | |
| 7 | BB3 | 47.5 | 27.2 | 25.3 | Sandy clay loam | |
| 8 | BB4 | 73.8 | 17.6 | 8.6 | Sandy loam | |
| 9 | CC1 | 72.5 | 19.0 | 8.5 | Sandy loam | |
| 10 | CC2 | 51.1 | 25.3 | 23.6 | Sandy clay loam | |
| 11 | CC3 | 45.7 | 28.2 | 26.1 | Loam | |
| 12 | CC4 | 70.1 | 20.3 | 9.6 | Sandy loam | |
| 13 | DD1 | 62.1 | 24.2 | 13.7 | Sandy loam | |
| 14 | DD2 | 59.5 | 24.1 | 16.4 | Sandy loam | |
| 15 | DD3 | 49.6 | 27.0 | 23.4 | Sandy clay loam | |
| 16 | DD4 | 73.3 | 17.4 | 8.9 | Sandy loam | |
| 30–60 | 17 | aa1 | 43.1 | 29.4 | 27.5 | Clay loam |
| 18 | aa2 | 69.8 | 19.1 | 11.2 | Sandy loam | |
| 19 | aa3 | 42.6 | 29.5 | 27.9 | Clay loam | |
| 20 | aa4 | 68.0 | 21.7 | 10.3 | Sandy loam | |
| 21 | bb1 | 70.1 | 20.4 | 9.5 | Sandy loam | |
| 22 | bb2 | 59.5 | 24.0 | 16.5 | Sandy loam | |
| 23 | bb3 | 48.9 | 26.5 | 24.6 | Sandy clay loam | |
| 24 | bb4 | 71.2 | 19.5 | 9.3 | Sandy loam | |
| 25 | cc1 | 73.7 | 18.4 | 7.9 | Sandy loam | |
| 26 | cc2 | 48.1 | 27.0 | 24.9 | Sandy clay loam | |
| 27 | cc3 | 43.3 | 29.5 | 27.2 | Clay loam | |
| 28 | cc4 | 69.9 | 20.8 | 9.3 | Sandy loam | |
| 29 | dd1 | 64.3 | 23.5 | 13.2 | Sandy loam | |
| 30 | dd2 | 51.5 | 26.1 | 22.4 | Sandy clay loam | |
| 31 | dd3 | 49.4 | 26.5 | 24.1 | Sandy clay loam | |
| 32 | dd4 | 70.8 | 19.3 | 9.9 | Sandy loam | |
| Depth (cm) | Sample No. | Symbol | Soil pH | CaCO3 | SOM | ECe | CEC |
|---|---|---|---|---|---|---|---|
| (%) | (dS·m−1) | (Cmolc·kg−1) | |||||
| 0–30 | 1 | AA1 | 7.96 | 15.80 | 0.19 | 6.50 | 16.30 |
| 2 | AA2 | 7.85 | 12.60 | 0.15 | 5.00 | 14.00 | |
| 3 | AA3 | 7.91 | 13.40 | 0.16 | 8.10 | 16.50 | |
| 4 | AA4 | 7.77 | 15.30 | 0.14 | 6.00 | 13.00 | |
| 5 | BB1 | 7.85 | 17.20 | 0.11 | 10.70 | 14.60 | |
| 6 | BB2 | 7.77 | 18.60 | 0.11 | 8.10 | 15.70 | |
| 7 | BB3 | 7.81 | 13.20 | 0.13 | 8.20 | 17.00 | |
| 8 | BB4 | 7.79 | 17.30 | 0.14 | 12.60 | 13.00 | |
| 9 | CC1 | 7.87 | 15.10 | 0.12 | 9.50 | 12.80 | |
| 10 | CC2 | 7.86 | 13.80 | 0.13 | 5.80 | 15.30 | |
| 11 | CC3 | 7.83 | 12.60 | 0.12 | 8.40 | 17.80 | |
| 12 | CC4 | 7.85 | 12.50 | 0.13 | 7.50 | 14.00 | |
| 13 | DD1 | 7.82 | 13.30 | 0.12 | 5.60 | 15.30 | |
| 14 | DD2 | 7.85 | 13.30 | 0.15 | 9.20 | 15.30 | |
| 15 | DD3 | 7.80 | 12.40 | 0.15 | 7.90 | 16.50 | |
| 16 | DD4 | 7.76 | 12.30 | 0.12 | 8.60 | 13.60 | |
| 30–60 | 17 | aa1 | 7.87 | 13.80 | 0.13 | 5.40 | 16.50 |
| 18 | aa2 | 7.81 | 14.80 | 0.11 | 4.30 | 12.90 | |
| 19 | aa3 | 7.97 | 16.50 | 0.19 | 12.50 | 17.00 | |
| 20 | aa4 | 7.86 | 14.80 | 0.12 | 8.20 | 14.20 | |
| 21 | bb1 | 7.81 | 15.10 | 0.13 | 6.60 | 13.30 | |
| 22 | bb2 | 7.99 | 15.80 | 0.17 | 9.40 | 16.40 | |
| 23 | bb3 | 7.25 | 16.80 | 0.16 | 12.60 | 18.60 | |
| 24 | bb4 | 7.81 | 15.20 | 0.12 | 10.30 | 12.60 | |
| 25 | cc1 | 7.83 | 14.20 | 0.11 | 7.60 | 12.20 | |
| 26 | cc2 | 7.91 | 16.20 | 0.15 | 10.20 | 15.60 | |
| 27 | cc3 | 8.19 | 13.50 | 0.15 | 10.30 | 18.60 | |
| 28 | cc4 | 7.81 | 11.80 | 0.11 | 7.60 | 13.80 | |
| 29 | dd1 | 7.87 | 14.50 | 0.13 | 9.10 | 14.40 | |
| 30 | dd2 | 8.10 | 12.60 | 0.12 | 8.30 | 14.60 | |
| 31 | dd3 | 7.85 | 11.30 | 0.12 | 7.20 | 18.00 | |
| 32 | dd4 | 7.82 | 11.70 | 0.13 | 7.80 | 12.60 | |
| Depth (cm) | Sample No. | Symbol | Macronutrients | Micronutrients | |||||
|---|---|---|---|---|---|---|---|---|---|
| AvN | AvP | AvK | AvFe | AvMn | AvZn | AvCu | |||
| (mg·kg−1) | |||||||||
| 0–30 | 1 | AA1 | 23.6 | 12.8 | 380.7 | 3.9 | 1.8 | 1.1 | 0.4 |
| 2 | AA2 | 21.4 | 9.4 | 223.2 | 2.9 | 1.5 | 0.9 | 0.3 | |
| 3 | AA3 | 29.6 | 12.9 | 227.8 | 3.6 | 1.6 | 0.9 | 0.2 | |
| 4 | AA4 | 18.7 | 11.6 | 244.7 | 2.2 | 1.8 | 0.7 | 0.5 | |
| 5 | BB1 | 19.3 | 6.8 | 206.7 | 2.7 | 1.4 | 0.6 | 1.4 | |
| 6 | BB2 | 31.4 | 7.6 | 264.2 | 3.2 | 1.4 | 1.1 | 1.4 | |
| 7 | BB3 | 14.8 | 6.3 | 239.8 | 3.6 | 1.5 | 1.3 | 1.5 | |
| 8 | BB4 | 19.5 | 7.5 | 236.5 | 3.8 | 1.8 | 0.9 | 1.8 | |
| 9 | CC1 | 14.5 | 5.3 | 246.2 | 3.4 | 1.6 | 0.9 | 0.4 | |
| 10 | CC2 | 21.6 | 6.2 | 274.5 | 3.5 | 1.3 | 1.2 | 0.3 | |
| 11 | CC3 | 21.8 | 5.7 | 239.6 | 3.6 | 1.5 | 0.7 | 0.2 | |
| 12 | CC4 | 31.3 | 7.2 | 216.8 | 3.6 | 1.7 | 1.3 | 0.2 | |
| 13 | DD1 | 21.1 | 5.9 | 271.1 | 3.2 | 1.3 | 1.1 | 0.4 | |
| 14 | DD2 | 27.9 | 11.2 | 266.2 | 3.2 | 1.5 | 1.1 | 0.4 | |
| 15 | DD3 | 28.3 | 8.0 | 236.5 | 3.6 | 1.5 | 1.1 | 0.2 | |
| 16 | DD4 | 33.3 | 7.6 | 221.2 | 3.4 | 1.8 | 1.3 | 0.3 | |
| 30–60 | 17 | aa1 | 17.2 | 7.3 | 336.8 | 3.1 | 1.4 | 1.0 | 0.3 |
| 18 | aa2 | 20.6 | 8.6 | 310.7 | 3.1 | 1.7 | 0.8 | 0.2 | |
| 19 | aa3 | 31.5 | 13.0 | 211.3 | 3.8 | 1.5 | 1.0 | 0.5 | |
| 20 | aa4 | 28.8 | 12.5 | 206.5 | 3.1 | 1.3 | 0.8 | 0.4 | |
| 21 | bb1 | 22.1 | 6.5 | 219.4 | 3.8 | 1.6 | 0.8 | 1.6 | |
| 22 | bb2 | 28.3 | 11.5 | 315.9 | 3.5 | 1.5 | 1.2 | 1.5 | |
| 23 | bb3 | 35.3 | 11.4 | 309.1 | 4.2 | 1.7 | 1.1 | 1.7 | |
| 24 | bb4 | 16.5 | 8.6 | 259.4 | 3.5 | 1.6 | 0.8 | 1.6 | |
| 25 | cc1 | 17.4 | 6.8 | 255.3 | 3.7 | 1.6 | 1.1 | 0.4 | |
| 26 | cc2 | 28.2 | 10.6 | 377.8 | 4.1 | 1.5 | 1.1 | 0.5 | |
| 27 | cc3 | 30.2 | 9.5 | 331.2 | 3.9 | 1.8 | 0.9 | 0.5 | |
| 28 | cc4 | 18.7 | 6.3 | 224.5 | 3.2 | 1.8 | 1.1 | 0.2 | |
| 29 | dd1 | 30.2 | 11.8 | 349.4 | 4.3 | 1.4 | 1.3 | 0.5 | |
| 30 | dd2 | 26.3 | 8.2 | 255.9 | 3.0 | 1.4 | 1.0 | 0.3 | |
| 31 | dd3 | 27.5 | 7.6 | 227.2 | 3.4 | 1.6 | 0.9 | 0.4 | |
| 32 | dd4 | 29.6 | 7.3 | 220.7 | 3.2 | 1.7 | 1.1 | 0.2 | |
| Parameter | Sand | Silt | Clay | pH | ECe | CaCO3 | SOM | CEC | AvN | AvP | AvK | AvFe | AvMn | AvZn | AvCu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (0–30 cm) | |||||||||||||||
| 1 | 1.00 | −0.978 ** | −0.993 ** | −0.338 | 0.251 | 0.326 | −0.328 | −0.922 ** | −0.068 | −0.145 | −0.314 | −0.463 | 0.349 | −0.167 | 0.365 |
| 2 | 1.00 | 0.947 ** | 0.405 | −0.328 | −0.301 | 0.381 | 0.932 ** | 0.091 | 0.196 | 0.413 | 0.445 | −0.369 | 0.174 | −0.311 | |
| 3 | 1.00 | 0.294 | −0.302 | −0.333 | 0.292 | 0.898 ** | 0.054 | 0.113 | 0.253 | 0.465 | −0.331 | 0.160 | −0.388 | ||
| 4 | 1.00 | −0.173 | −0.063 | 0.612 * | 0.271 | −0.098 | 0.395 | 0.516 * | 0.358 | −0.003 | −0.062 | −0.103 | |||
| 5 | 1.00 | 0.102 | −0.248 | −0.181 | −0.067 | −0.211 | −0.319 | 0.239 | 0.237 | −0.233 | −0.111 | ||||
| 6 | 1.00 | −0.148 | −0.978 ** | −0.197 | 0.039 | 0.223 | −0.184 | 0.051 | −0.366 | 0.302 | |||||
| 7 | 1.00 | −0.248 | 0.102 | 0.803 ** | 0.580 * | 0.303 | 0.426 | 0.084 | 0.195 | ||||||
| 8 | 1.00 | 0.142 | 0.055 | 0.279 | 0.416 | −0.419 | 0.123 | −0.487 | |||||||
| 9 | 1.00 | 0.304 | −0.051 | 0.201 | 0.126 | 0.419 | −0.394 | ||||||||
| 10 | 1.00 | 0.371 | −0.122 | 0.450 | −0112 | 0.241 | |||||||||
| 11 | 1.00 | 0.319 | −0.093 | 0.216 | 0.456 | ||||||||||
| 12 | 1.00 | 0.125 | 0.505 * | −0.299 | |||||||||||
| 13 | 1.00 | −0.004 | 0.309 | ||||||||||||
| 14 | 1.00 | −0.039 | |||||||||||||
| 15 | 1.00 | ||||||||||||||
| (30–60 cm) | |||||||||||||||
| 1 | 1.00 | −0.990 ** | −0.997 ** | −0.179 | −0.407 | −0.156 | −0.618 * | −0.886 ** | −0.461 | −0.347 | −0.377 | −0.236 | 0.147 | −0.100 | −0.542 * |
| 2 | 1.00 | 0.978 ** | 0.229 | 0.418 | 0.151 | 0.629 ** | 0.886 ** | 0.459 | 0.376 | 0.351 | 0.233 | −0.192 | 0.108 | 0.563 * | |
| 3 | 1.00 | 0.152 | 0.399 | 0.157 | 0.608 * | 0.879 ** | 0.458 | 0.329 | 0.388 | 0.237 | −0.123 | 0.095 | 0.528 * | ||
| 4 | 1.00 | −0.141 | −0.297 | 0.028 | −0.018 | −0.106 | −0.028 | 0.033 | 0.254 | −0.216 | −0.090 | 0.042 | |||
| 5 | 1.00 | 0.508 * | 0.730 ** | 0.475 | 0.623 ** | 0.652 ** | 0.084 | 0.629 ** | 0.020 | 0.294 | 0.703 ** | ||||
| 6 | 1.00 | 0.603 * | 0.180 | 0.239 | 0.660 ** | 0.350 | 0.551 * | −0.242 | 0.030 | 0.560 * | |||||
| 7 | 1.00 | 0.645 ** | 0.647 ** | 0.673 ** | 0.211 | 0.501 * | −0.073 | 0.295 | 0.693 ** | ||||||
| 8 | 1.00 | 0.608 * | 0.432 | 0.325 | 0.352 | 0.011 | 0.110 | 0.664 ** | |||||||
| 9 | 1.00 | 0.697 ** | 0.109 | 0.461 | −0.082 | 0.312 | 0.630 ** | ||||||||
| 10 | 1.00 | 0.244 | 0.426 | −0.437 | 0.214 | 0.737 ** | |||||||||
| 11 | 1.00 | 0.446 | −0.062 | 0.394 | 0.413 | ||||||||||
| 12 | 1.00 | 0.103 | 0.403 | 0.742 ** | |||||||||||
| 13 | 1.00 | −0.086 | −0.274 | ||||||||||||
| 14 | 1.00 | 0.364 | |||||||||||||
| 15 | 1.00 | ||||||||||||||
| Depth (cm) | Sample No. | Symbol | Cations | Anions | TSC/TSA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| K+ | Na+ | Ca++ | Mg++ | CO3−− | HCO3− | SO4−− | Cl− | ||||
| mmol L−1 | |||||||||||
| 0–30 | 1 | AA1 | 0.40 | 42.80 | 15.20 | 7.60 | 0.00 | 1.00 | 7.40 | 57.60 | 66.00 |
| 2 | AA2 | 0.20 | 32.00 | 12.00 | 5.80 | 0.00 | 0.80 | 6.10 | 43.10 | 50.00 | |
| 3 | AA3 | 0.30 | 54.50 | 17.30 | 10.30 | 0.00 | 0.80 | 6.10 | 75.50 | 82.40 | |
| 4 | AA4 | 0.30 | 31.70 | 15.50 | 12.50 | 0.00 | 1.00 | 5.70 | 53.30 | 60.00 | |
| 5 | BB1 | 0.40 | 65.70 | 25.30 | 15.60 | 0.00 | 0.90 | 8.50 | 97.60 | 107.00 | |
| 6 | BB2 | 0.20 | 55.10 | 16.40 | 9.30 | 0.00 | 0.70 | 7.30 | 73.00 | 81.00 | |
| 7 | BB3 | 0.30 | 54.20 | 17.30 | 10.20 | 0.00 | 0.80 | 6.00 | 75.20 | 82.00 | |
| 8 | BB4 | 0.40 | 89.50 | 22.60 | 14.40 | 0.00 | 1.30 | 9.80 | 115.80 | 126.90 | |
| 9 | CC1 | 0.30 | 66.10 | 18.60 | 10.00 | 0.00 | 1.30 | 9.50 | 84.20 | 95.00 | |
| 10 | CC2 | 0.20 | 38.30 | 12.40 | 7.10 | 0.00 | 0.60 | 5.20 | 52.20 | 58.00 | |
| 11 | CC3 | 0.30 | 53.00 | 24.20 | 11.50 | 0.00 | 0.90 | 7.00 | 81.10 | 89.00 | |
| 12 | CC4 | 0.20 | 46.90 | 18.60 | 9.30 | 0.00 | 1.10 | 7.40 | 66.50 | 75.00 | |
| 13 | DD1 | 0.20 | 38.20 | 12.20 | 7.10 | 0.00 | 0.60 | 5.00 | 52.10 | 57.70 | |
| 14 | DD2 | 0.30 | 57.30 | 27.40 | 14.50 | 0.00 | 1.20 | 8.10 | 90.20 | 99.50 | |
| 15 | DD3 | 0.20 | 49.50 | 18.10 | 9.50 | 0.00 | 1.10 | 7.30 | 68.90 | 77.30 | |
| 16 | DD4 | 0.30 | 52.50 | 22.10 | 11.20 | 0.00 | 1.00 | 7.90 | 77.20 | 86.10 | |
| 30–60 | 17 | aa1 | 0.30 | 34.10 | 13.20 | 6.40 | 0.00 | 1.00 | 6.40 | 46.60 | 54.00 |
| 18 | aa2 | 0.30 | 26.20 | 10.30 | 6.20 | 0.00 | 0.60 | 5.50 | 36.90 | 43.00 | |
| 19 | aa3 | 0.30 | 84.80 | 25.80 | 14.10 | 0.00 | 1.40 | 10.10 | 113.50 | 125.00 | |
| 20 | aa4 | 0.40 | 50.10 | 20.20 | 11.10 | 0.00 | 1.30 | 7.20 | 73.30 | 81.80 | |
| 21 | bb1 | 0.20 | 46.40 | 11.80 | 7.60 | 0.00 | 0.80 | 6.80 | 58.40 | 66.00 | |
| 22 | bb2 | 0.30 | 61.10 | 21.20 | 11.40 | 0.00 | 1.00 | 8.20 | 84.80 | 94.00 | |
| 23 | bb3 | 0.40 | 87.20 | 23.10 | 14.30 | 0.00 | 1.10 | 8.30 | 115.60 | 125.00 | |
| 24 | bb4 | 0.30 | 71.10 | 20.40 | 11.20 | 0.00 | 1.00 | 9.40 | 92.60 | 103.00 | |
| 25 | cc1 | 0.30 | 52.00 | 15.30 | 8.40 | 0.00 | 0.90 | 6.40 | 68.70 | 76.00 | |
| 26 | cc2 | 0.40 | 67.20 | 23.10 | 11.30 | 0.00 | 0.90 | 9.00 | 92.10 | 102.00 | |
| 27 | cc3 | 0.40 | 58.90 | 28.30 | 15.40 | 0.00 | 1.30 | 8.30 | 93.40 | 103.00 | |
| 28 | cc4 | 0.30 | 44.10 | 20.20 | 11.40 | 0.00 | 1.00 | 7.20 | 67.80 | 76.00 | |
| 29 | dd1 | 0.40 | 51.80 | 25.10 | 13.80 | 0.00 | 1.50 | 7.30 | 82.30 | 91.10 | |
| 30 | dd2 | 0.40 | 52.10 | 23.80 | 11.20 | 0.00 | 0.90 | 6.90 | 79.70 | 87.50 | |
| 31 | dd3 | 0.30 | 44.60 | 20.10 | 10.60 | 0.00 | 1.00 | 7.10 | 67.50 | 75.60 | |
| 32 | dd4 | 0.30 | 49.10 | 18.50 | 9.20 | 0.00 | 0.90 | 6.30 | 69.90 | 77.10 | |
| Depth (cm) | r | R2 | Adjusted R2 | SEE | Significance | Fitted Equation |
|---|---|---|---|---|---|---|
| 0–30 | 0.994 | 0.988 | 0.987 | 0.223 | *** | ECe = 0.141+0.097 TSC or TSA |
| 30–60 | 0.998 | 0.996 | 0.996 | 0.148 | *** | ECe = −0.120+0.101 TSC or TSA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Soghir, M.M.A.; Mohamed, A.G.; El-Desoky, M.A.; Awad, A.A.M. Comprehensive Assessment of Soil Chemical Properties for Land Reclamation Purposes in the Toshka Area, EGYPT. Sustainability 2022, 14, 15611. https://doi.org/10.3390/su142315611
Al-Soghir MMA, Mohamed AG, El-Desoky MA, Awad AAM. Comprehensive Assessment of Soil Chemical Properties for Land Reclamation Purposes in the Toshka Area, EGYPT. Sustainability. 2022; 14(23):15611. https://doi.org/10.3390/su142315611
Chicago/Turabian StyleAl-Soghir, Mostafa M. A., Ahmed G. Mohamed, Mohamed A. El-Desoky, and Ahmed A. M. Awad. 2022. "Comprehensive Assessment of Soil Chemical Properties for Land Reclamation Purposes in the Toshka Area, EGYPT" Sustainability 14, no. 23: 15611. https://doi.org/10.3390/su142315611
APA StyleAl-Soghir, M. M. A., Mohamed, A. G., El-Desoky, M. A., & Awad, A. A. M. (2022). Comprehensive Assessment of Soil Chemical Properties for Land Reclamation Purposes in the Toshka Area, EGYPT. Sustainability, 14(23), 15611. https://doi.org/10.3390/su142315611





