Elemental Composition of Palm Kernel Expeller Used as Supplementary Stock Fodder
Abstract
:1. Introduction
2. Materials and Methods
2.1. PKE Collection
2.2. Pasture Sampling
2.3. Pseudo-Total Element Concentrations
2.4. Total C and N
2.5. pH
2.6. Cattle Liver Data
2.7. Calculation of Nutrient Inputs to Soils through PKE
2.8. Statistical Analysis
3. Results and Discussion
3.1. Elements in Palm Kernel Expeller
3.1.1. Essential Plant or Animal Nutrients
Macronutrients
| PKE Batch | 1 (n = 5) | 2 (n = 5) + | 3 (n = 6) ǂ | 4 (n = 6) ǂ | 5 (n = 6) ǂ | Concentrations Reported from Other Sources | Pasture Concentration | Cattle Feed MTL ٨ | Cattle Requirements ‡ |
|---|---|---|---|---|---|---|---|---|---|
| C (%) | 47 (0.45) a | 49 (0.81) b | 49 (0.36) b | 48 (0.31) ab | 47 (0.26) ab | — | 43 ꭗ | — | — |
| N (%) | 2.2 (0.09) a | 2.1 (0.11) a | 2.0 (0.12) a | 2.2 (0.03) a | 2.1 (0.02) a | 2.2 ҂ 2.9 Ʇ | 2.0 ꭗ | — | — |
| P ** | 5251 (107) a | 5229 (90) a | 5923 (100) ab | 7265 (160) c | 6325 (330) b | 6500 Ⴡ ǁ 7900 ɣ 4800–7100 ǁ | 3404 (148) | 6000 | 3100 |
| K * | 17,034 (950) c | 18,927 (496) c | 6065 (45) ab | 6757 (187) b | 5834 (243) a | 8000 Ⴡ ǁ 7600–9300 ǁ | 22,428 (1265) | 20,000 | 10,000 |
| Mg ** | 5948 (277) b | 6209 (146) b | 2826 (24) a | 3083 (128) a | 2723 (107) a | 3000 Ⴡ ǁ 2700 ɣ 1600–3300 ǁ | 998 (48) | 6000 | 1400 |
| S * | 2038 (44) ab | 1916 (37) a | 2148 (27) ab | 2745 (92) c | 2325 (136) b | 1900–2300 ǁ | 2538 (105) | 3000 (high concentrate) 5000 (high forage) | 2000 |
| B | 18 (3.5) a | 20 (2) a | 24 (1.4) a | 21 (1.9) a | 20 (2.6) a | — | 6.4 (0.41) | 150 | — |
| Na | 280 (71) b | 63 (35) a | 145 (17) ab | 131 (18) a | 126 (14) a | 200 Ⴡ | 2226 (282) | — | 1200 |
| Cr | 4.8 (0.2) a | 5.5 (3.1) a | 2.5 (0.1) a | 4.0 (0.58) a | 2.3 (0.49) a | — | 0.90 (0.13) | 100 | — |
| Mn | 317 (7.6) a | 298 (9.3) a | 271 (0.92) a | 468 (40) b | 302 (31) a | 225 ɣ 132–340 ǁ | 119 (10) | 2000 | 25 |
| Fe ** | 2406 (174) c | 786 (29) b | 624 (49) ab | 624 (28) ab | 447 (107) a | 4.1 ɣ 835–6130 ǁ | 433 (97) | 500 | 40 |
| Co | 0.68 (0.07) c | 0.1 (0) ab | 0.07 (0.02) ab | 0.13 (0.02) b | 0.03 (0.02) a | — | 0.93 (0.16) | 25 | 0.06 |
| Cu * | 28 (0.43) b | 29 (0.61) b | 22 (0.32) a | 36 (1.1) c | 27 (1.8) b | 22 Ⴡ ǁ 29 ɣ 21–29 ǁ | 6.9 (0.28) | 40 ꟻ | 9–11 |
| Zn | 54 (0.65) bc | 47 (1.2) ab | 44 (0.53) a | 59 (1.4) c | 49 (3.1) ab | 77 ɣ 41–50 ǁ | 27 (1.5) | 500 | 25 |
| Mo | 0.42 (0.04) a | 0.42 (0.02) a | 0.33 (0.02) a | 0.35 (0.03) a | 0.43 (0.03) a | 0.70–0.79 ǁ | 0.43 (0.061) | 5 | — |
Micronutrients
3.1.2. Non-Essential Elements
3.2. Implications for Stock Fodder
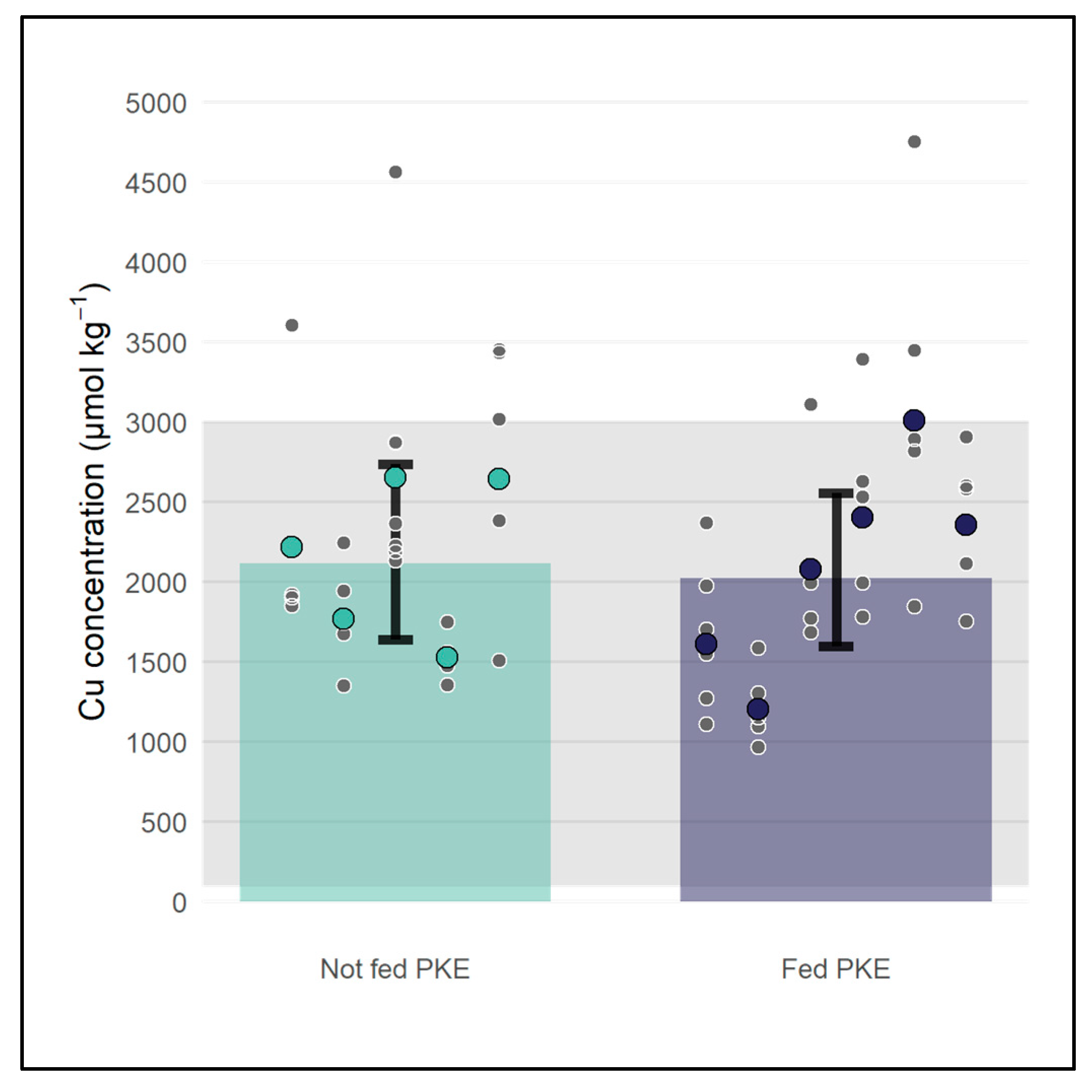
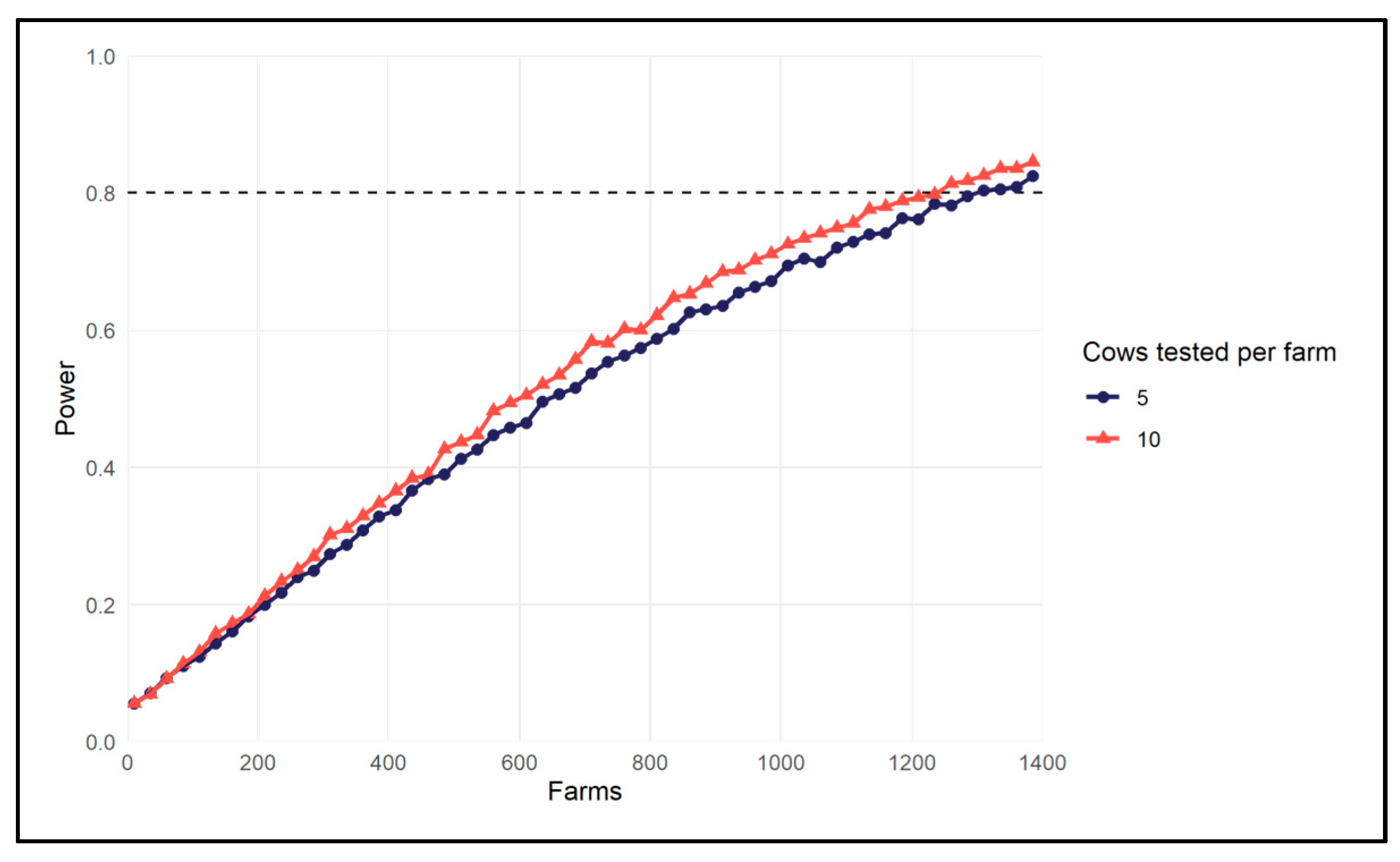
3.3. Pilot Study Using Dairy Cattle Liver Data
3.4. Elements in PKE as Soil Nutrient Inputs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Index Mundi. Palm Kernel Meal Imports by Country in 1000 MT. 2022. Available online: https://www.indexmundi.com/agriculture/?commodity=palm-kernel-meal&graph=imports (accessed on 10 May 2022).
- Index Mundi. Palm Kernel Meal Production by Country in 1000 MT. 2022. Available online: https://www.indexmundi.com/agriculture/?commodity=palm-kernel-meal&graph=production (accessed on 9 June 2022).
- DairyNZ. DairyNZ PKE Pricing Analysis; DairyNZ: Hamilton, New Zealand, 2020. [Google Scholar]
- MPI. Importation of Palm Kernel Expeller from Indonesia: MPI Audit Report; MPI: Wellington, New Zealand, 2015. [Google Scholar]
- Morgans, C.L.; Meijaard, E.; Santika, T.; Law, E.; Budiharta, S.; Ancrenaz, M.; Wilson, K.A. Evaluating the effectiveness of palm oil certification in delivering multiple sustainability objectives. Environ. Res. Lett. 2018, 13, 064032. [Google Scholar] [CrossRef] [Green Version]
- Grace, N.D.; Knowles, S.O. Trace element supplementation of livestock in New Zealand: Meeting the challenges of free-range grazing systems. Vet. Med. Int. 2012, 2012, 639472. [Google Scholar] [CrossRef]
- Grace, N.; Knowles, S.; Hittmann, A. High and variable copper status identified among dairy herds in the Waikato region by concentrations of Cu in liver sourced from biopsies and cull cows. N. Z. Vet. J. 2010, 58, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Satter, L.; Blohowiak, A.; Stauffacher, R.; Wilson, J. Milk production, estimated phosphorus excretion, and bone characteristics of dairy cows fed different amounts of phosphorus for two or three years. J. Dairy Sci. 2001, 84, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Thompson-Morrison, H.; Ariantiningsih, F.; Arief, S.M.; Gaw, S.; Robinson, B. Chemical elements in plants and oils from current and former palm oil production systems. Sci. Rep. 2022. submitted. [Google Scholar]
- Yeong, S. The nutritive value of palm kernel cake as a feedstuff for poultry. In Proceedings of the A Nutritional Workshop on Oil Palm by-Products Utilization, Kualalumpur, Malysia, 14–15 December 1981. [Google Scholar]
- Alimon, A.R. The nutritive value of palm kernel cake for animal feed. Palm Oil Dev. 2004, 40, 12–14. [Google Scholar]
- Hammid, A.A.; Kuntom, A.; Ismail, R.; Pardi, N. Arsenic in palm kernel expeller using microwave digestion and graphite furnace atomic absorption spectrophotometry method. Int. J. Basic Appl. Sci. 2013, 1, 641–649. [Google Scholar]
- National Research Council. Mineral Tolerance of Animals, 2nd ed.; National Academic Press: Washington, DC, USA, 2005. [Google Scholar]
- European Union. Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed-Council statement; European Union: Maastricht, The Netherlands, 2002. [Google Scholar]
- Thompson-Morrison, H.; Ariantiningsih, F.; Arief, S.M.; Gaw, S.; Robinson, B. Chemical elements in soils of current and former palm oil production systems. Geoderma Regional. 2022. submitted. [Google Scholar]
- Szydłowska-Czerniak, A.; Trokowski, K.; Karlovits, G.; Szłyk, E. Spectroscopic determination of metals in palm oils from different stages of the technological process. J. Agric. Food Chem. 2013, 61, 2276–2283. [Google Scholar] [CrossRef]
- Tangendjaja, B. Quality Control of Feed Ingredients for Aquaculture, in Feed and Feeding Practices in Aquaculture; Davis, D.A., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 141–169. [Google Scholar]
- US EPA. Method 3051A (SW-846): Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils, Revision 1; US EPA: Washington, DC, USA, 2007. [Google Scholar]
- NIST. Certificate of Analysis: Standard Reference Material 1573a Tomato Leaves; National Institute of Standards & Technology: Gaithersburg, MD, USA, 1995. [Google Scholar]
- NIST. Certificate of Analysis: Standard Reference Material 2706 New Jersey Soil; National Institute of Standards & Technology: Gaithersburg, MD, USA, 2018. [Google Scholar]
- NIST. Certificate of Analysis: Standard Reference Material 2710 Montana soil; National Institute of Standards & Technology: Gaithersburg, MD, USA, 2003. [Google Scholar]
- INCT. Polish Certified Reference Material for Multielement Trace Analysis: Oriental Basma Tobacco Leaves (INCT-OBTL-5); Institute of Nuclear Chemistry and Technology: Warszawa, Poland, 2010. [Google Scholar]
- LECO. Instrument: CN828 Determination of Carbon and Nitrogen in Soil; LECO Corportaion: St. Joseph, MI, USA, 2021. [Google Scholar]
- LECO. Instrument: CNS928 Determination of Carbon, Nitrogen and Sulfur in Plant Tissue; LECO Corportaion: St. Joseph, MI, USA, 2019. [Google Scholar]
- Blakemore, L.C.; Searle, P.L.; Daly, B.K. Methods for Chemical Analysis of Soils; New Zealand Soil Bureau Scientific Report; New Zealand Soil Bureau: Lower Hutt, New Zealand, 1987; p. 80. [Google Scholar]
- Index Mundi. New Zealand Palm Kernel Meal Imports by Year. 2022. Available online: https://www.indexmundi.com/agriculture/?country=nz&commodity=palm-kernel-meal&graph=imports (accessed on 1 June 2021).
- Stats NZ. Fertilisers—Nitrogen and Phosphorus. 2021. Available online: https://www.stats.govt.nz/indicators/fertilisers-nitrogen-and-phosphorus (accessed on 1 June 2022).
- FANZ. Fertiliser Use in NZ. 2018. Available online: https://www.fertiliser.org.nz/Site/about/fertiliser_use_in_nz.aspx#:~:text=exposure%20to%20cadmium-,Nitrogen%20use,form%20of%20nitrogen%20fertiliser%20used (accessed on 3 June 2022).
- R Core Team. R: A Language and Environment for Statistical Computing. 2021. Available online: https://www.R-project.org/ (accessed on 1 June 2022).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Hall, N.G.; Schönfeldt, H.C. Total nitrogen vs. amino-acid profile as indicator of protein content of beef. Food Chem. 2013, 140, 608–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donough, C.R.; Cahyo, A.; Wandri, R.; Fisher, M.; Oberthür, T. Plant nutrients in palm oil. Better Crops Plant Food 2016, 100, 19–22. [Google Scholar]
- Corley, R.H.V.; Tinker, P.B.H. The Oil Palm, 5th ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Woittiez, L.S.; Slingerland, M.; Giller, K.E. Yield gaps in Indonesian smallholder plantations: Causes and solutions. In Proceedings of the International Palm Oil Congress and Exhibition, Kuala Lumpur, Malaysia, 6–8 October 2015. [Google Scholar]
- Rafflegeau, S.; Michel-Dounias, I.; Tailliez, B.; Ndigui, B.; Papy, F. Unexpected N and K nutrition diagnosis in oil palm smallholdings using references of high-yielding industrial plantations. Agron. Sustain. Dev. 2010, 30, 777–787. [Google Scholar] [CrossRef] [Green Version]
- Law, C.C.; Zaharah, A.R.; Husni, M.H.A.; Siti Nor Akmar, A. Leaf nitrogen content in oil palm seedlings and their relationship to SPAD chlorophyll meter readings. J. Oil Palm Environ. Health 2014, 5, 8–17. [Google Scholar]
- Zhong, J.; Wang, X. Evaluation Technologies for Food Quality; Elsevier Science: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Battaglia, M.; Cruywagen, C.; Bertuzzi, T.; Gallo, A.; Moschini, M.; Piva, G.; Masoero, F. Transfer of melamine from feed to milk and from milk to cheese and whey in lactating dairy cows fed single oral doses. J. Dairy Sci. 2010, 93, 5338–5347. [Google Scholar] [CrossRef]
- DairyNZ. Farmfact: 1-71 Palm Kernel Extract (PKE); DairyNZ: Hamilton, New Zealand, 2008. [Google Scholar]
- Grace, N.D.; Knowles, S.O.; Sykes, A. ; New Zealand Society of Animal Production. Managing Mineral Deficiencies in Grazing Livestock; New Zealand Society of Animal Production: Palmerston North, New Zealand, 2010. [Google Scholar]
- Reiser, R.; Simmler, M.; Portmann, D.; Clucas, L.; Schulin, R.; Robinson, B. Cadmium concentrations in New Zealand pastures: Relationships to soil and climate variables. J. Environ. Qual. 2014, 43, 917–925. [Google Scholar] [CrossRef]
- DairyNZ. Common Feed Supplements. 2022. Available online: https://www.dairynz.co.nz/feed/supplements/common-feed-supplements/ (accessed on 2 June 2022).
- Kolade, O.O.; Coker, A.O.; Sridhar, M.K.C.; Adeoye, G.O. Palm kernel waste management through composting and crop production. J. Environ. Health Res. 2006, 5, 81–85. [Google Scholar]
- MPI. Imported Feed Commodities: ACVM (Imported Feed Commodities) Notice 2014; MPI: Wellingotn, New Zealand, 2014. [Google Scholar]
- MPOB. Chemical Fertiliser Ganocare™ as Preventive Treatment in Controlling Ganoderma Disease of Oil Palm; MPOB: Kajang, Malaysia, 2015. [Google Scholar]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer: Berlin, Germany, 2007. [Google Scholar]
- Abdollahi, M.; Hosking, B.; Ravindran, V. Nutrient analysis, metabolisable energy and ileal amino acid digestibility of palm kernel meal for broilers. Anim. Feed Sci. Technol. 2015, 206, 119–125. [Google Scholar] [CrossRef]
- Thompson-Morrison, H.; Gaw, S.; Robinson, B. An assessment of trace element accumulation in palm oil production. Sustainability 2022, 14, 4553. [Google Scholar] [CrossRef]
- Nokes, C. A Guide to the Ministry of Health Drinking-Water Standards for New Zealand; Environmental Science & Research Ltd.: Christchurch, New Zealand, 2008. [Google Scholar]
- Goselink, R.M.A.; Klop, G.; Dijkstra, J.; Bannink, A. Phosphorus Metabolism in Dairy Cattle: A Literature Study on Recent Developments and Gaps in Knowledge; Wageningen UR Livestock Research: Wageningen, The Netherlands, 2015. [Google Scholar]
- Crawford, G. Avoiding Mineral Toxicity in Cattle. 2007. Available online: https://fyi.extension.wisc.edu/wbic/files/2010/12/Factsheet_MineralToxicity.pdf (accessed on 20 August 2022).
- Ward, G.M. Potassium metabolism of domestic ruminants—A review. J. Dairy Sci. 1966, 49, 268–276. [Google Scholar] [CrossRef]
- Martín-Tereso, J.; Martens, H. Calcium and magnesium physiology and nutrition in relation to the prevention of milk fever and tetany (dietary management of macrominerals in preventing disease). Vet. Clin. North Am. : Food Anim. Pract. 2014, 30, 643–670. [Google Scholar] [CrossRef]
- Luckey, T.D.; Venugopal, B. Metal Toxicity in Mammals: Physiologic and Chemical Basis for Metal Toxicity; Springer: New York, NY, USA, 1977. [Google Scholar]
- Coup, M.R.; Campbell, A.G. The effect of excessive iron intake upon the health and production of dairy cows. N. Z. J. Agric. Res. 1964, 7, 624–638. [Google Scholar] [CrossRef]
- Oruc, H.H.; Uzunoglu, I.; Cengiz, M. Suspected iron toxicity in dairy cattle. Uludag Univ. J. Fac. Vet. Med. 2009, 28, 75–77. [Google Scholar]
- Phillippo, M.; Humphries, W.; Garthwaite, P. The effect of dietary molybdenum and iron on copper status and growth in cattle. J. Agric. Sci. 1987, 109, 315–320. [Google Scholar] [CrossRef]
- Phillippo, M.; Humphries, W.R.; Atkinson, T.; Henderson, G.D.; Garthwaite, P.H. The effect of dietary molybdenum and iron on copper status, puberty, fertility and oestrous cycles in cattle. J. Agric. Sci. 1987, 109, 321–336. [Google Scholar] [CrossRef]
- Hair-Bejo, M.; Davis, M.P.; Alimon, A.R.; Moonafizad, M. Chronic copper toxicosis: Utilization of palm kernel cake in sheep fed solely on concentrate diets. In Proceedings of the First Symposium on Integration of Livestock to Palm Oil Production, Kuala Lumpur, Malaysia, 22–27 May 1995. [Google Scholar]
- Jelsma, I.; Woittiez, L.S.; Ollivier, J.; Dharmawan, A.H. Do wealthy farmers implement better agricultural practices? An assessment of implementation of Good Agricultural Practices among different types of independent oil palm smallholders in Riau, Indonesia. Agric. Syst. 2019, 170, 63–76. [Google Scholar] [CrossRef]
- Ravensdown. Fertiliser Prices; Ravensdown: Christchurch, New Zealand, 2022. [Google Scholar]
- Abraham, E. Cadmium in New Zealand agricultural soils. N. Z. J. Agric. Res. 2020, 63, 202–219. [Google Scholar] [CrossRef]
- Rys, G.J. A National Cadmium Management Strategy for New Zealand Agriculture; Cadmium Working Group: Wellington, New Zealand, 2011. [Google Scholar]
- Vermeulen, V. Use of Zinc in Agriculture: An Assessment of Data for Evidence of Accumulation in Waikato Soils Surface Water and Sediments: A Thesis Presented in Partial Fulfillment of the Requirements for the Degree of Master of Health Science in Environmental Health at Massey University, Wellington, New Zealand; Massey University: Wellington, New Zealand, 2015. [Google Scholar]
- Selbie, D.R.; Buckthought, L.E.; Shepherd, M.A. The challenge of the urine patch for managing nitrogen in grazed pasture systems. Adv. Agron. 2015, 129, 229–292. [Google Scholar]
- University of Canterbury. New Kiwi Research to Turn Biowaste into Economic Boost; University of Canterbury: Christchurch, New Zealand, 2021. [Google Scholar]
- Mason, B.H.; Moore, C.B. Principles of Geochemistry; Wiley: Hoboken, NJ, USA, 1982. [Google Scholar]
| Batch | 1 (n = 5) | 2 (n = 5) | 3 (n = 6) + | 4 (n = 6) + | 5 (n = 6) ǂ | Concentrations Reported from other Sources | Pasture Concentration | Cattle Feed MTL |
|---|---|---|---|---|---|---|---|---|
| Al ** | 2767 (97) c | 867 (211) b | 420 (88) ab | 226 (34) a | 257 (82) a | 178 ٨ | 469 (127) | 1000 ‡ |
| Ti | 179 (13) e | 25 (0.95) d | 19 (1.5) c | 15 (0.38) b | 11 (0.51) a | — | — | — |
| Ni | 2.3 (0.05) ab | 1.8 (0.2) a | 1.4 (0.05) a | 4.2 (0.77) b | 1.6 (0.16) a | — | 0.77 (0.070) | 100 ‡ |
| As | 0.52 (0.05) b | 0.31 (0.04) ab | 0.29 (0.02) a | 0.20 (0.04) a | 0.24 (0.07) a | 0.18–3.1 ꭗ | 0.41 (0.030) | 30 ‡ 4 Ʇ |
| Sr | 26 (0.94) b | 12 (0.3) a | 11 (0.41) a | 21 (2.7) b | 11 (0.5) a | — | — | 2000 ‡ |
| Zr | 0.49 (0.06) c | 0.025 (0.02) a | 0.13 (0.02) b | 0.17 (0.01) b | 0.12 (0.02) ab | — | — | — |
| Ag | <0.01a | <0.01a | <0.01a | <0.01a | <0.01a | — | — | — |
| Cd | 0.024 (0.0024) bc | <0.01a | 0.023 (0.0021) bc | 0.027 (0.0021) c | 0.017 (0.0021) ab | — | 0.14 (0.014) | 10 ‡ 1 Ʇ |
| Sb | 0.018 (0.0012) a | 0.021 (0.0028) a | 0.021 (0.0030) a | 0.022 (0.0039) a | 0.014 (0.0021) a | — | — | — |
| Te | 0.014 (0.0012) c | <0.01b | <0.01ab | <0.01a | <0.01a | — | — | — |
| Cs | 1.1 (0.037) d | 0.80 (0.032) c | 0.43 (0.021) b | 0.22 (0.060) a | 0.35 (0.022) ab | — | — | — |
| Ce | 3.7 (0.28) b | 0.24 (0.024) a | 0.22 (0.060) a | 0.15 (0.022) a | 0.10 (0) a | — | — | — |
| Au | <0.01b | <0.01b | <0.01a | <0.01a | <0.01a | — | — | — |
| Hg | <0.01a | <0.01a | <0.01a | <0.01a | <0.01a | — | — | 2 ‡ 0.1 Ʇ |
| Pb | 1.2 (0.084) b | 0.35 (0.024) a | 0.44 (0.10) a | 0.30 (0.058) a | 0.27 (0.067) a | — | 0.37 (0.054) | 100 ‡ 10 Ʇ |
| Element | NZ Dairy Farm Fertiliser Use (t yr−1) | Mean NZ Imports through PKE (t yr−1) | Mean Fertiliser Equivalent Imports through PKE to NZ Dairy Farms (t yr−1) | Fertiliser Equivalent Cost (USD) |
|---|---|---|---|---|
| N | 284,760 | 39,258 | 14% | 77,879,500 |
| P | 55,440 | 11,097 | 20% | 38,500,729 |
| K | 73,360 | 20,208 | 28% | 21,756,711 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson-Morrison, H.; Moltchanova, E.; Gaw, S.; Robinson, B. Elemental Composition of Palm Kernel Expeller Used as Supplementary Stock Fodder. Sustainability 2022, 14, 15752. https://doi.org/10.3390/su142315752
Thompson-Morrison H, Moltchanova E, Gaw S, Robinson B. Elemental Composition of Palm Kernel Expeller Used as Supplementary Stock Fodder. Sustainability. 2022; 14(23):15752. https://doi.org/10.3390/su142315752
Chicago/Turabian StyleThompson-Morrison, Hadee, Elena Moltchanova, Sally Gaw, and Brett Robinson. 2022. "Elemental Composition of Palm Kernel Expeller Used as Supplementary Stock Fodder" Sustainability 14, no. 23: 15752. https://doi.org/10.3390/su142315752
APA StyleThompson-Morrison, H., Moltchanova, E., Gaw, S., & Robinson, B. (2022). Elemental Composition of Palm Kernel Expeller Used as Supplementary Stock Fodder. Sustainability, 14(23), 15752. https://doi.org/10.3390/su142315752






