Distribution Pattern and Structure of Vascular Plant Communities in Riparian Areas and Their Response to Soil Factors: A Case Study of Baoan Lake, Hubei Province, China
Abstract
1. Introduction
2. Materials and Methods
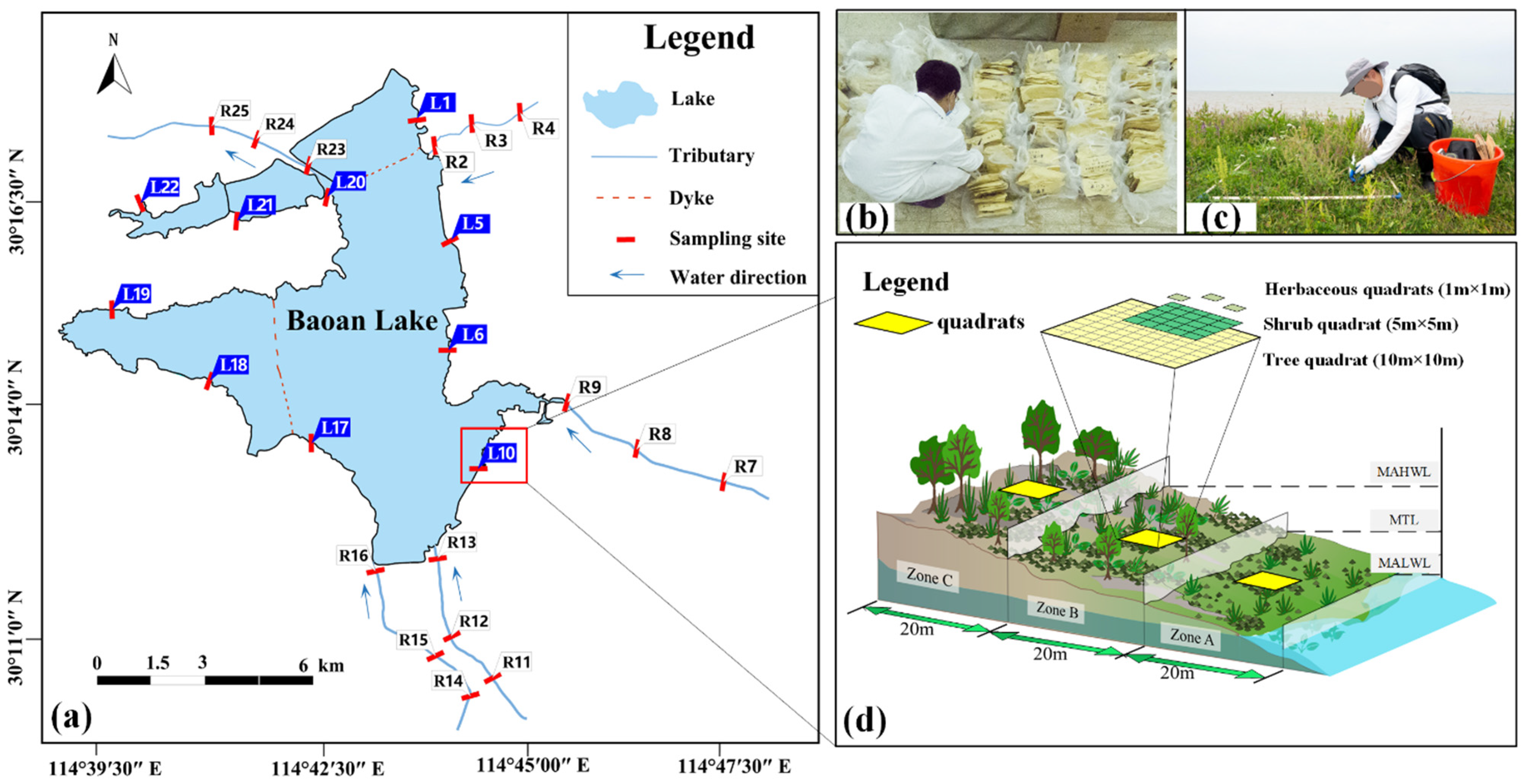
2.1. Study Area
2.2. Vegetation Investigation
2.3. Investigation and Measurement of Soil Factors
2.4. Data Analysis
3. Results
3.1. Soil Habitat Characteristics of Lakeshore and Tributary
3.2. Distribution and Composition of Vascular Plant Communities
3.3. Structural Characteristics of the Vascular Plant Community
4. Discussion
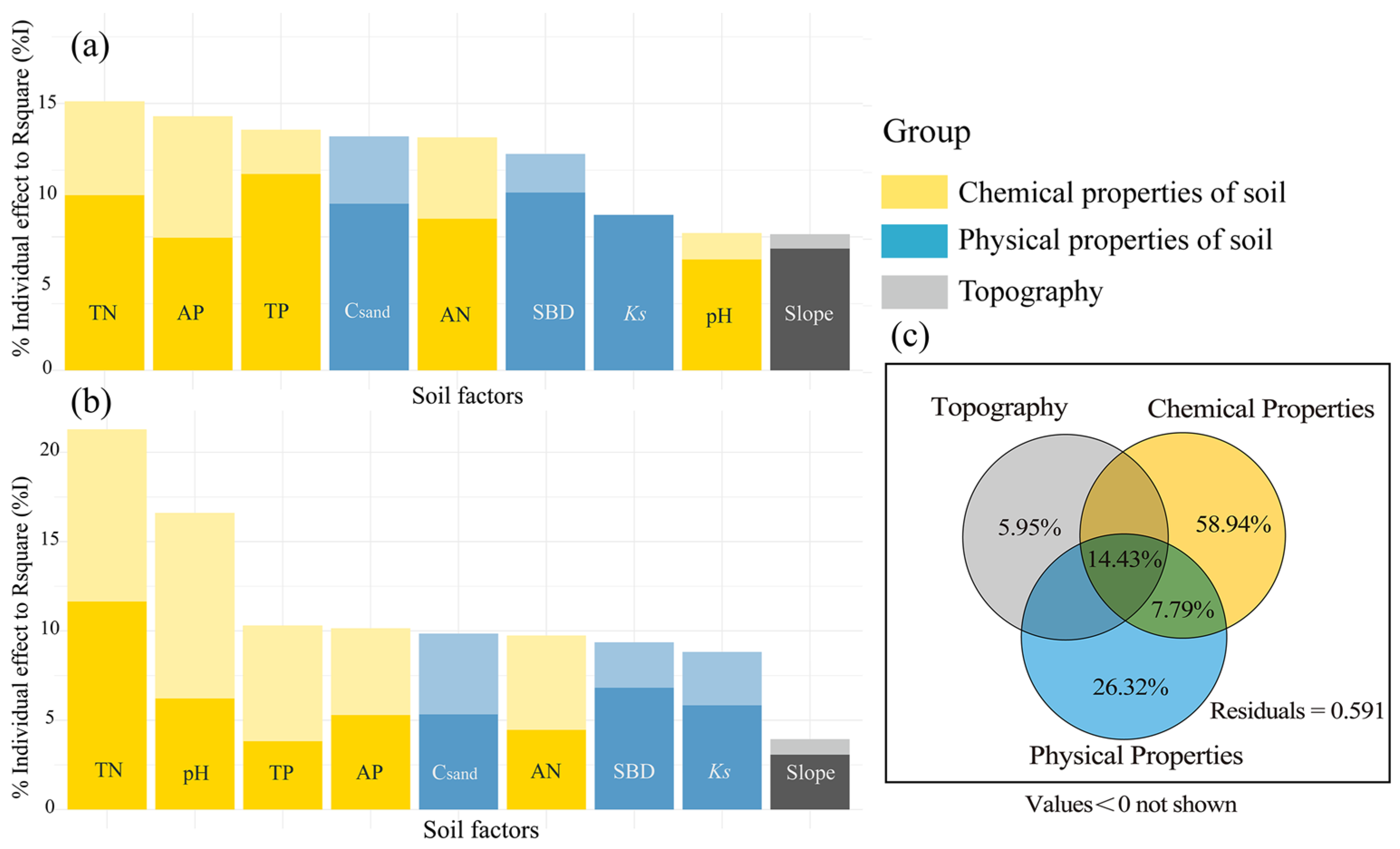
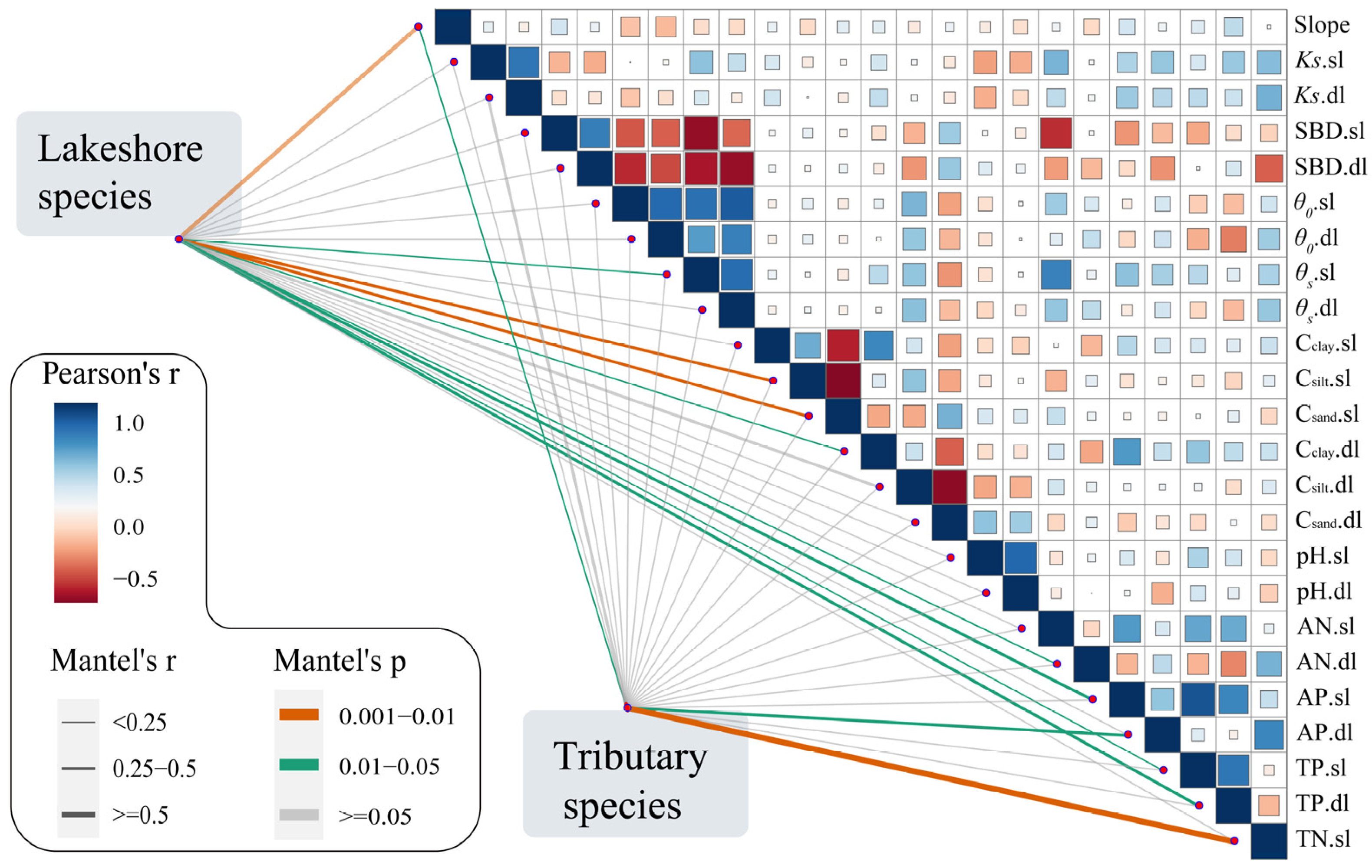
4.1. The Importance of Habitat Factors
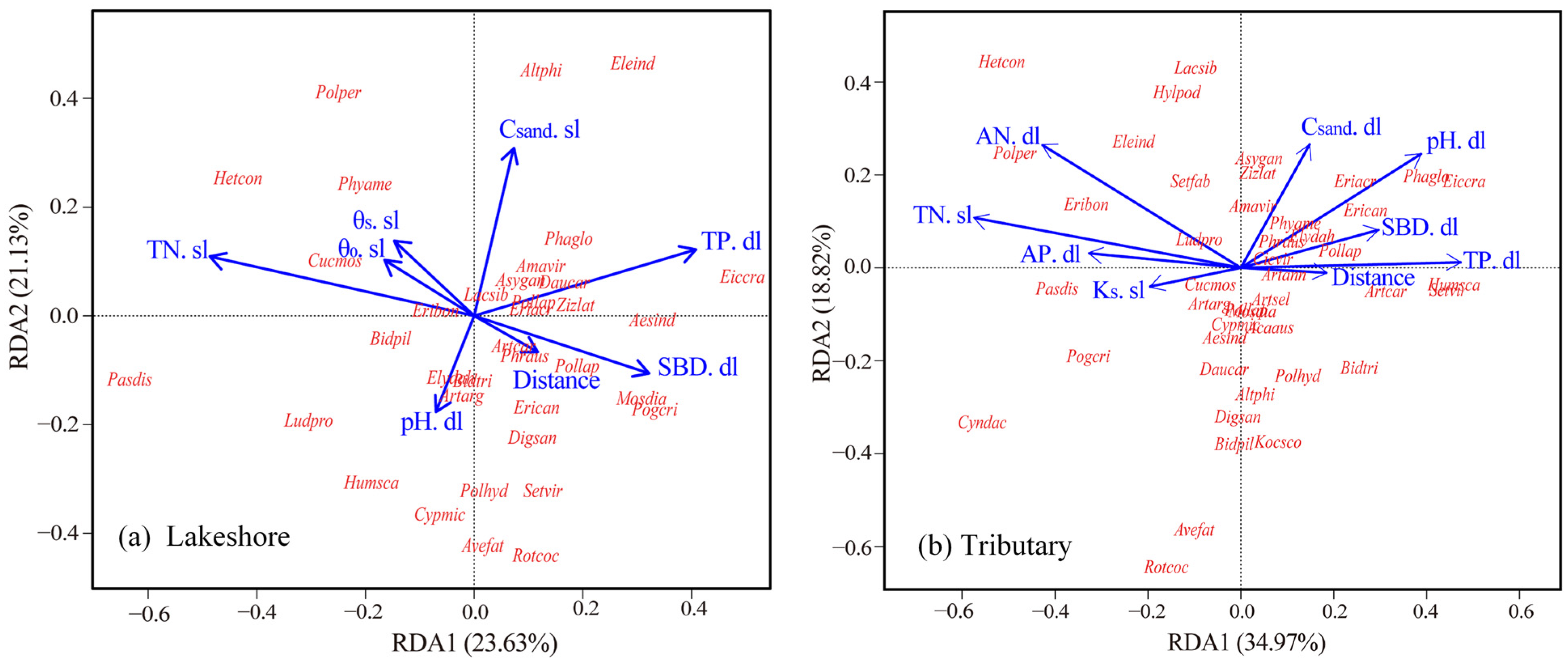
4.2. Distribution Pattern of Vascular Plant Communities in Response to Soil Factors
4.3. Community Structure of Vascular Plants in Response to Soil Factors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Abbreviated Code | Species | Abbreviated Code |
|---|---|---|---|
| Abutilon theophrasti Medicus | Abuthe | Kummerowia stipulacea (Maxim.) Makino | Kumsti |
| Acalypha australis L. | Acaaus | Kummerowia striata (Thunb.) Schindl. | Kumstr |
| Aeschynomene indica L. | Aesind | Lactuca sibirica (L.) Benth. ex Maxim. | Lacsib |
| Alternanthera philoxeroides (Mart.) Griseb. | Altphi | Leonurus pseudomacranthus Kitagawa | Leopse |
| Amaranthus viridis L. | Amavir | Leonurus sibiricus L. | Leosib |
| Artemisia annua L. | Artann | Lolium perenne L. | Lolper |
| Artemisia argyi Lévl. et Van. | Artarg | Ludwigia prostrata Roxb. | Ludpro |
| Artemisia caruifolia Buch.-Ham. ex Roxb. | Artcar | Melia azedarach L. | Melaze |
| Artemisia selengensis Turcz. ex Bess. | Artsel | Melochia corchorifolia L. | Melcor |
| Asystasia gangetica (L.) T. Anders. | Asygan | Morus alba L. | Moralb |
| Avena fatua L. | Avefat | Mosla dianthera (Buch.-Ham. ex Roxburgh) Maxim. | Mosdia |
| Bidens pilosa L. | Bidpil | Nelumbo nucifera Gaertn. | Nelnuc |
| Bidens tripartita L. | Bidtri | Oxalis corniculata L. | Oxacor |
| Broussonetia papyrifera (Linnaeus) L’Heritier ex Ventenat | Bropap | Pachyrhizus erosus (L.) Urb. | Pacero |
| Cicuta virosa L. | Cicvir | Paederia cruddasiana Prain | Paecru |
| Cinnamomum bodinieri Lévl. | Cinbod | Paspalum distichum Linnaeus | Pasdis |
| Cirsium vlassovianum Fisch. ex DC. | Cirvla | Phaenosperma globosa Munro ex Benth. | Phaglo |
| Cocculus orbiculatus (L.) DC. | Cocorb | Phragmites australis (Cav.) Trin. ex Steud. | Phraus |
| Cucumis melo var. agrestis Naud. | Cucmel | Phytolacca americana L. | Phyame |
| Cucurbita moschata (Duch. ex Lam.) Duch. ex Poiret | Cucmos | Pinus elliottii Engelmann | Pinell |
| Cynodon dactylon (L.) Pers. | Cyndac | Pogonatherum crinitum (Thunb.) Kunth | Pogcri |
| Cyperus difformis L. | Cypdif | Polygonum hydropiper L. | Polhyd |
| Cyperus haspan L. | Cyphas | Polygonum lapathifolium var. salicifolium Sibth. | Pollap |
| Cyperus microiria Steud. | Cypmic | Polygonum lapathifolium L. | Pollap |
| Daucus carota L. | Daucar | Polygonum perfoliatum L. | Polper |
| Digitaria sanguinalis (L.) Scop. | Digsan | Populus adenopoda Maxim. | Popade |
| Discocleidion rufescens (Franch.) Pax et Hoffm. | Disruf | Prunus × subhirtella (Miq.) Sok. | Prusub |
| Echinochloa caudata Roshev. | Echcau | Pterocarya stenoptera C. DC. | Pteste |
| Echinochloa crus-galli var. austrojaponensis Ohwi | Echcru | Rosa multiflora Thunb. | Rosmul |
| Eichhornia crassipes (Mart.) Solme | Eiccra | Rosa sertata Rolfa | Rosser |
| Eleusine indica (L.) Gaertn. | Eleind | Rottboellia cochinchinensis (Loureiro) Clayton | Rotcoc |
| Elymus dahuricus Turcz. | Elydah | Rumex acetosa L. | Rumace |
| Erigeron acris L. | Eriacr | Salix matsudana Koidz. | Salmat |
| Erigeron bonariensis L. | Eribon | Sesbania cannabina (Retz.) Poir. | Sescan |
| Erigeron canadensis L. | Erican | Setaria faberi R. A. W. Herrmann | Setfab |
| Fatoua villosa (Thunb.) Nakai | Fatvil | Setaria viridis (L.) Beauv. | Setvir |
| Glycine soja Siebold & Zucc. | Glysoj | Solanum americanum Miller | Solame |
| Heteropogon contortus (L.) P. Beauv. ex Roem. et Schult. | Hetcon | Torenia fordii Hook. f. | Torfor |
| Humulus scandens (Lour.) Merr. | Humsca | Trapa natans | Tranat |
| Hylodesmum podocarpum (Candolle) H. Ohashi & R. R. Mill | Hylpod | Triadica sebifera (Linnaeus) Small | Triseb |
| Ipomoea nil (Linnaeus) Roth | Iponil | Typha orientalis Presl | Typori |
| Kochia scoparia (L.) Schrad. | Kocsco | Vigna angularis (Willd.) Ohwi et Ohashi | Vigang |
| Koelreuteria paniculata Laxm. | Koepan | Vitex negundo var. cannabifolia (Sieb.et Zucc.) Hand.-Mazz. | Vitneg |
| Zizania latifolia (Griseb.) Stapf | Zizlat |
| Parameters | Layer | Lakeshore | Tributary | ||
|---|---|---|---|---|---|
| Mean (SE) | Min–Max | Mean (SE) | Min–Max | ||
| Ks(cm min−1) | 0–20 cm | 1.94 ± 0.36 | 0–6.38 | 1.10 ± 0.25 | 0–5.13 |
| 20–40 cm | 1.25 ± 0.31 | 0–5.89 | 0.74 ± 0.12 | 0–1.96 | |
| SBD (g cm−3) | 0–20 cm | 1.35 ± 0.03 | 1.14–1.60 | 1.40 ± 0.02 | 1.20–1.57 |
| 20–40 cm | 1.39 ± 0.02 | 1.22–1.60 | 1.45 ± 0.01 | 1.28–1.54 | |
| θ0 (g g−1) | 0–20 cm | 0.29 ± 0.01 | 0.15–0.41 | 0.26 ± 0.01 | 0.21–0.34 |
| 20–40 cm | 0.30 ± 0.02 | 0.15–0.45 | 0.25 ± 0.01 | 0.18–0.35 | |
| θS (g g−1) | 0–20 cm | 0.33 ± 0.01 | 0.23–0.43 | 0.31 ± 0.01 | 0.24–0.38 |
| 20–40 cm | 0.33 ± 0.01 | 0.24–0.45 | 0.30 ± 0.01 | 0.24–0.37 | |
| Cclay | 0–20 cm | 13.9 ± 0.43 | 9.49–17.03 | 13.28 ± 0.43 | 10.12–19.51 |
| 0–20 cm | 14.25 ± 0.26 | 11.18–15.61 | 13.62 ± 0.33 | 10.37–17.01 | |
| Csilt | 0–20 cm | 66.36 ± 0.39 | 61.66–68.77 | 66.20 ± 0.85 | 56.30–72.62 |
| 20–40 cm | 67.46 ± 0.39 | 63.30–70.42 | 67.37 ± 0.63 | 59.30–74.33 | |
| Csand | 20–40 cm | 19.27 ± 0.78 | 15.78–28.85 | 20.49 ± 1.14 | 7.87–33.16 |
| 20–40 cm | 18.39 ± 0.62 | 14.56–25.52 | 19.00 ± 0.75 | 13.04–29.27 | |
| pH | 0–20 cm | 6.47 ± 0.15 | 5.51–7.63 | 6.95 ± 0.09 | 5.51–7.78 |
| 20–40 cm | 6.62 ± 0.11 | 5.58–7.76 | 6.94 ± 0.09 | 5.51–7.78 | |
| AN (mg L−1) | 0–20 cm | 113.97 ± 2.94 | 90.81–135.81 | 114.31 ± 2.39 | 81.88–135.28 |
| 20–40 cm | 62.32 ± 0.94 | 56.35–72.90 | 61.84 ± 0.87 | 55.01–69.91 | |
| AP (mg L−1) | 0–20 cm | 14.22 ± 0.97 | 8.32–21.97 | 14.19 ± 1.82 | 1.16–35.42 |
| 20–40 cm | 8.86 ± 0.73 | 6.03–24.70 | 5.58 ± 0.35 | 0.58–8.37 | |
| TP (mg L−1) | 0–20 cm | 0.46 ± 0.01 | 0.36–0.56 | 0.57 ± 0.04 | 0.35–1.25 |
| 20–40 cm | 0.22 ± 0.01 | 0.13–0.38 | 0.37 ± 0.03 | 0.21–0.75 | |
| TN (mg L−1) | 0–20 cm | 1.57 ± 0.07 | 1.13–2.22 | 1.05 ± 0.03 | 0.75–1.33 |
| Distance (m) | / | 2.29 ± 0.54 | −0.50–11.00 | 2.71 ± 0.58 | −0.70–13.50 |
| Slope (◦) | / | 0.45 ± 0.05 | 0.01–0.80 | 0.45 ± 0.08 | 0.01–1.50 |
| Zones | Dominance | Lakeshore | Tributary | ||||
|---|---|---|---|---|---|---|---|
| Dominant Families | Dominant Species | Biomass | Dominant Families | Dominant Species | Biomass | ||
| A | First | Amaranthaceae | Altphi | 349.98 | Polygonaceae | Polhyd | 212.08 |
| 33.67% | 33.50% | 48.04% | 14.29% | ||||
| Second | Gramineae | Setvir | Amaranthaceae | Altphi | |||
| 26.73% | 13.71% | 42.64% | 48.97% | ||||
| B | First | Gramineae | Eleind | 352.61 | Polygonaceae | Polper | 295.03 |
| 62.63% | 13.91% | 51.13% | 8.84% | ||||
| Second | Amaranthaceae | Setvir | Gramineae | Artarg | |||
| 24.24% | 23.48% | 45.54% | 8.84% | ||||
| C | First | Gramineae | Eleind | 302.01 | Gramineae | Polper | 265.95 |
| 41.68% | 61.54% | 44.69% | 8.78% | ||||
| Second | Compositae | Bidtri | Polygonaceae | Elydah | |||
| 20.22% | 9.35% | 44.01% | 2.74% | ||||
References
- Naiman, R.J.; Decamps, H.; Pollock, M. The Role of Riparian Corridors in Maintaining Regional Biodiversity. Ecol. Appl. 1993, 3, 209–212. [Google Scholar] [CrossRef]
- Naiman, R.J.; Decamps, H. The Ecology of Interfaces: Riparian Zones. Annu. Rev. Ecol. Syst. 1997, 28, 621–658. [Google Scholar] [CrossRef]
- Sabo, J.L.; Sponseller, R.; Dixon, M.; Gade, K.; Harms, T.; Heffernan, J.; Jani, A.; Katz, G.; Soykan, C.; Watts, J.; et al. Riparian Zones Increase Regional Species Richness by Harboring Different, Not More, Species. Ecology 2005, 86, 56–62. [Google Scholar] [CrossRef]
- Kominoski, J.S.; Shah, J.J.F.; Canhoto, C.; Fischer, D.G.; Giling, D.P.; González, E.; Griffiths, N.A.; Larrañaga, A.; LeRoy, C.J.; Mineau, M.M.; et al. Forecasting Functional Implications of Global Changes in Riparian Plant Communities. Front. Ecol. Environ. 2013, 11, 423–432. [Google Scholar] [CrossRef]
- Osborne, L.L.; Kovacic, D.A. Riparian Vegetated Buffer Strips in Water-Quality Restoration and Stream Management. Freshw. Biol. 1993, 29, 243–258. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, F.; Lu, Z.; Zhang, M.; Zhang, L.; Hao, W.; Zhao, L.; Jiang, Y.; Gao, B.; Chen, R.; et al. Community Differentiation and Ecological Influencing Factors along Environmental Gradients: Evidence from 1200 Km Belt Transect across Inner Mongolia Grassland, China. Sustainability 2021, 14, 361. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Liu, Y.; Guo, H.; Chen, G.; Fu, Z.; Fu, Y.; Ge, G. Effects of Sediment Types on the Distribution and Diversity of Plant Communities in the Poyang Lake Wetlands. Diversity 2022, 14, 491. [Google Scholar] [CrossRef]
- Ledesma, J.L.J.; Futter, M.N.; Blackburn, M.; Lidman, F.; Grabs, T.; Sponseller, R.A.; Laudon, H.; Bishop, K.H.; Köhler, S.J. Towards an Improved Conceptualization of Riparian Zones in Boreal Forest Headwaters. Ecosystems 2018, 21, 297–315. [Google Scholar] [CrossRef]
- Henriques, M.; McVicar, T.R.; Holland, K.L.; Daly, E. Riparian Vegetation and Geomorphological Interactions in Anabranching Rivers: A Global Review. Ecohydrology 2022, 15, e2370. [Google Scholar] [CrossRef]
- Fagundes, N.C.A.; de Ávila, M.A.; de Souza, S.R.; de Azevedo, I.F.P.; Nunes, Y.R.F.; Fernandes, G.W.; Fernandes, L.A.; dos Santos, R.M.; Veloso, M.D.D.M. Riparian Vegetation Structure and Soil Variables in Pandeiros River, Brazil. Rodriguesia 2019, 70. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, W.; Daryanto, S.; Wang, L.; Fan, H.; Feng, Q.; Wang, Y. The Spatial Distribution and Temporal Variation of Desert Riparian Forests and Their Influencing Factors in the Downstream Heihe River Basin, China. Hydrol. Earth Syst. Sci. 2017, 21, 2405–2419. [Google Scholar] [CrossRef]
- Zhao, Q.; Ding, S.; Liu, Q.; Wang, S.; Jing, Y.; Lu, M. Vegetation Influences Soil Properties along Riparian Zones of the Beijiang River in Southern China. PeerJ 2020, 8, e9699. [Google Scholar] [CrossRef]
- Shen, R.; Lan, Z.; Chen, Y.; Leng, F.; Jin, B.; Fang, C.; Chen, J. The Effects of Flooding Regimes and Soil Nutrients on Lakeshore Plant Diversity in a Pristine Lake and a Human Managed Lake in Subtropical China. J. Freshw. Ecol. 2019, 34, 757–769. [Google Scholar] [CrossRef]
- Zhang, J.T. Quantitative Ecology, 2nd ed.; Science Press: Beijing, China, 2004. [Google Scholar]
- Reynolds, W.D.; Elrick, D.E.; Youngs, E.G.; Amoozegar, A.; Booltink, H.W.G.; Bouma, J. Saturated and field-saturated water flow parameters. In Methods of Soil Analysis; Part 4, Physical Methods; Dane, J.H., Topp, G.C., Eds.; Soil Science Society of America Inc.: Madison, WI, USA, 2002; pp. 797–878. [Google Scholar]
- Lu, R.K. Soil and Agricultural Chemistry Analysis; Chinese Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Zhou, X.; Tao, Y.; Yin, B.; Tucker, C.; Zhang, Y. Nitrogen Pools in Soil Covered by Biological Soil Crusts of Different Successional Stages in a Temperate Desert in Central Asia. Geoderma 2020, 366, 114166. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, D.; Chen, X.; Zhang, Y.; Maisupova, B.; Tao, Y. The Spatiotemporal Patterns of Vegetation Coverage and Biomass of the Temperate Deserts in Central Asia and Their Relationships with Climate Controls. Remote Sens. Environ. 2016, 175, 271–281. [Google Scholar] [CrossRef]
- Zhang, J.; Xi, Y.; Li, J. The Relationships between Environment and Plant Communities in the Middle Part of Taihang Mountain Range, North China. Community Ecol. 2007, 7, 155–163. [Google Scholar] [CrossRef]
- Strong, W.L. Biased Richness and Evenness Relationships within Shannon–Wiener Index Values. Ecol. Indic. 2016, 67, 703–713. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Levene, H. Robust tests for the equality of variances. In Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling; Olkin, I., Ed.; Stanford University Press: Palo Alto, CA, USA, 1960; pp. 278–292. [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: New York, NY, USA, 2003; ISBN 978-0-521-89108-0. [Google Scholar]
- Lai, J.; Zou, Y.; Zhang, J.; Peres-Neto, P.R. Generalizing Hierarchical and Variation Partitioning in Multiple Regression and Canonical Analyses Using the Rdacca.Hp R Package. Methods Ecol. Evol. 2022, 13, 782–788. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. Corrplot: Visualization of a Correlation Matrix 2016. Available online: https://CRAN.R-project.org/package=corrplot (accessed on 17 July 2022).
- Dufour, S.; Rodríguez-González, P.M.; Laslier, M. Tracing the Scientific Trajectory of Riparian Vegetation Studies: Main Topics, Approaches and Needs in a Globally Changing World. Sci. Total Environ. 2019, 653, 1168–1185. [Google Scholar] [CrossRef]
- Liu, S.; Hou, X.; Yang, M.; Cheng, F.; Coxixo, A.; Wu, X.; Zhang, Y. Factors Driving the Relationships between Vegetation and Soil Properties in the Yellow River Delta, China. CATENA 2018, 165, 279–285. [Google Scholar] [CrossRef]
- Zhang, Z.; Wan, C.; Zheng, Z.; Hu, L.; Feng, K.; Chang, J.; Xie, P. Plant Community Characteristics and Their Responses to Environmental Factors in the Water Level Fluctuation Zone of the Three Gorges Reservoir in China. Environ. Sci. Pollut. Res. 2013, 20, 7080–7091. [Google Scholar] [CrossRef]
- Zhang, X.; Nian, L.; Liu, X.; Li, X.; Adingo, S.; Liu, X.; Wang, Q.; Yang, Y.; Zhang, M.; Hui, C.; et al. Spatial–Temporal Correlations between Soil PH and NPP of Grassland Ecosystems in the Yellow River Source Area, China. Int. J. Environ. Res. Public Health 2022, 19, 8852. [Google Scholar] [CrossRef]
- Zhang, X.; Xiang, D.-Q.; Yang, C.; Wu, W.; Liu, H.-B. The Spatial Variability of Temporal Changes in Soil PH Affected by Topography and Fertilization. CATENA 2022, 218, 106586. [Google Scholar] [CrossRef]
- Ma, X.; Liu, M.; Li, Z. Changes in Microbial Properties and Community Composition in Acid Soils Receiving Wastewater from Concentrated Animal Farming Operations. Appl. Soil Ecol. 2015, 90, 11–17. [Google Scholar] [CrossRef]
- Adhikari, K.; Owens, P.R.; Ashworth, A.J.; Sauer, T.J.; Libohova, Z.; Richter, J.L.; Miller, D.M. Topographic Controls on Soil Nutrient Variations in a Silvopasture System. Agrosyst. Geosci. Environ. 2018, 1, 180008. [Google Scholar] [CrossRef]
- Jiao, S.; Zhang, M.; Wang, Y.; Liu, J.; Li, Y. Variation of Soil Nutrients and Particle Size under Different Vegetation Types in the Yellow River Delta. Acta Ecol. Sin. 2014, 34, 148–153. [Google Scholar] [CrossRef]
- Ohdo, T.; Takahashi, K. Plant Species Richness and Community Assembly along Gradients of Elevation and Soil Nitrogen Availability. AoB PLANTS 2020, 12, plaa014. [Google Scholar] [CrossRef]
- Bai, J.; Tang, H.; Chen, F.; Lou, Y. Functional Traits Response to Flooding Depth and Nitrogen Supply in the Helophyte Glyceria Spiculosa (Gramineae). Aquat. Bot. 2021, 175, 103449. [Google Scholar] [CrossRef]
- Xia, J.; Ren, J.; Zhang, S.; Wang, Y.; Fang, Y. Forest and Grass Composite Patterns Improve the Soil Quality in the Coastal Saline-Alkali Land of the Yellow River Delta, China. Geoderma 2019, 349, 25–35. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Wu, J.; Kong, Z.; Feinstein, L.M.; Ding, X.; Ge, G.; Wu, L. Bacterial and Fungal Community Composition and Functional Activity Associated with Lake Wetland Water Level Gradients. Sci. Rep. 2018, 8, 760. [Google Scholar] [CrossRef]
- Feng, W.; Santonja, M.; Bragazza, L.; Buttler, A. Shift in Plant-Soil Interactions along a Lakeshore Hydrological Gradient. Sci. Total Environ. 2020, 742, 140254. [Google Scholar] [CrossRef]
- Aguiar, F.C.; Segurado, P.; Martins, M.J.; Bejarano, M.D.; Nilsson, C.; Portela, M.M.; Merritt, D.M. The Abundance and Distribution of Guilds of Riparian Woody Plants Change in Response to Land Use and Flow Regulation. J. Appl. Ecol. 2018, 55, 2227–2240. [Google Scholar] [CrossRef]
- Capon, S.J. Flood Variability and Spatial Variation in Plant Community Composition and Structure on a Large Arid Floodplain. J. Arid Environ. 2005, 60, 283–302. [Google Scholar] [CrossRef]
- Ke, P.-J.; Miki, T. Incorporating the Soil Environment and Microbial Community into Plant Competition Theory. Front. Microbiol. 2015, 6, 1066. [Google Scholar] [CrossRef] [PubMed]
- Sanders, H.L. Marine Benthic Diversity: A Comparative Study. Am. Nat. 1968, 102, 243–282. [Google Scholar] [CrossRef]
- Li, C.; Lou, H.; Yang, S.; Li, X.; Zhang, J.; Pan, Z.; Zhang, Y.; Yi, Y.; Gong, J. Effect of Human Disturbances and Hydrologic Elements on the Distribution of Plant Diversity within the Shamu Watershed, Mt. Yuntai Nature Reserve, China. J. Environ. Manag. 2022, 311, 114833. [Google Scholar] [CrossRef]
- Flora of China. Available online: http://www.iplant.cn/foc/ (accessed on 5 March 2021).
- Zhou, T.; Hou, G.; Sun, J.; Zong, N.; Shi, P. Degradation Shifts Plant Communities from S- to R-Strategy in an Alpine Meadow, Tibetan Plateau. Sci. Total Environ. 2021, 800, 149572. [Google Scholar] [CrossRef]
- Nilsson, C.; Andersson, E.; Merritt, D.M.; Johansson, M.E. Differences in Riparian Flora Between Riverbanks and River Lakeshores Explained by Dispersal Traits. Ecology 2002, 83, 2878–2887. [Google Scholar] [CrossRef]
- You, W.; Yu, D.; Xie, D.; Yu, L.; Xiong, W.; Han, C. Responses of the Invasive Aquatic Plant Water Hyacinth to Altered Nutrient Levels under Experimental Warming in China. Aquat. Bot. 2014, 119, 51–56. [Google Scholar] [CrossRef]
- Liu, Z.; Ge, X.; Fu, Z.; Liu, J. Alternanthera Philoxeroides Invasion Affects the Soil Seed Bank of Reed Community. Environ. Exp. Bot. 2020, 180, 104196. [Google Scholar] [CrossRef]
- Xi, D.-G.; You, W.-H.; Hu, A.-A.; Huang, P.; Du, D.-L. Developmentally Programmed Division of Labor in the Aquatic Invader Alternanthera Philoxeroides Under Homogeneous Soil Nutrients. Front. Plant Sci. 2019, 10, 485. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.M.S.; Schaefer, C.E.G.R.; de Oliveira Silva, J.; Ferreira Júnior, W.G.; dos Santos, R.M.; Neri, A.V. The Influence of Soil on Vegetation Structure and Plant Diversity in Different Tropical Savannic and Forest Habitats. J. Plant Ecol. 2018, 11, 226–236. [Google Scholar] [CrossRef]
- Conner, W.H.; Gosselink, J.G.; Parrondo, R.T. Comparison of the Vegetation of Three Louisiana Swamp Sites with Different Flooding Regimes. Am. J. Bot. 1981, 68, 320–331. [Google Scholar] [CrossRef]
- D’Odorico, P.; Engel, V.; Carr, J.A.; Oberbauer, S.F.; Ross, M.S.; Sah, J.P. Tree–Grass Coexistence in the Everglades Freshwater System. Ecosystems 2011, 14, 298–310. [Google Scholar] [CrossRef]
- Castanho, C.T.; Oliveira, A.A.; Prado, P.I. The Importance of Plant Life Form on Spatial Associations along a Subtropical Coastal Dune Gradient. J. Veg. Sci. 2012, 23, 952–961. [Google Scholar] [CrossRef]
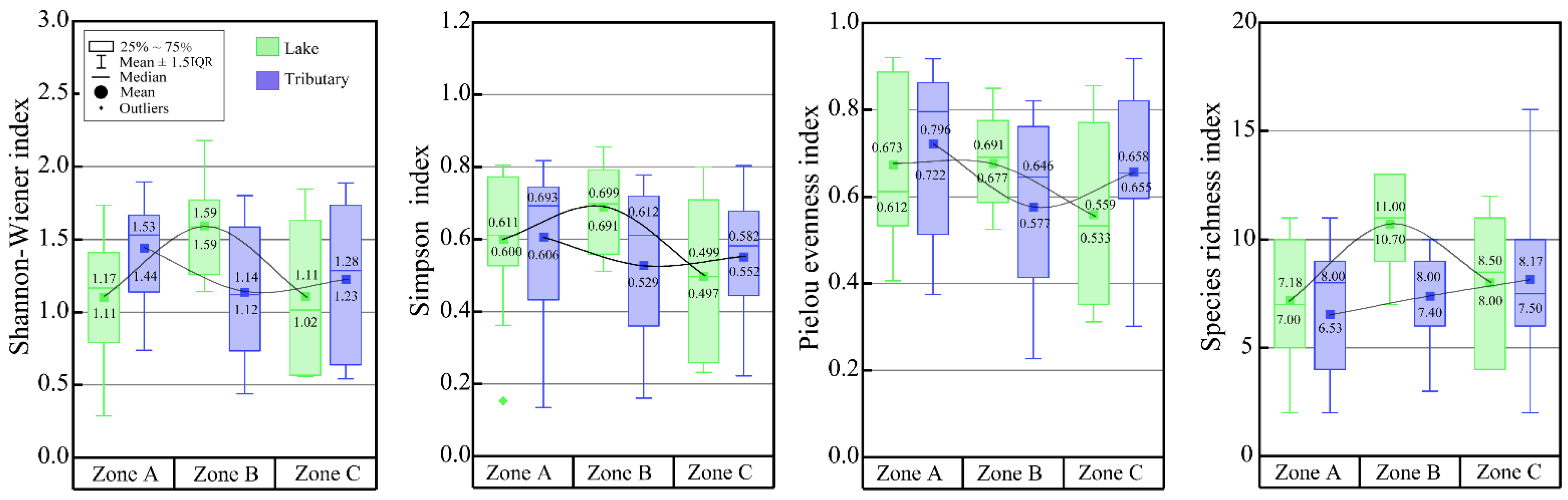
| Zone | Lakeshore | Tributary | t | Sig.(2-Tailed) | ||
|---|---|---|---|---|---|---|
| Mean (SD) | Min–Max | Mean (SD) | Min–Max | |||
| A | 1.11 ± 0.40 | 0.29–1.74 | 1.44 ± 0.35 | 0.74–1.90 | −2.178 | 0.040 |
| B | 1.59 ± 0.34 | 1.14–2.18 | 1.14 ± 0.48 | 0.44–1.80 | 2.524 | 0.022 |
| C | 1.11 ± 0.55 | 0.56–1.85 | 1.23 ± 0.55 | 0.54–1.89 | −0.385 | 0.708 |
| Factors | Diversity Indices | Community Species Characteristics | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R | H′ | E | D | Biomass | Tree | Density | Perennial | Annual | |
| Csand.sl | −0.19 | −0.17 | −0.13 | −0.21 | 0.12 | 0.48 ** | −0.05 | 0.01 | −0.09 |
| Csand.dl | 0.18 | 0.17 | 0.06 | 0.21 | 0.31 | 0.39 | −0.03 | −0.13 | 0.15 |
| SBD | 0.08 | −0.04 | −0.07 | 0.23 | 0.23 | 0.04 | 0.23 | −0.01 | 0.46 * |
| Form | 0.13 | −0.21 | −0.31 | −0.23 | 0.37 * | −0.15 | 0.14 | 0.01 | 0.25 |
| Slope | −0.48 * | 0.01 | 0.21 | 0.07 | −0.44 * | 0.54 * | −0.08 | −0.17 | 0.13 |
| AN.sl | −0.25 | 0.11 | 0.26 | 0.10 | −0.31 | 0.27 | −0.34 | −0.22 | −0.29 |
| AN.dl | −0.03 | 0.12 | 0.1 | 0.12 | 0.08 | −0.2 | −0.11 | 0.05 | −0.28 |
| AP.sl | −0.17 | 0.37 * | 0.45 ** | 0.38 * | −0.38 | 0.15 | −0.39 | −0.23 | −0.35 |
| AP.dl | −0.3 | 0.15 | 0.38 | 0.19 | −0.36 | −0.14 | −0.27 | −0.02 | −0.45 * |
| TP.sl | −0.07 | 0.35 * | 0.33 | 0.35 * | −0.29 | 0.2 | −0.37 | −0.32 | −0.21 |
| TP.dl | −0.15 | 0.26 | 0.34 | 0.32 | −0.33 | 0.34 | −0.31 | −0.42 * | 0.06 |
| TN.sl | −0.15 | 0.22 | 0.32 | 0.25 | −0.24 | −0.18 | −0.21 | 0.08 | −0.48 * |
| Variables | Habitats Difference | Zoning Difference | ||||||
|---|---|---|---|---|---|---|---|---|
| Lakeshore (Reference: Tributary) | Zone A (Reference: Zone B) | Zone C (Reference: Zone B) | Zone A (Reference: Zone C) | |||||
| CE (%) | p | CE (%) | p | CE (%) | p | CE (%) | p | |
| Preponderant families of vascular plant species | ||||||||
| Asteraceae | 0.205 | 0.962 | −10.925 * | 0.003 | 0.854 | 0.849 | −11.779 * | 0.010 |
| Poaceae | 7.767 | 0.258 | −28.206 * | 0.001 | −8.751 | 0.233 | 28.614 * | 0.007 |
| Amaranthaceae | −5.409 | 0.498 | 11.445 | 0.176 | −17.169 | 0.104 | 28.614 * | 0.007 |
| Apiaceae | 0.498 | 0.684 | −2.906 * | 0.035 | −2.681 | 0.114 | −0.224 | 0.890 |
| Polygonaceae | 16.168 * | 0.009 | 12.877 | 0.058 | 0.499 | 0.952 | 12.378 | 0.128 |
| Lifeforms of vascular plant species | ||||||||
| Woody plants | 0.364 | 0.244 | −1.797 * | 0.001 | 1.348 * | 0.001 | −3.146 * | 0.001 |
| Annual herbaceous | −2.739 | 0.760 | −12.458 * | 0.049 | −0.265 | 0.973 | −12.193 | 0.123 |
| Perennial herbaceous | 29.472 * | 0.015 | 5.493 | 0.655 | −15.551 | 0.321 | 21.044 | 0.173 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zu, J.; Xia, J.; Zeng, Z.; Liu, X.; Cai, W.; Li, J.; Wang, Q.; Wang, Y.; Dou, C. Distribution Pattern and Structure of Vascular Plant Communities in Riparian Areas and Their Response to Soil Factors: A Case Study of Baoan Lake, Hubei Province, China. Sustainability 2022, 14, 15769. https://doi.org/10.3390/su142315769
Zu J, Xia J, Zeng Z, Liu X, Cai W, Li J, Wang Q, Wang Y, Dou C. Distribution Pattern and Structure of Vascular Plant Communities in Riparian Areas and Their Response to Soil Factors: A Case Study of Baoan Lake, Hubei Province, China. Sustainability. 2022; 14(23):15769. https://doi.org/10.3390/su142315769
Chicago/Turabian StyleZu, Jiayi, Jihong Xia, Zhuo Zeng, Xiujun Liu, Wangwei Cai, Jingjiang Li, Qihua Wang, Yue Wang, and Chuanbin Dou. 2022. "Distribution Pattern and Structure of Vascular Plant Communities in Riparian Areas and Their Response to Soil Factors: A Case Study of Baoan Lake, Hubei Province, China" Sustainability 14, no. 23: 15769. https://doi.org/10.3390/su142315769
APA StyleZu, J., Xia, J., Zeng, Z., Liu, X., Cai, W., Li, J., Wang, Q., Wang, Y., & Dou, C. (2022). Distribution Pattern and Structure of Vascular Plant Communities in Riparian Areas and Their Response to Soil Factors: A Case Study of Baoan Lake, Hubei Province, China. Sustainability, 14(23), 15769. https://doi.org/10.3390/su142315769






