Influence of Different Pretreatment Methods and Conditions on the Anaerobic Digestion Efficiency of Spent Mushroom Substrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Material (SMS) and Inoculum for the BMP Test
2.2. Pretreatment of SMS
2.3. BMP Test
2.4. Analytical Methods
3. Results and Discussion
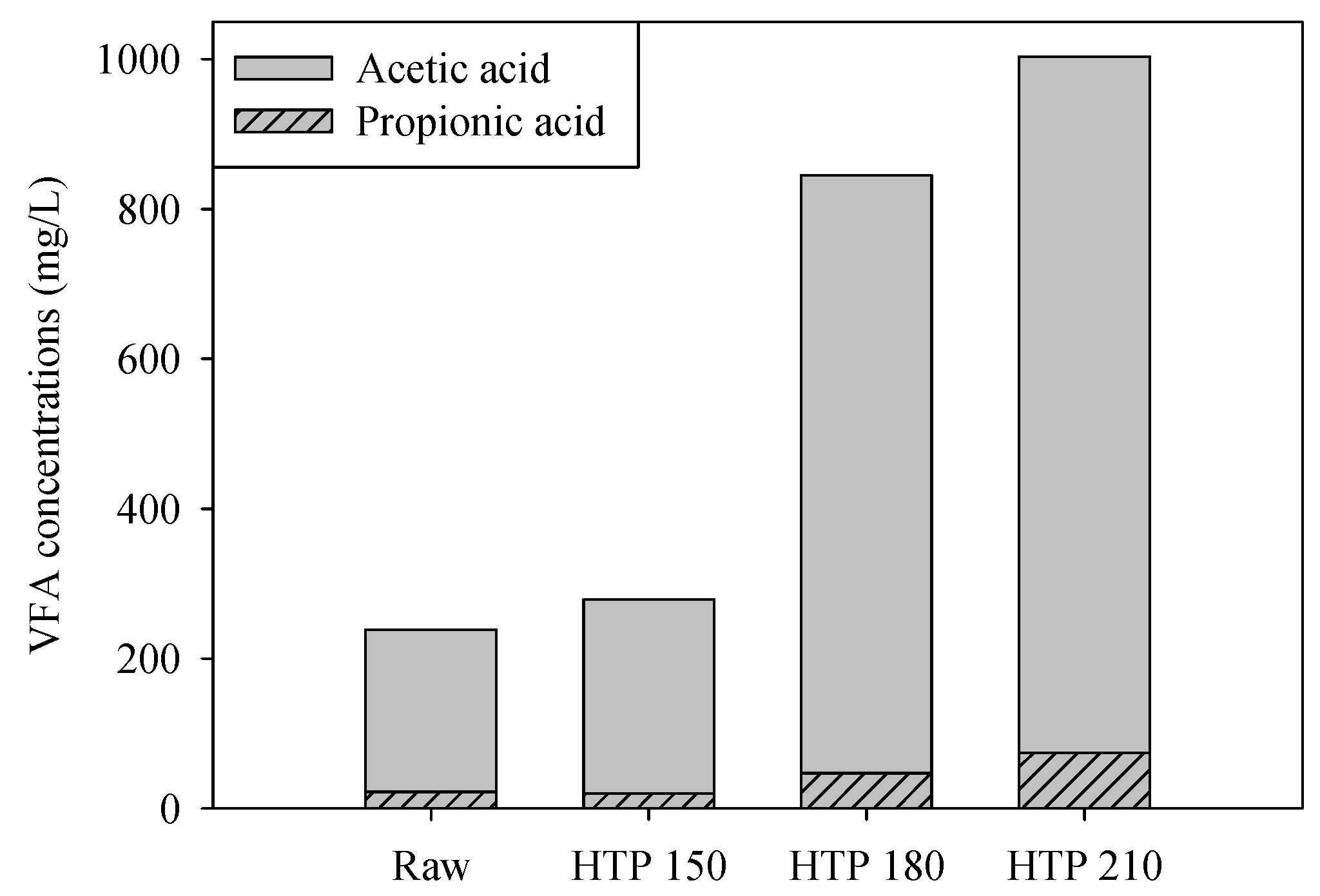
3.1. Effects of Pretreatment on SMS Characteristics
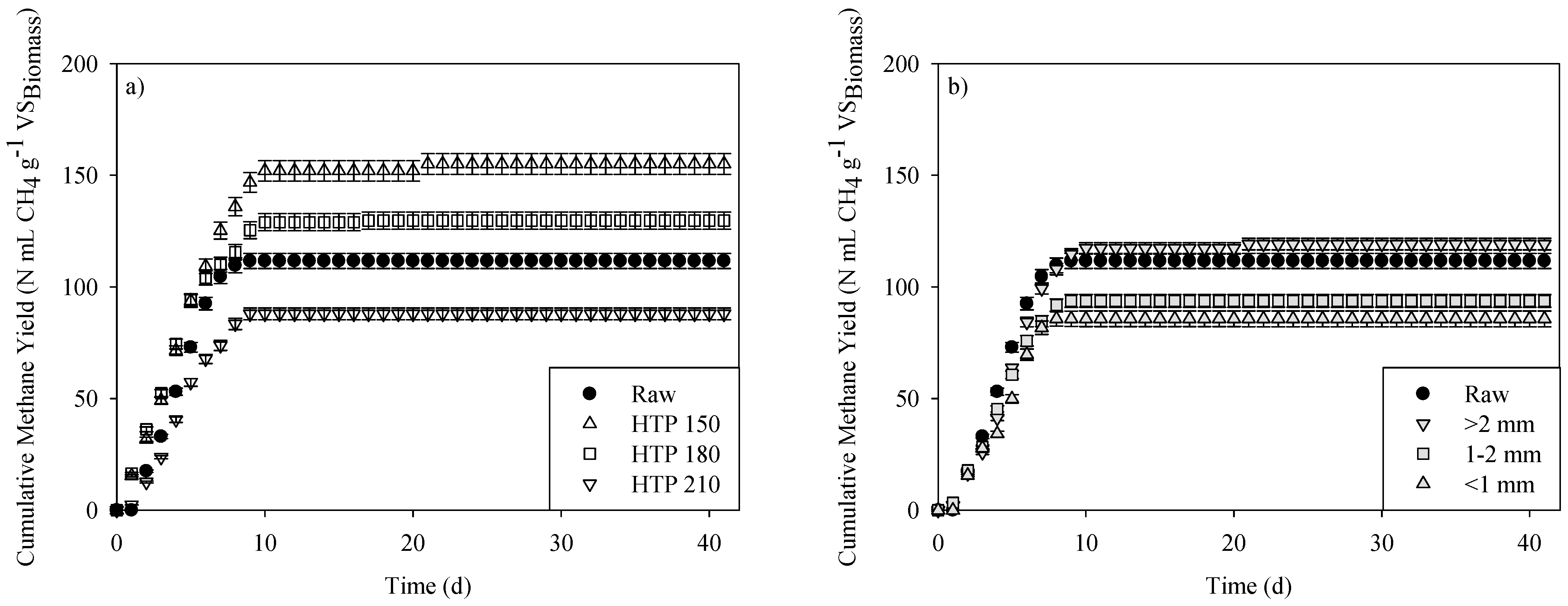
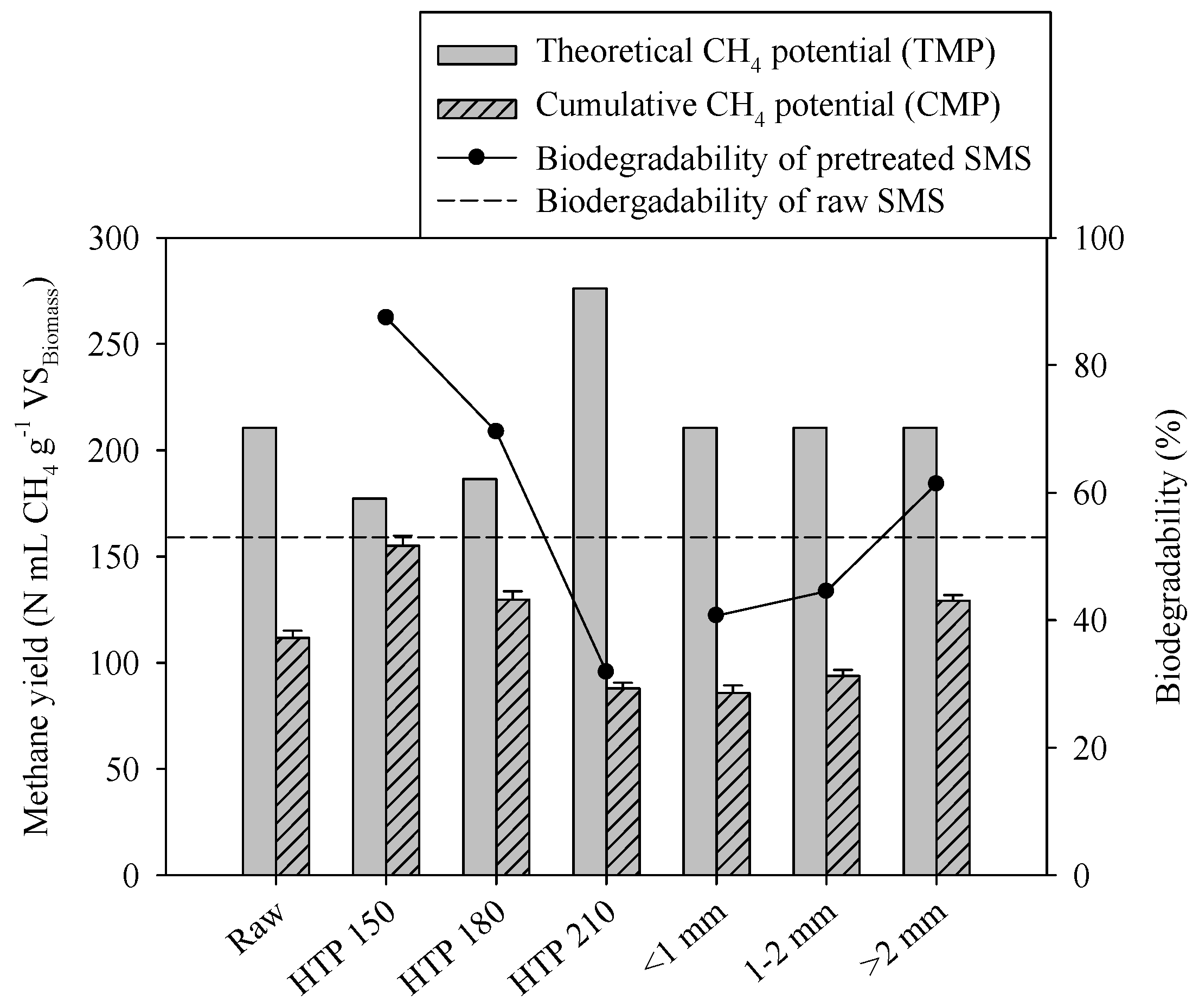
3.2. Effects of Pretreatment on AD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Semple, K.T.; Watts, N.U.; Fermor, T.R. Factors affecting the mineralization of [U-14C] benzene in spent mushroom substrate. FEMS Microbiol. Lett. 1998, 164, 317–321. [Google Scholar] [CrossRef]
- Williams, B.C.; McMullan, J.T.; McCahey, S. An initial assessment of spent mushroom compost as a potential energy feedstock. Bioresour. Technol. 2001, 79, 227–230. [Google Scholar] [CrossRef]
- Kang, D.-S.; Min, K.-J.; Kwak, A.-M.; Lee, S.-Y.; Kang, H.-W. Defense response and suppression of Phytophthora blight disease of pepper by water extract from spent mushroom substrate of Lentinula edodes. Plant Pathol. J. 2017, 33, 264. [Google Scholar] [CrossRef]
- Ehaliotis, C.; Zervakis, G.I.; Karavitis, P. Residues and by-products of olive-oil mills for root-zone heating and plant nutrition in organic vegetable production. Sci. Hortic. 2005, 106, 293–308. [Google Scholar] [CrossRef]
- Kakkar, V.; Dhanda, S. Comparative evaluation of wheat and paddy straws for mushroom production and feeding residual straws to ruminants. Bioresour. Technol. 1998, 66, 175–177. [Google Scholar] [CrossRef]
- Subramaniyam, R.; Vimala, R. Solid state and submerged fermentation for the production of bioactive substances: A comparative study. Int. J. Sci. Nat. 2012, 3, 480–486. [Google Scholar]
- Phan, C.-W.; Sabaratnam, V. Potential uses of spent mushroom substrate and its associated lignocellulosic enzymes. Appl. Microbiol. Biotechnol. 2012, 96, 863–873. [Google Scholar] [CrossRef]
- Jonathan, S.G.; Lawal, M.M.; Oyetunji, O.J. Effect of spent mushroom compost of Pleurotus pulmonarius on growth performance of four Nigerian vegetables. Mycobiology 2011, 39, 164–169. [Google Scholar] [CrossRef] [Green Version]
- Medina, E.; Paredes, C.; Bustamante, M.; Moral, R.; Moreno-Caselles, J. Relationships between soil physico-chemical, chemical and biological properties in a soil amended with spent mushroom substrate. Geoderma 2012, 173, 152–161. [Google Scholar] [CrossRef]
- Tian, X.; Li, C.; Yang, H.; Ye, Z.; Xu, H. Spent mushroom: A new low-cost adsorbent for removal of congo red from aqueous solutions. Desalination Water Treat. 2011, 27, 319–326. [Google Scholar] [CrossRef]
- Leong, Y.K.; Varjani, S.; Lee, D.-J.; Chang, J.-S. Valorization of spent mushroom substrate for low-carbon biofuel production: Recent advances and developments. Bioresour. Technol. 2022, 128012. [Google Scholar] [CrossRef]
- Atallah, E.; Zeaiter, J.; Ahmad, M.N.; Leahy, J.J.; Kwapinski, W. Hydrothermal carbonization of spent mushroom compost waste compared against torrefaction and pyrolysis. Fuel Process. Technol. 2021, 216, 106795. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.; Sohn, D.; Kim, Y.M.; Park, K.Y. Hydrothermal carbonization of lipid extracted algae for hydrochar production and feasibility of using hydrochar as a solid fuel. Energy 2018, 153, 913–920. [Google Scholar] [CrossRef]
- Kim, D.; Park, S.; Park, K.Y. Upgrading the fuel properties of sludge and low rank coal mixed fuel through hydrothermal carbonization. Energy 2017, 141, 598–602. [Google Scholar] [CrossRef]
- Lee, J.; Park, K.Y. Impact of hydrothermal pretreatment on anaerobic digestion efficiency for lignocellulosic biomass: Influence of pretreatment temperature on the formation of biomass-degrading byproducts. Chemosphere 2020, 256, 127116. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Surendra, K.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic digestion of lignocellulosic biomass: Challenges and opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef]
- Atelge, M.; Atabani, A.; Banu, J.R.; Krisa, D.; Kaya, M.; Eskicioglu, C.; Kumar, G.; Lee, C.; Yildiz, Y.; Unalan, S. A critical review of pretreatment technologies to enhance anaerobic digestion and energy recovery. Fuel 2020, 270, 117494. [Google Scholar]
- Varjani, S.; Sivashanmugam, P.; Tyagi, V.K.; Gunasekaran, M. Breakthrough in hydrolysis of waste biomass by physico-chemical pretreatment processes for efficient anaerobic digestion. Chemosphere 2022, 294, 133617. [Google Scholar]
- Shrestha, S.; Fonoll, X.; Khanal, S.K.; Raskin, L. Biological strategies for enhanced hydrolysis of lignocellulosic biomass during anaerobic digestion: Current status and future perspectives. Bioresour. Technol. 2017, 245, 1245–1257. [Google Scholar] [CrossRef]
- Guo, J.; Wang, W.; Liu, X.; Lian, S.; Zheng, L. Effects of thermal pre-treatment on anaerobic co-digestion of municipal biowastes at high organic loading rate. Chemosphere 2014, 101, 66–70. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, P.; Li, F.; Jin, S.; Wang, S.; Zhou, S. A review of lignocellulose change during hydrothermal pretreatment for bioenergy production. Curr. Org. Chem. 2016, 20, 2799–2809. [Google Scholar] [CrossRef]
- Ghasimi, D.S.; Aboudi, K.; de Kreuk, M.; Zandvoort, M.H.; van Lier, J.B. Impact of lignocellulosic-waste intermediates on hydrolysis and methanogenesis under thermophilic and mesophilic conditions. Chem. Eng. J. 2016, 295, 181–191. [Google Scholar] [CrossRef]
- BS 812-103.1; Testing Aggregates: Methods for Determination of Particle Size Distribution. British Standards Institution (BSI): London, UK, 1985.
- Owen, W.; Stuckey, D.; Healy, J.; Young, L.; McCarty, P. Bioassay for monitoring biochemical methane potential and anaerobic toxicity. Water Res. 1979, 13, 485–492. [Google Scholar] [CrossRef]
- Shelton, D.R.; Tiedje, J.M. General method for determining anaerobic biodegradation potential. Appl. Environ. Microb. 1984, 47, 850–857. [Google Scholar] [CrossRef] [Green Version]
- ASTM E871-82(2019); Standard Test Method for Moisture Analysis of Particulate Wood Fuels. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2006.
- ASTM E872-82(2019); Standard Test Method for Volatile Matter in the Analysis of Particulate Wood Fuels. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2006.
- ASTM E1755-01; Standard Test Method for Determination of Ash in Biomass. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2007.
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 8th ed.; Association of Official Analytical Chemists (AOAC): Rockville, MD, USA, 2005. [Google Scholar]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A review of the processes, parameters, and optimization of anaerobic digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, J.; Huang, G.; Yang, Z.; Han, L. Understanding the synergistic effect and the main factors influencing the enzymatic hydrolyzability of corn stover at low enzyme loading by hydrothermal and/or ultrafine grinding pretreatment. Bioresour. Technol. 2018, 264, 327–334. [Google Scholar] [CrossRef]
- Phuttaro, C.; Sawatdeenarunat, C.; Surendra, K.; Boonsawang, P.; Chaiprapat, S.; Khanal, S.K. Anaerobic digestion of hydrothermally-pretreated lignocellulosic biomass: Influence of pretreatment temperatures, inhibitors and soluble organics on methane yield. Bioresour. Technol. 2019, 284, 128–138. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Yousefifar, A.; Baroutian, S.; Farid, M.M.; Gapes, D.J.; Young, B.R. Fundamental mechanisms and reactions in non-catalytic subcritical hydrothermal processes: A review. Water Res. 2017, 123, 607–622. [Google Scholar] [CrossRef]
- Palmowski, L.; Müller, J. Influence of the size reduction of organic waste on their anaerobic digestion. Water Sci. Technol. 2000, 41, 155–162. [Google Scholar] [CrossRef]
- Ahmed, B.; Aboudi, K.; Tyagi, V.K.; Álvarez-Gallego, C.J.; Fernández-Güelfo, L.A.; Romero-García, L.I.; Kazmi, A. Improvement of anaerobic digestion of lignocellulosic biomass by hydrothermal pretreatment. Appl. Sci. 2019, 9, 3853. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Wachemo, A.C.; Zhang, L.; Bai, T.; Li, X.; Zuo, X.; Yuan, H. Effect of hydrothermal pretreatment severity on the pretreatment characteristics and anaerobic digestion performance of corn stover. Bioresour. Technol. 2019, 289, 121646. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.K.; Um, Y.; Park, Y.-C.; Seo, J.-H.; Kim, K.H. Compounds inhibiting the bioconversion of hydrothermally pretreated lignocellulose. Appl. Microbiol. Biotechnol. 2015, 99, 4201–4212. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Luo, T.; Mei, Z. Can hydrothermal pretreatment improve anaerobic digestion for biogas from lignocellulosic biomass? Bioresour. Technol. 2018, 249, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Dutta, A. Challenges and opportunities of lignocellulosic biomass for anaerobic digestion. Resour. Conserv. Recycl. 2018, 130, 164–174. [Google Scholar] [CrossRef]


| Raw Sample | |||
|---|---|---|---|
| Proximate analysis | % by wet weight | Moisture | 71.8 |
| Total solid (TS) | 28.2 | ||
| % by dry weight | Volatile solid (VS) | 87.5 | |
| Ultimate analysis | % by dry weight | C | 41 |
| H | 5.33 | ||
| O | 43.1 | ||
| N | 2.66 | ||
| S | 0.2 | ||
| unitless | C/N | 15.4 | |
| Structural analysis | % by dry weight | Lignin | 12.3 |
| Cellulose | 60.1 | ||
| Hemicellulose | 27.6 | ||
| Hydrothermal (°C) | Mechanical (mm) | ||||||
|---|---|---|---|---|---|---|---|
| 150 | 180 | 210 | <1 | 1–2 | >2 | ||
| Proximate analysis (% w/w) | Moisture a | 84.8 | 85.0 | 85.3 | 44.8 | 63.6 | 65.1 |
| Total Solid (TS) a | 15.2 | 15.0 | 14.7 | 55.2 | 36.4 | 34.9 | |
| Volatile Solid (VS) b | 89.6 | 89.0 | 87.6 | 83.4 | 87.6 | 88.7 | |
| Elemental analysis (% w/w) | C b | 39.0 | 39.7 | 44.7 | 41.0 | 41.0 | 41.0 |
| H b | 5.12 | 4.81 | 4.70 | 5.33 | 5.33 | 5.33 | |
| O b | 45.5 | 43.9 | 37.8 | 43.1 | 43.1 | 43.1 | |
| N b | 2.28 | 2.95 | 3.13 | 2.66 | 2.66 | 2.66 | |
| S b | 0.220 | 0.240 | 0.270 | 0.200 | 0.200 | 0.200 | |
| C/N | 17.1 | 13.5 | 14.3 | 15.4 | 15.4 | 15.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Ryu, D.-y.; Jang, K.H.; Lee, J.W.; Kim, D. Influence of Different Pretreatment Methods and Conditions on the Anaerobic Digestion Efficiency of Spent Mushroom Substrate. Sustainability 2022, 14, 15854. https://doi.org/10.3390/su142315854
Lee J, Ryu D-y, Jang KH, Lee JW, Kim D. Influence of Different Pretreatment Methods and Conditions on the Anaerobic Digestion Efficiency of Spent Mushroom Substrate. Sustainability. 2022; 14(23):15854. https://doi.org/10.3390/su142315854
Chicago/Turabian StyleLee, Jongkeun, Do-yoon Ryu, Kye Hwan Jang, Jong Wook Lee, and Daegi Kim. 2022. "Influence of Different Pretreatment Methods and Conditions on the Anaerobic Digestion Efficiency of Spent Mushroom Substrate" Sustainability 14, no. 23: 15854. https://doi.org/10.3390/su142315854
APA StyleLee, J., Ryu, D.-y., Jang, K. H., Lee, J. W., & Kim, D. (2022). Influence of Different Pretreatment Methods and Conditions on the Anaerobic Digestion Efficiency of Spent Mushroom Substrate. Sustainability, 14(23), 15854. https://doi.org/10.3390/su142315854








