Sustainable Grain Protectants: Recruiting Entomopathogenic Nematodes against Stored-Product Coleopterans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects and Commodity
2.2. Entomopathogenic Nematodes
2.3. Bioassays
2.4. Data Analysis
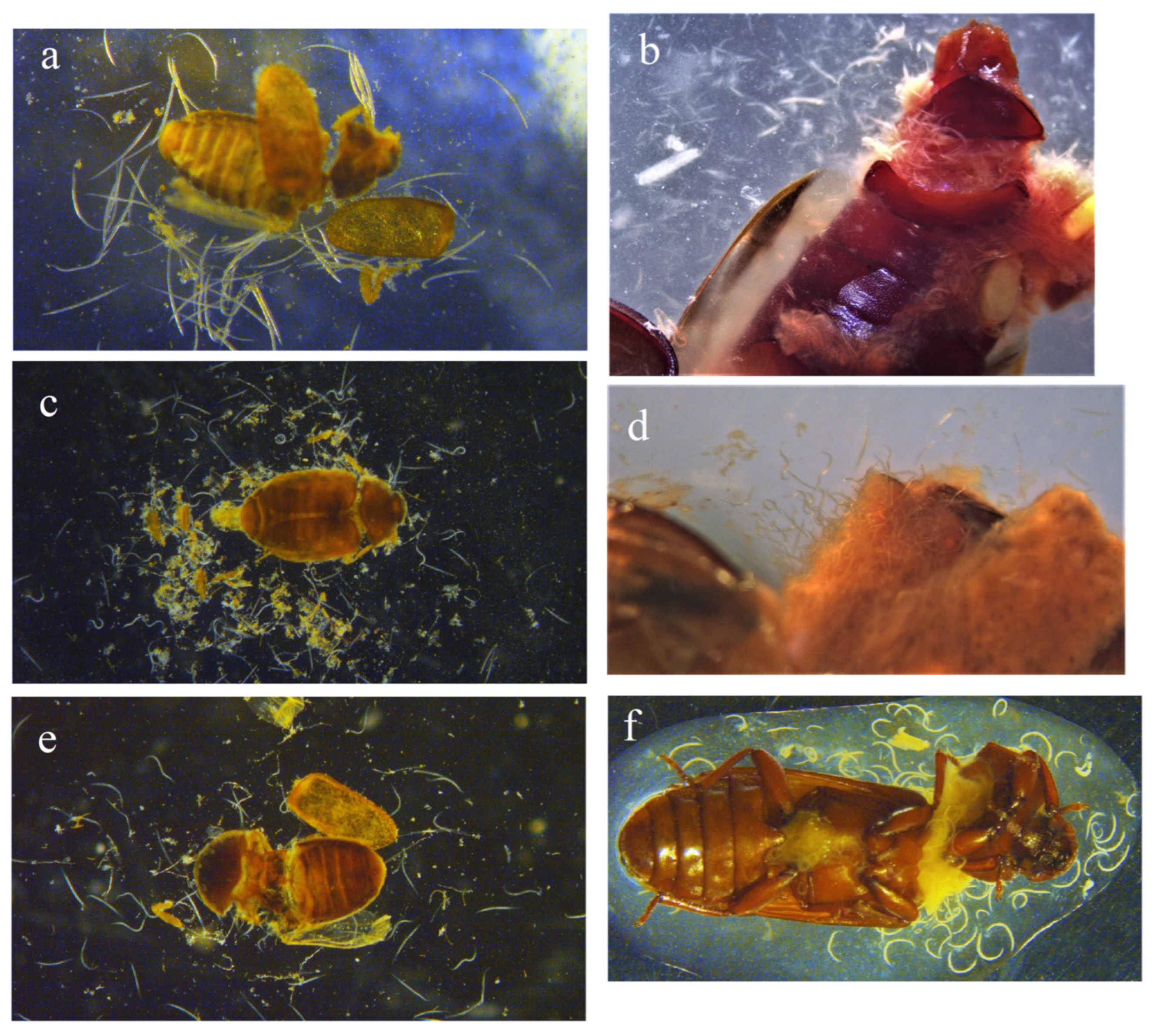
3. Results
3.1. Mortality of Trogoderma granarium Adults
3.2. Mortality of Tenebrio molitor Adults
3.3. Mortality of Alphitobius diaperinus Adults
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alves, L.F.A.; Rohde, C.; Alves, V.S. Patogenicidade de Steinernema glaseri e S. carpocapsae (Nematoda: Rhabdita) contra o cascudinho, Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae). Neotrop. Entomol. 2005, 34, 139–141. [Google Scholar] [CrossRef] [Green Version]
- Alves, V.S.; Neves, P.M.J.D.O.; Alves, L.F.A.; Moino, A., Jr.; Holz, N. Entomopathogenic nematodes (Rhabditida: Heterorhabditidae and Steinernematidae) screening for lesser mealworm Alphitobius diaperinus (Coleoptera: Tenebrionidae) control. Rev. Colomb. Entomol. 2012, 38, 76–80. [Google Scholar] [CrossRef]
- Mbata, G.N.; Shapiro-Ilan, D.I. Laboratory evaluation of virulence of Heterorhabditid nematodes to Plodia interpunctella Hübner (Lepidoptera: Pyralidae). Environ. Entomol. 2005, 34, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Rodríguez, O.; Campbell, J.F.; Ramaswamy, S.B. Pathogenicity of three species of entomopathogenic nematodes to some major stored-product insect pests. J. Stored Prod. Res. 2006, 42, 241–252. [Google Scholar] [CrossRef]
- Athanassiou, C.G.; Kavallieratos, N.G.; Menti, H.; Karanastasi, E. Mortality of four stored product pests in stored wheat when exposed to doses of three entomopathogenic nematodes. J. Econ. Entomol. 2010, 103, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Boemare, N.; Laumond, C.; Mauleon, H. The entomopathogenic nematode-bacterium complex: Biology, life cycle and vertebrate safety. Biocontrol Sci. Technol. 2010, 6, 333–346. [Google Scholar] [CrossRef]
- de Carvalho Barbosa Negrisoli, C.R.; Negrisoli, A.S.J.; Berbardi, D.; Garcia, M.S. Activity of eight strains of entomopathogenic nematodes (Rhabditida: Steinernematidae, Heterorhabditidae) against five stored product pests. Exp. Parasitol. 2013, 134, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, E.E.; Frizzo, L.S.; Malmierca, M.; Zbrun, M.V.; Lax, P.; Doucet, M.E. Biological control of Alphitobius diaperinus with Steinernema rarum CUL and Heterorhabditis bacteriophora SMC and feasibility of application in rice hull. J. Pest Sci. 2016, 89, 161–170. [Google Scholar] [CrossRef]
- Gulcu, B.; Cimen, H.; Raja, R.K.; Hazir, S. Entomopathogenic nematodes and their mutualistic bacteria: Their ecology and application as microbial control agents. Biopestic. Int. 2017, 3, 79–112. [Google Scholar]
- Javed, S.; Khanum, T.A.; Khan, S. Biocontrol potential of entomopathogenic nematode species against Tribolium confusum (Jac.) (Coleoptera: Tenebrionidae) and Rhyzopertha dominica (Fab.) (Coleoptera: Bostrichidae) under laboratory conditions. Egypt. J. Biol. Pest Control 2020, 30, 5. [Google Scholar] [CrossRef]
- Karanastasi, E.; Kavallieratos, N.G.; Boukouvala, M.C.; Christodoulopoulou, A.D.; Papadopoulou, A.A. Effect of three entomopathogenic nematode species to Trogoderma granarium Everts (Coleoptera: Dermestidae) larvae on stored-wheat. J. Stored Prod. Res. 2020, 88, 101641. [Google Scholar] [CrossRef]
- Erdoğuş, F.D. On the efficiency of entomopathogenic nematodes (Rhabditida: Heterorhabditidae and Steinernematidae) on rust red flour beetle, Tribolium castaneum (Herbst.) (Coleoptera: Tenebrionidae). Egypt. J. Biol. Pest Control 2021, 31, 116. [Google Scholar] [CrossRef]
- Qader, F.; Mohammed, B.; Ameen, H. Efficacy of entomopathogenic nematodes against three species of stored product insects. IOP Conf. Ser. Earth Environ. Sci. 2021, 910, 012047. [Google Scholar] [CrossRef]
- Kaya, H.K.; Gaugler, R. Entomopathogenic nematodes. Annu. Rev. Entomol. 1993, 38, 181–206. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, M.; Ahuja, A.; Vinay, B.K.; Kommu, K.K.; Thakur, S.; Paschapur, A.U.; Jeeven, B.; Mishra, K.K.; Meena, R.P.; et al. Entomopathogenic nematodes: A sustainable option for insect pest management. In Biopesticides; Rakshit, A., Meena, V.S., Abhilash, P.C., Sarma, B.K., Singh, H.B., Fraceto, L., Parihar, M., Singh, A.K., Eds.; Elsevier Academic Press Inc.: Duxford, UK, 2022; pp. 73–92. [Google Scholar]
- Boemare, N. Interactions between the partners of the entomopathogenic bacterium nematode complexes, Steinernema-Xenorhabdus and Heterorhabditis-Photorhabdus. Nematology 2002, 4, 601–603. [Google Scholar] [CrossRef] [Green Version]
- Ablel Razek, A.S. Pathogenic effects of Xenorhabdus nematophilus and Photorhabdus luminescens (Enterobacteriaceae) against pupae of the diamond backmoth, Plutella xylostella (L.). J. Pest Sci. 2003, 76, 108–111. [Google Scholar] [CrossRef]
- Ciche, T.A.; Ensign, J.C. For the insect pathogen Photorhabdus luminescens, which end of a nematode is out? Appl. Environ. Microbiol. 2003, 69, 1890–1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, D.; Macchietto, M.; Chang, D.; Barros, M.M.; Baldwin, J.; Mortazavi, A.; Dillman, A.R. Activated entomopathogenic nematode infective juveniles release lethal venom proteins. PLoS Pathog. 2017, 13, e1006302. [Google Scholar] [CrossRef] [Green Version]
- Martens, E.C.; Heungens, K.; Goodrich-Blair, H. Early colonization events in the mutualistic association between Steinernema carpocapsae nematodes and Xenorhabdus nematophila bacteria. J. Bacteriol. 2003, 185, 3147–3154. [Google Scholar] [CrossRef] [Green Version]
- Ciche, T.A.; Darby, C.; Ehlers, R.U.; Forst, S.; Goodrich-Blair, H. Dangerous liaisons: The symbiosis of entomopathogenic nematodes and bacteria. Biol. Control 2006, 38, 22–46. [Google Scholar] [CrossRef]
- Bowen, D.J.; Ensign, J.C. Purification and characterization of a high-molecular-weight insecticidal protein complex produced by the entomopathogenic bacterium Photorhabdus luminescens. Appl. Environ. Microbiol. 1998, 64, 3029–3035. [Google Scholar] [CrossRef] [PubMed]
- Bowen, D.; Rocheleau, T.A.; Blackburn, M.; Andreev, O.; Golubeva, E.; Bhartia, R.; French-Constant, R.H. Insecticidal toxins from the bacterium Photorhabdus luminescens. Science 1998, 280, 2129–2132. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Fatig, R.O., III; Orr, G.L.; Schafer, B.W.; Strickland, J.A.; Sukhapinda, K.; Woodsworth, A.T.; Petell, J.K. Photorhabdus luminescens W-14 insecticidal activity consists of at least two similar but distinct proteins: Purification and characterization of toxin A and toxin B. J. Biol. Chem. 1999, 274, 9836–9842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.; Kim, Y. Eicosanoids rescue Spodoptera exigua infected with Xenorhabdus nematophilus, the symbiotic bacteria to the entomopathogenic nematode Steinernema carpocapsae. J. Insect Physiol. 2000, 46, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Patterson, S.S.; Ripp, S.; Simpson, M.L.; Sayler, G.S. Expression of the Photorhabdus luminescens lux genes (luxA, B, C, D, and E) in Saccharomyces cerevisiae. FEMS Yeast Res. 2003, 4, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Boemare, N.; Akhurst, R. The genera Photorhabdus and Xenorhabdus. Prokaryotes 2006, 6, 451–494. [Google Scholar]
- Yuksel, E.; Canhilal, R.; Imren, M. Potential of four Turkish isolates of entomopathogenic nematodes against three major stored products insect pests. J. Stored Prod. Res. 2019, 83, 317–321. [Google Scholar] [CrossRef]
- Laznik, Ž.; Tóth, T.; Lakatos, T.; Vidrih, M.; Trdan, S. The activity of three new strains of Steinernema feltiae against adults of Sitophilus oryzae under laboratory conditions. J. Food Agric. Environ. 2010, 8, 150–154. [Google Scholar]
- Okumura, G.T. A report of canthariasis and allergy caused by Trogoderma (Coleoptera: Dermestidae). Calif. Vector Views 1967, 14, 19–22. [Google Scholar]
- Jood, S.; Kapoor, A.C.; Singh, R. Effect of insect infestation and storage on lipids of cereal grains. J. Agric. Food Chem. 1996, 44, 1502–1506. [Google Scholar] [CrossRef]
- Rees, D. Insects of Stored Products; Manson Publishing: London, UK, 2004. [Google Scholar]
- Hagstrum, D.W.; Subramanyam, B. Stored-Product Insect Resource; AACC International: St. Paul, MN, USA, 2009. [Google Scholar]
- Agabou, A.; Alloui, N. Importance of Alphitobius diaperinus (Panzer) as a reservoir for pathogenic bacteria in Algerian broiler houses. Vet. World 2010, 3, 71–73. [Google Scholar]
- Van Broekhoven, S.; Bastiaan-Net, S.; de Jong, N.W.; Witchers, H.J. Influence of processing and in vitro digestion on the allergic cross-reactivity of three mealworm species. Food Chem. 2016, 196, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, W.L. Beetles (Coleoptera). In Medical and Veterinary Entomology; Mullen, G.R., Durden, L.A., Eds.; Academic Press: London, UK, 2019; pp. 129–143. [Google Scholar]
- Garino, C.; Mielke, H.; Knüppel, S.; Selhorst, T.; Broll, H.; Braeuning, A. Quantitative allergenicity risk assessment of food products containing yellow mealworm (Tenebrio molitor). Food Chem. Toxicol. 2020, 142, 111460. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.S. Pests of Stored Foodstuffs and their Control; Kluwer Academic Publishers: New York, NY, USA, 2003. [Google Scholar]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.S.; Moraiet, M.A.; Ahmad, S. Insecticides: Impact on the environment and human health. In Environmental Deterioration and Human Health; Malik, A., Grohmann, E., Akhtar, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 99–123. [Google Scholar]
- Benelli, G.; Wilke, A.B.; Bloomquist, J.R.; Desneux, N.; Beier, J.C. Overexposing mosquitoes to insecticides under global warming: A public health concern? Sci. Total Environ. 2021, 762, 143069. [Google Scholar] [CrossRef]
- Wakil, W.; Kavallieratos, N.G.; Ghazanfar, M.U.; Usman, M.; Habib, A.; El-Shafie, H.A.F. Efficacy of different entomopathogenic fungal isolates against four key stored-grain beetle species. J. Stored Prod. Res. 2021, 93, 101845. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Nika, E.P.; Skourti, A.; Perinelli, D.R.; Spinozzi, E.; Bonacucina, G.; Cappellacci, L.; Morshedloo, M.R.; Canale, A.; Benelli, G.; et al. Apiaceae essential oil nanoemulsions as effective wheat protectants against five arthropod pests. Ind. Crops Prod. 2022, 186, 115001. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Michail, E.J.; Boukouvala, M.C.; Nika, E.P.; Skourti, A. Efficacy of pirimiphos-methyl, deltamethrin, spinosad and silicoSec against adults and larvae of Tenebrio molitor L. on wheat, barley and maize. J. Stored Prod. Res. 2019, 83, 161–167. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Boukouvala, M.C.; Ntalli, N.; Skourti, A.; Karagianni, E.S.; Nika, E.P.; Kontodimas, D.C.; Cappellacci, L.; Petrelli, R.; Cianfaglione, K.; et al. Effectiveness of eight essential oils against two key stored-product beetles, Prostephanus truncatus (Horn) and Trogoderma granarium Everts. Food Chem. Toxicol. 2020, 139, 111255. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Boukouvala, M.C.; Ntalaka, C.T.; Skourti, A.; Nika, E.P.; Maggi, F.; Spinozzi, E.; Mazzara, E.; Petrelli, R.; Lupidi, G.; et al. Efficacy of 12 commercial essential oils as wheat protectants against stored-product beetles, and their acetylcholinesterase inhibitory activity. Entomol. Gen. 2021, 41, 385–414. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Skourti, A.; Nika, E.P.; Mártonfi, P.; Spinozzi, E.; Maggi, F. Tanacetum vulgare essential oil as grain protectant against adults and larvae of four major stored-product insect pests. J. Stored Prod. Res. 2021, 94, 101882. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Nika, E.P.; Skourti, A.; Spinozzi, E.; Ferrati, M.; Petrelli, R.; Maggi, F.; Benelli, G. Carlina acaulis essential oil: A candidate product for agrochemical industry due to its pesticidal capacity. Ind. Crops Prod. 2022, 188, 115572. [Google Scholar] [CrossRef]
- Papanikolaou, N.E.; Kavallieratos, N.G.; Iliopoulos, V.; Evergetis, E.; Skourti, A.; Nika, E.P.; Haroutounian, S.A. Essential oil coating: Mediterranean culinary plants as grain protectants against larvae and adults of Tribolium castaneum and Trogoderma granarium. Insects 2022, 13, 165. [Google Scholar] [CrossRef]
- Sagheer, M.; Aman, Y.; Mansoor-ul-Hasan; Ahmed, F.; Ranjha, M.H.; Ali, Q.; Ali, K.; Sidra-tul-Muntaha. Fumigant bioactivity of extracts of Citrulus colocynthes, Moringa oleifera and Azadirachta indica against Tribolium castaneum and Alphitobius diaperinus under laboratory conditions. In Proceedings of the 10th International Conference on Controlled Atmosphere and Fumigation in Stored Products (CAF2016), New Delhi, India, 7–11 November 2016; Navarro, S., Jayas, D.S., Alagusundaram, K., Eds.; CAF Permanent Committee Secretariat: Winnipeg, MB, Canada, 2016; pp. 459–464. [Google Scholar]
- Kavallieratos, N.G.; Nika, E.P.; Skourti, A.; Filintas, C.S.; Goumenou, T.D. Short- and long-term mortalities of small and large larvae of Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae) on concrete surfaces treated with three insecticides: Impact of food. Insects 2022, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Zar, J.H. Biostatistical Analysis; Pearson Education Limited: Essex, UK, 2014. [Google Scholar]
- Scheff, D.S.; Arthur, F.H. Fecundity of Tribolium castaneum and Tribolium confusum adults after exposure to deltamethrin packaging. J. Pest Sci. 2018, 91, 717–725. [Google Scholar] [CrossRef]
- Sall, J.; Lehman, A.; Creighton, L. JMP start statistics. In A Guide to Statistics and Data Analysis Using JMP and JMP in Software; Duxbury Press: Belmont, ON, Canada, 2001. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry; Freeman & Company: New York, NY, USA, 1995. [Google Scholar]
- SAS Institute Inc. Using JMP 16.2; SAS Institute Inc.: Cary, NC, USA, 2021. [Google Scholar]
- Kousar, N.; Riaz, T.; Feroz, A.; Shakoori, A.R.; Rauf, F. Efficacy of diatomaceous earth and deltamethrin alone and in combinations on mortality and energy reserves of insecticide resistant strains of stored grain pest, Trogoderma granarium. Pakistan J. Zool. 2021, 53, 2183–2193. [Google Scholar] [CrossRef]
- Ali, H.; Raza, A.B.M.; Majeed, M.Z.; Hamid, M.I. Laboratory evaluation of selected botanical and microbial formulations against khapra beetle Trogoderma granarium Everts (Coleoptera: Dermestidae). Pakistan J. Agric. Res. 2022, 35, 154–164. [Google Scholar] [CrossRef]
- Saad, M.M.G.; El Gendy, A.N.G.; Elkhateeb, A.M.; Abdelgaleil, S.A.M. Insecticidal properties and grain protective efficacy of essential oils against stored product insects. Int. J. Trop. Insect Sci. 2022, 42, 3639–3648. [Google Scholar] [CrossRef]
- Koc, S.; Polat, B.; Cengiz, A.; Kahraman, S.; Tufan-Cetin, O.; Cetin, H. Pathogenicity of an entomopathogenic nematode, Steinernema carpocapsae on Alphitobius diaperinus (Coleoptera: Tenebrionidae) strains from Turkey. J. Econ. Entomol. 2022, 115, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Laznik, Ž.; Trdan, S. Intraspecific variability of Steinernema feltiae (Filipjev) (Rhabditida: Steinernematidae) as biological control agent of rice weevil (Sitophilus oryzae [L.], Coleoptera, Curculionidae) adults. Acta Agric. Slov. 2010, 95, 51–59. [Google Scholar]
- Kung, S.P.; Gaugler, R.; Kaya, H.K. Effects of soil temperature, moisture and relative humidity on entomopathogenic nematode persistence. J. Invertabr. Pathol. 1991, 57, 242–249. [Google Scholar] [CrossRef]
| Effect | Trogoderma granarium | Tenebrio molitor | Alphitobius diaperinus | ||||
|---|---|---|---|---|---|---|---|
| Between exposure intervals | |||||||
| Source | DF | F | p | F | p | F | p |
| Intercept | 1 | 39,131.4 | <0.01 | 2600.6 | <0.01 | 29.9 | <0.01 |
| Nematode species | 2 | 26.3 | <0.01 | 4.3 | 0.02 | 4.4 | 0.02 |
| Nematode dose | 5 | 27.0 | <0.01 | 33.4 | <0.01 | 8.3 | <0.01 |
| Nematode species × nematode dose | 10 | 2.0 | 0.04 | 1.4 | 0.19 | 1.0 | 0.41 |
| Within exposure intervals | |||||||
| Exposure | 1 | 571.0 | <0.01 | 371.5 | <0.01 | 25.3 | <0.01 |
| Exposure × nematode species | 2 | 10.6 | <0.01 | 18.2 | <0.01 | 9.2 | <0.01 |
| Exposure × nematode dose | 5 | 5.5 | <0.01 | 1.4 | 0.21 | 7.5 | <0.01 |
| Exposure × nematode species × nematode dose | 10 | 1.1 | 0.36 | 9.1 | <0.01 | 1.9 | 0.05 |
| 100 IJs/mL | 500 IJs/mL | 1000 IJs/mL | 5000 IJs/mL | 10,000 IJs/mL | 50,000 IJs/mL | F | p | ||
|---|---|---|---|---|---|---|---|---|---|
| 4 Days | H. bacteriophora | 21.1 ± 3.5 Cc | 23.3 ± 3.7 BCc | 28.9 ± 3.1 ABCe | 51.1 ± 7.2 ABb | 53.3 ± 4.4 Acd | 55.6 ± 3.4 Ac | 7.3 | <0.01 |
| S. carpocapsae | 36.7 ± 5.0 Bbc | 48.9 ± 5.9 ABb | 51.1 ± 5.6 ABcd | 52.2 ± 6.2 ABb | 68.9 ± 4.2 Abc | 72.2 ± 3.6 Ab | 7.0 | <0.01 | |
| S. feltiae | 23.3 ± 4.1 Cc | 24.4 ± 1.8 BCc | 37.8 ± 2.2 ABde | 38.9 ± 4.2 ABb | 42.2 ± 7.0 ABd | 46.7 ± 5.0 Ac | 5.7 | <0.01 | |
| 8 Days | H. bacteriophora | 55.6 ± 5.0 Bab | 57.8 ± 3.6 Bab | 58.9 ± 4.6 Bbc | 83.3 ± 4.7 Aa | 84.4 ± 2.4 Aab | 100.0 ± 0.0 Aa | 17.6 | <0.01 |
| S. carpocapsae | 76.7 ± 4.4 Ba | 78.9 ± 4.6 Ba | 86.7 ± 2.4 ABa | 96.7 ± 1.7 Aa | 97.8 ± 1.5 Aa | 100.0 ± 0.0 Aa | 11.4 | <0.01 | |
| S. feltiae | 61.1 ± 5.6 Cab | 73.3 ± 6.5 BCa | 80.0 ± 3.7 ABCab | 84.4 ± 2.9 ABa | 85.6 ± 4.4 ABab | 100.0 ± 0.0 Aa | 7.0 | <0.01 | |
| F | 11.1 | 31.1 | 26.2 | 16.1 | 18.9 | 34.6 | |||
| p | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| 100 IJs/mL | 500 IJs/mL | 1000 IJs/mL | 5000 IJs/mL | 10,000 IJs/mL | 50,000 IJs/mL | F | p | ||
|---|---|---|---|---|---|---|---|---|---|
| 4 Days | H. bacteriophora | 7.8 ± 2.2 Bab | 12.2 ± 4.7 ABab | 14.4 ± 1.8 ABa | 16.7 ± 1.7 ABc | 17.8 ± 2.8 ABd | 20.0 ± 4.1 Ab | 3.6 | 0.01 |
| S. carpocapsae | 7.8 ± 2.8 Bab | 13.3 ± 2.4 ABa | 16.7 ± 1.7 Aa | 21.1 ± 3.5 Abc | 24.4 ± 2.4 Ad | 26.7 ± 4.1 Ab | 6.4 | <0.01 | |
| S. feltiae | 2.2 ± 2.2 Bb | 3.3 ± 1.7 Bb | 3.3 ± 1.7 Bb | 23.3 ± 4.1 Abc | 26.7 ± 4.1 Acd | 27.8 ± 2.8 Ab | 24.0 | <0.01 | |
| 8 Days | H. bacteriophora | 16.7 ± 4.4 Ca | 20.0 ± 4.4 BCa | 25.6 ± 3.4 ABCa | 43.3 ± 5.5 ABa | 46.7 ± 2.4 Aab | 62.2 ± 8.5 Aa | 6.9 | <0.01 |
| S. carpocapsae | 12.2 ± 4.0 Dab | 18.9 ± 3.1 CDa | 27.8 ± 4.0 BCa | 51.1 ± 6.8 ABa | 70.0 ± 4.1 ABa | 85.6 ± 3.4 Aa | 13.4 | <0.01 | |
| S. feltiae | 15.6 ± 3.4 Ca | 20.0 ± 2.9 BCa | 24.4 ± 3.8 BCa | 40.0 ± 5.8 ABab | 42.2 ± 4.9 ABbc | 76.7 ± 6.7 Aa | 10.6 | <0.01 | |
| F | 3.3 | 4.8 | 20.0 | 8.1 | 19.8 | 24.7 | |||
| p | 0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| 100 IJs/mL | 500 IJs/mL | 1000 IJs/mL | 5000 IJs/mL | 10,000 IJs/mL | 50,000 IJs/mL | F | p | ||
|---|---|---|---|---|---|---|---|---|---|
| 4 Days | H. bacteriophora | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.1 ± 1.1 ab | 1.1 ± 1.1 b | 0.8 | 0.56 |
| S. carpocapsae | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.1 ± 1.1 | 2.2 ± 2.2 ab | 2.2 ± 2.2 b | 0.6 | 0.70 | |
| S. feltiae | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 b | 1.1 ± 1.1 b | 1.0 | 0.43 | |
| 8 Days | H. bacteriophora | 0.0 ± 0.0 B | 0.0 ± 0.0 B | 0.0 ± 0.0 B | 6.7 ± 3.3 AB | 7.8 ± 3.2 ABa | 11.1 ± 2.6 Aa | 6.7 | <0.01 |
| S. carpocapsae | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 2.2 ± 1.5 | 2.2 ± 2.2 ab | 5.6 ± 2.9 ab | 1.9 | 0.11 | |
| S. feltiae | 0.0 ± 0.0 B | 0.0 ± 0.0 B | 0.0 ± 0.0 B | 0.0 ± 0.0 B | 0.0 ± 0.0 Bb | 3.3 ± 1.7 Aab | 4.0 | <0.01 | |
| F | - | - | - | 2.2 | 2.4 | 3.6 | |||
| p | - | - | - | 0.07 | 0.05 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavallieratos, N.G.; Karanastasi, E.; Nika, E.P.; Skourti, A.; Boukouvala, M.C.; Sampazioti, I.E. Sustainable Grain Protectants: Recruiting Entomopathogenic Nematodes against Stored-Product Coleopterans. Sustainability 2022, 14, 16038. https://doi.org/10.3390/su142316038
Kavallieratos NG, Karanastasi E, Nika EP, Skourti A, Boukouvala MC, Sampazioti IE. Sustainable Grain Protectants: Recruiting Entomopathogenic Nematodes against Stored-Product Coleopterans. Sustainability. 2022; 14(23):16038. https://doi.org/10.3390/su142316038
Chicago/Turabian StyleKavallieratos, Nickolas G., Eirini Karanastasi, Erifili P. Nika, Anna Skourti, Maria C. Boukouvala, and Ioanneta E. Sampazioti. 2022. "Sustainable Grain Protectants: Recruiting Entomopathogenic Nematodes against Stored-Product Coleopterans" Sustainability 14, no. 23: 16038. https://doi.org/10.3390/su142316038
APA StyleKavallieratos, N. G., Karanastasi, E., Nika, E. P., Skourti, A., Boukouvala, M. C., & Sampazioti, I. E. (2022). Sustainable Grain Protectants: Recruiting Entomopathogenic Nematodes against Stored-Product Coleopterans. Sustainability, 14(23), 16038. https://doi.org/10.3390/su142316038










