Deterioration of Novel Silver Coated Mirrors on Polycarbonate Used for Concentrated Solar Power
Abstract
1. Introduction
2. Material and Methods
2.1. Deposition of the Metallic Layer
2.2. Deposition of the Protective Layer
2.3. Weathering Degradation Tests
2.4. Electrochemical Measurements
2.5. Characterization
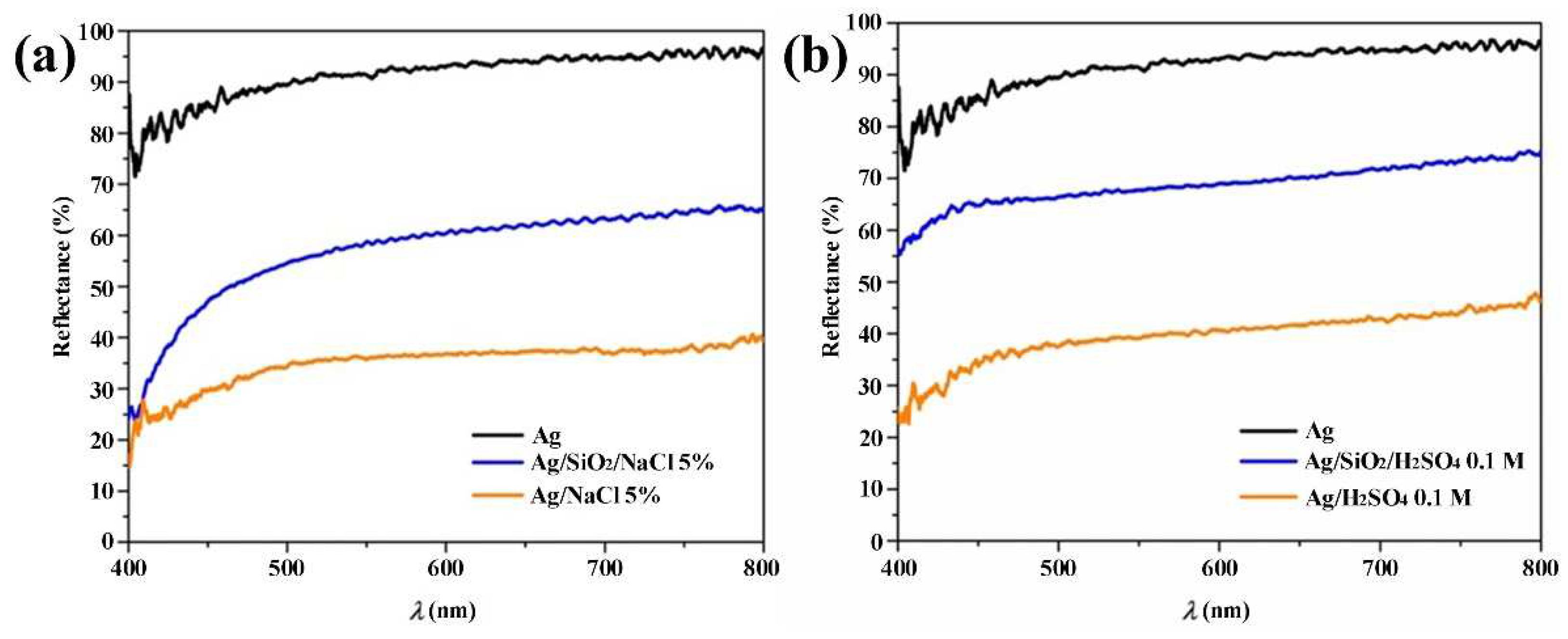
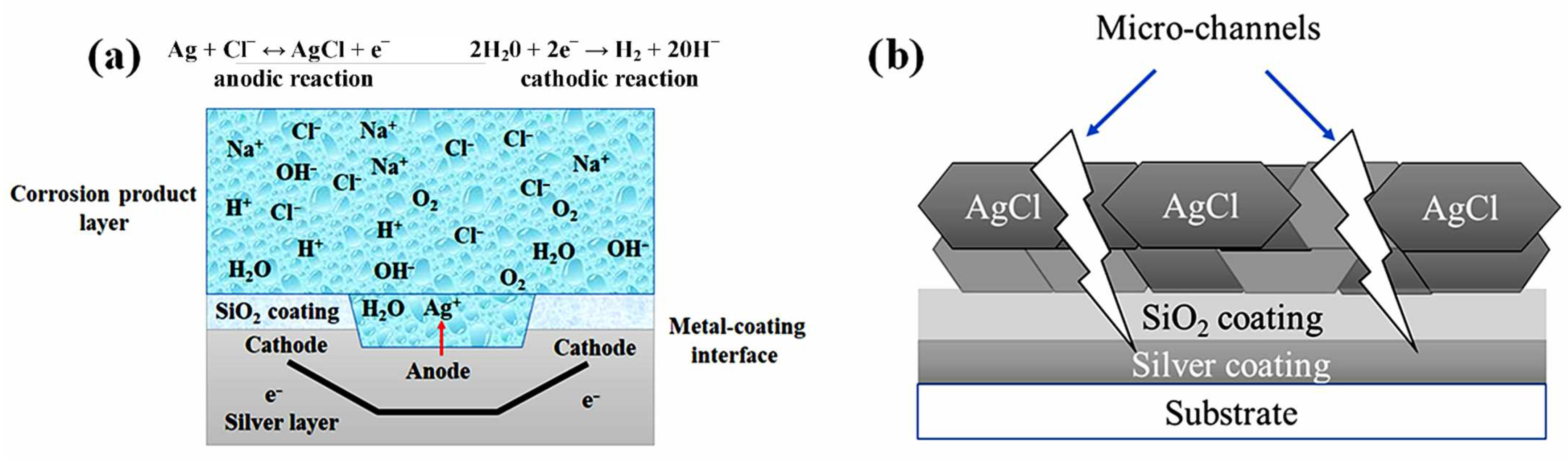
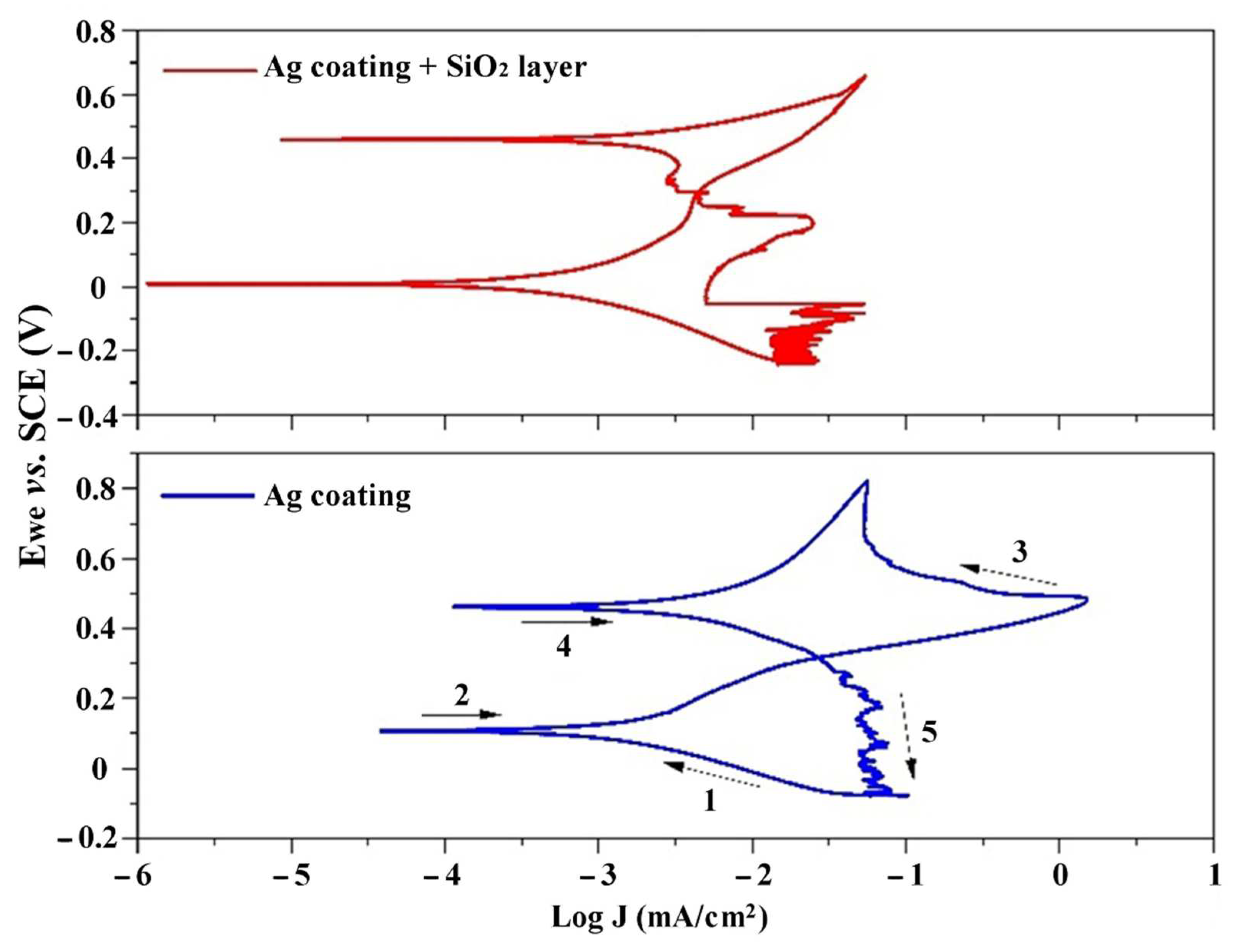
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernández-García, A.; Sutter, F.; Fernández-Reche, J.; Lüpfert, E. Mirrors. In The Performance of Concentrated Solar Power (CSP) Systems; Elsevier: Amsterdam, The Netherlands, 2017; pp. 67–98. [Google Scholar]
- Meyen, S.; Lüpfert, E.; Fernández-García, A.; Kennedy, C. Standardization of Solar Mirror Reflectance Measurements-Round Robin Test. Available online: http://www.nrel.gov/docs/fy11osti/49189.pdf (accessed on 3 October 2022).
- Barlev, D.; Vidu, R.; Stroeve, P. Innovation in concentrated solar power. Sol. Energy Mater. Sol. Cells 2011, 95, 2703–2725. [Google Scholar] [CrossRef]
- Desai, J.D.; Baviskar, P.K.; Hui, K.N.; Pathan, H.M. Quadrivalently Doped Hematite Thin Films for Solar Water Splitting. ES Energy Environ. 2018, 2, 21–30. [Google Scholar] [CrossRef][Green Version]
- Gao, Y.; Wang, Z.; Ding, D.; Li, W.; Ma, Y.; Hao, Y.; Zhang, H. Novel Methods to Harness Solar Radiation for Advanced Energy Applications. ES Energy Environ. 2019, 5, 1–7. [Google Scholar] [CrossRef]
- Kennedy, C.E.; Terwilliger, K. Optical Durability of Candidate Solar Reflectors. J. Sol. Energy Eng. 2005, 127, 262–269. [Google Scholar] [CrossRef]
- Quaschning, V. Technical and economical system comparison of photovoltaic and concentrating solar thermal power systems depending on annual global irradiation. Sol. Energy 2004, 77, 171–178. [Google Scholar] [CrossRef]
- Badán, J.A.; Navarrete-Astorga, E.; Henríquez, R.; Martín, F.; Marotti, R.E.; Ramos-Barrado, J.R.; Dalchiele, E.A. Optical properties of silver nanoparticles deposited onto silicon substrates by different soft-solution processing techniques. Opt. Mater. 2020, 100, 109651. [Google Scholar] [CrossRef]
- Sutter, F.; Fernández-García, A.; Heller, P.; Anderson, K.; Wilson, G.; Schmücker, M.; Marvig, P. Durability Testing of Silvered-Glass Mirrors. Energy Procedia 2015, 69, 1568–1577. [Google Scholar] [CrossRef]
- Bennett, J.M.; Ashley, E.J. Infrared Reflectance and Emittance of Silver and Gold Evaporated in Ultrahigh Vacuum. Appl. Opt. 1965, 4, 221. [Google Scholar] [CrossRef]
- James, R.A.; Stapleton, A.J.; Hughes, A.; Charrault, E.; Zuber, K.; Switalska, E.; Evans, D.; Murphy, P.; Llusca, M. Metallic Adhesive Layers for Ag-Based First Surface Mirrors. Adv. Eng. Mater. 2018, 20, 1800106. [Google Scholar] [CrossRef]
- Thomas, N.L.; Wolfe, J.D. UV-Shifted Durable Silver Coating for Astronomical Mirrors; Dierickx, P., Ed.; SPIE: Bellingham, DC, USA, 2000; pp. 312–323. [Google Scholar]
- Amor, Y.B.; Sutter, E.; Takenouti, H.; Tribollet, B.; Boinet, M.; Faure, R.; Balencie, J.; Durieu, G. Electrochemical study of the tarnish layer of silver deposited on glass. Electrochim. Acta 2014, 131, 89–95. [Google Scholar] [CrossRef]
- Liang, D.; Allen, H.C.; Frankel, G.S.; Chen, Z.Y.; Kelly, R.G.; Wu, Y.; Wyslouzil, B.E. Effects of Sodium Chloride Particles, Ozone, UV, and Relative Humidity on Atmospheric Corrosion of Silver. J. Electrochem. Soc. 2010, 157, C146. [Google Scholar] [CrossRef]
- Graedel, T.E.; Leygraf, C. Corrosion Mechanisms for Nickel Exposed to the Atmosphere. J. Electrochem. Soc. 2000, 147, 1010. [Google Scholar] [CrossRef]
- Avenel, C.; Raccurt, O.; Gardette, J.-L.; Therias, S. Review of accelerated ageing test modelling and its application to solar mirrors. Sol. Energy Mater. Sol. Cells 2018, 186, 29–41. [Google Scholar] [CrossRef]
- Girard, R.; Delord, C.; Disdier, A.; Raccurt, O. Critical Constraints Responsible to Solar Glass Mirror Degradation. Energy Procedia 2015, 69, 1519–1528. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Yu, H.; He, Y.-L. A feedforward-feedback hybrid control strategy towards ordered utilization of concentrating solar energy. Renew. Energy 2020, 154, 305–315. [Google Scholar] [CrossRef]
- Fernández-García, A.; Sutter, F.; Montecchi, M.; Sallaberry, F.; Heimsath, A.; Heras, C.; Baron, E.L.; Soum-Glaude, A. Parameters and Method to Evaluate the Reflectance Properties of Reflector Materials for Concentrating Solar Power Technology under Laboratory Conditions. Available online: https://www.solarpaces.org/wp-content/uploads/Document-1_SolarPACES_Reflectance-Guideline_V3.1.pdf (accessed on 3 October 2022).
- Utkin, A.I.; Zavitaev, E.V.; Yushkanov, A.A. Calculation of the electrical conductivity of a thin metal layer in the case of different specular reflectances of its surfaces. J. Surf. Investig. X-Ray Synchrotron Neutron Tech. 2016, 10, 962–968. [Google Scholar] [CrossRef]
- Sutter, F.; Fernández-García, A.; Heimsath, A.; Montecchi, M.; Pelayo, C. Advanced measurement techniques to characterize the near-specular reflectance of solar mirrors. AIP Conf. Proc. 2019, 2126, 110003. [Google Scholar]
- Heimsath, A.; Nitz, P. Scattering and specular reflection of solar reflector materials—Measurements and method to determine solar weighted specular reflectance. Sol. Energy Mater. Sol. Cells 2019, 203, 110191. [Google Scholar] [CrossRef]
- Alder, F.A.; Charrault, E.; Zuber, K.; Fabretto, M.; Patil, A.; Murphy, P.; Llusca, M. Fabrication of robust solar mirrors on polymeric substrates by physical vapor deposition technique. Sol. Energy Mater. Sol. Cells 2020, 209, 110476. [Google Scholar] [CrossRef]
- Anjum, F.; Fryauf, D.M.; Ahmad, R.; Phillips, A.C.; Kobayashi, N.P. Improving silver mirrors with aluminum oxynitride protection layers: Variation in refractive index with controlled oxygen content by radiofrequency magnetron sputtering. J. Astron. Telesc. Instrum. Syst. 2018, 4, 044004. [Google Scholar] [CrossRef]
- Limam, E.; Maurice, V.; Seyeux, A.; Zanna, S.; Klein, L.H.; Chauveau, G.; Grèzes-Besset, C.; Savin De Larclause, I.; Marcus, P. Local Degradation Mechanisms by Tarnishing of Protected Silver Mirror Layers Studied by Combined Surface Analysis. J. Phys. Chem. B 2018, 122, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Fryauf, D.M. Effect of intermediate layers on atomic layer deposition-aluminum oxide protected silver mirrors. J. Astron. Telesc. Instrum. Syst. 2017, 3, 034001. [Google Scholar] [CrossRef]
- Leygarf, C.; Wallinder, I.O.; Tidblad, J.; Graedel, T. Atmospheric Corrosion; The Electrochemical Society Series; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 337–347. [Google Scholar]
- Wan, Y.; Wang, X.; Wang, X.; Li, Y.; Sun, H.; Zhang, K. Determination and generation of the corrosion compounds on silver exposed to the atmospheres. Int. J. Electrochem. Sci. 2015, 10, 2336–2354. [Google Scholar]
- Folgner, K.A.; Chu, C.-T.; Lingley, Z.R.; Kim, H.I.; Yang, J.-M.; Barrie, J.D. Environmental durability of protected silver mirrors prepared by plasma beam sputtering. Appl. Opt. 2017, 56, C75. [Google Scholar] [CrossRef]
- Chiou, A.-H.; Chien, T.-C.; Su, C.-K.; Lin, J.-F.; Hsu, C.-Y. The effect of differently sized Ag catalysts on the fabrication of a silicon nanowire array using Ag-assisted electroless etching. Curr. Appl. Phys. 2013, 13, 717–724. [Google Scholar] [CrossRef]
- Limam, E.; Maurice, V.; Seyeux, A.; Zanna, S.; Klein, L.H.; Chauveau, G.; Grèzes-Besset, C.; Savin De Larclause, I.; Marcus, P. Role of SiC substrate surface on local tarnishing of deposited silver mirror stacks. Appl. Surf. Sci. 2018, 436, 1147–1156. [Google Scholar] [CrossRef]
- Mishra, S.K.; Kumar, V.; Tiwari, S.K.; Mishra, T.; Angula, G.; Adhikari, S. Development and degradation behavior of protective multilayer coatings for aluminum reflectors for solar thermal applications. Thin Solid Film. 2016, 619, 202–207. [Google Scholar] [CrossRef]
- García-Segura, A.; Fernández-García, A.; Ariza, M.J.; Sutter, F.; Valenzuela, L. Effects of reduced sulphur atmospheres on reflector materials for concentrating solar thermal applications. Corros. Sci. 2018, 133, 78–93. [Google Scholar] [CrossRef]
- Xie, P.; Li, Y.; Hou, Q.; Sui, K.; Liu, C.; Fu, X.; Zhang, J.; Murugadoss, V.; Fan, J.; Wang, Y.; et al. Tunneling-induced negative permittivity in Ni/MnO nanocomposites by a bio-gel derived strategy. J. Mater. Chem. C 2020, 8, 3029–3039. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, Q.; Zhang, Z.; Kang, Z.; Liao, Q.; Ding, Y.; Ma, M.; Gao, F.; Zhao, X.; Zhang, Y. Electromagnetic Shielding Hybrid Nanogenerator for Health Monitoring and Protection. Adv. Funct. Mater. 2018, 28, 1703801. [Google Scholar] [CrossRef]
- Kong, L.; Yin, X.; Xu, H.; Yuan, X.; Wang, T.; Xu, Z.; Huang, J.; Yang, R.; Fan, H. Powerful absorbing and lightweight electromagnetic shielding CNTs/RGO composite. Carbon 2019, 145, 61–66. [Google Scholar] [CrossRef]
- Kargar, F.; Barani, Z.; Balinskiy, M.; Magana, A.S.; Lewis, J.S.; Balandin, A.A. Dual-Functional Graphene Composites for Electromagnetic Shielding and Thermal Management. Adv. Electron. Mater. 2019, 5, 1800558. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, C.; Feng, A.; Shi, P.; Zhang, C.; Liu, X.; Wang, K.; Wu, G. A low-dielectric decoration strategy to achieve absorption dominated electromagnetic shielding material. Compos. Part B Eng. 2020, 183, 107690. [Google Scholar] [CrossRef]
- Gilliam, M.A.; Farhat, S.A.; Garner, G.E.; Stubbs, B.P.; Peterson, B.B. Characterization of the deposition behavior and changes in bonding structures of hexamethyldisiloxane and decamethylcyclopentasiloxane atmospheric plasma-deposited films. Plasma Process. Polym. 2019, 16, e1900024. [Google Scholar] [CrossRef]
- Hossain, M.M.; Trinh, Q.H.; Nguyen, D.B.; Sudhakaran, M.S.P.; Mok, Y.S. Robust hydrophobic coating on glass surface by an atmospheric-pressure plasma jet for plasma-polymerisation of hexamethyldisiloxane conjugated with (3-aminopropyl) triethoxysilane. Surf. Eng. 2019, 35, 466–475. [Google Scholar] [CrossRef]
- Trinh, Q.H.; Nguyen, D.B.; Hossain, M.M.; Mok, Y.S. Deposition of superhydrophobic coatings on glass substrates from hexamethyldisiloxane using a kHz-powered plasma jet. Surf. Coat. Technol. 2019, 361, 377–385. [Google Scholar] [CrossRef]
- Gotoh, K.; Shohbuke, E.; Ryu, G. Application of atmospheric pressure plasma polymerization for soil guard finishing of textiles. Text. Res. J. 2018, 88, 1278–1289. [Google Scholar] [CrossRef]
- Shohbuke, E.; Gotoh, K. Control of Wettability and Antifouling Property of Polyester Fabric by Atmospheric Pressure Plasma Jet Treatments. J. Jpn. Res. Assoc. Text. 2018, 59, 359–368. [Google Scholar] [CrossRef]
- Pérez-Bueno, J.J.; Vasquez-García, S.R.; García-González, L.; Vorobiev, Y.V.; Luna-Bárcenas, G.; González-Hernández, J. Optical processes in PMMA, SiO2, and hybrid organic-inorganic sol-gel films colored with rhodamine 6GDN. J. Phys. Chem. B 2002, 106, 1550–1556. [Google Scholar] [CrossRef]
- Díaz-Flores, L.L.; Pérez-Bueno, J.J.; Ramírez-Bon, R.; Espinoza-Beltrán, F.J.; Vorobiev, Y.V.; González-Hernández, J. Improved light stability of colored SiO2 coatings containing organic and metalorganic dye molecules. J. Vac. Sci. Technol. A Vac. Surf. Film. 2000, 18, 1579–1583. [Google Scholar] [CrossRef]
- ASTM D1654-08(2016)e1; Standard Test Method for Evaluation of Painted or Coated Specimens Subjected to Corrosive Environments. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM B117-19; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM International: West Conshohocken, PA, USA, 2019.
- Keast, V.J. Atmospheric Corrosion of Silver and Silver Nanoparticles. Corros. Mater. Degrad. 2022, 3, 221–234. [Google Scholar] [CrossRef]
- Payer, J.H.; Ball, G.; Rickett, B.I.; Kim, H.S. Role of transport properties in corrosion product growth. Mater. Sci. Eng. A 1995, 198, 91–102. [Google Scholar] [CrossRef]
- Jin, X.; Lu, J.; Liu, P.; Tong, H. The electrochemical formation and reduction of a thick AgCl deposition layer on a silver substrate. J. Electroanal. Chem. 2003, 542, 85–96. [Google Scholar] [CrossRef]
- Cruz-Manzo, S.; Greenwood, P. Analytical transfer function to simulate the dynamic response of the finite-length Warburg impedance in the time-domain. J. Energy Storage 2022, 55, 105529. [Google Scholar] [CrossRef]







| Parameter | Value |
|---|---|
| UV index | 8.1 index (high) |
| Relative humidity (%) | 45 |
| Temperature (°C) | 24 |
| Solar radiation index | 610 W/m2 |
| Samples with a Protective Coating | Samples without Coating | |||
|---|---|---|---|---|
| Environmental weathering degradation | 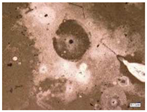 |  | 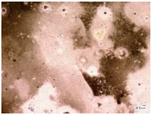 | 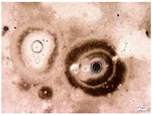 |
| Accelerated weathering degradation (NaCl 5%) | 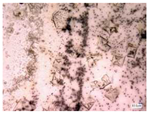 |  |  | 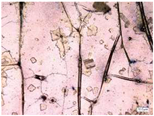 |
| Accelerated weathering degradation (H2SO4 0.1 M) |  | 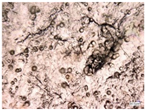 | 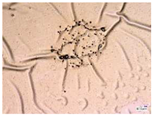 |  |
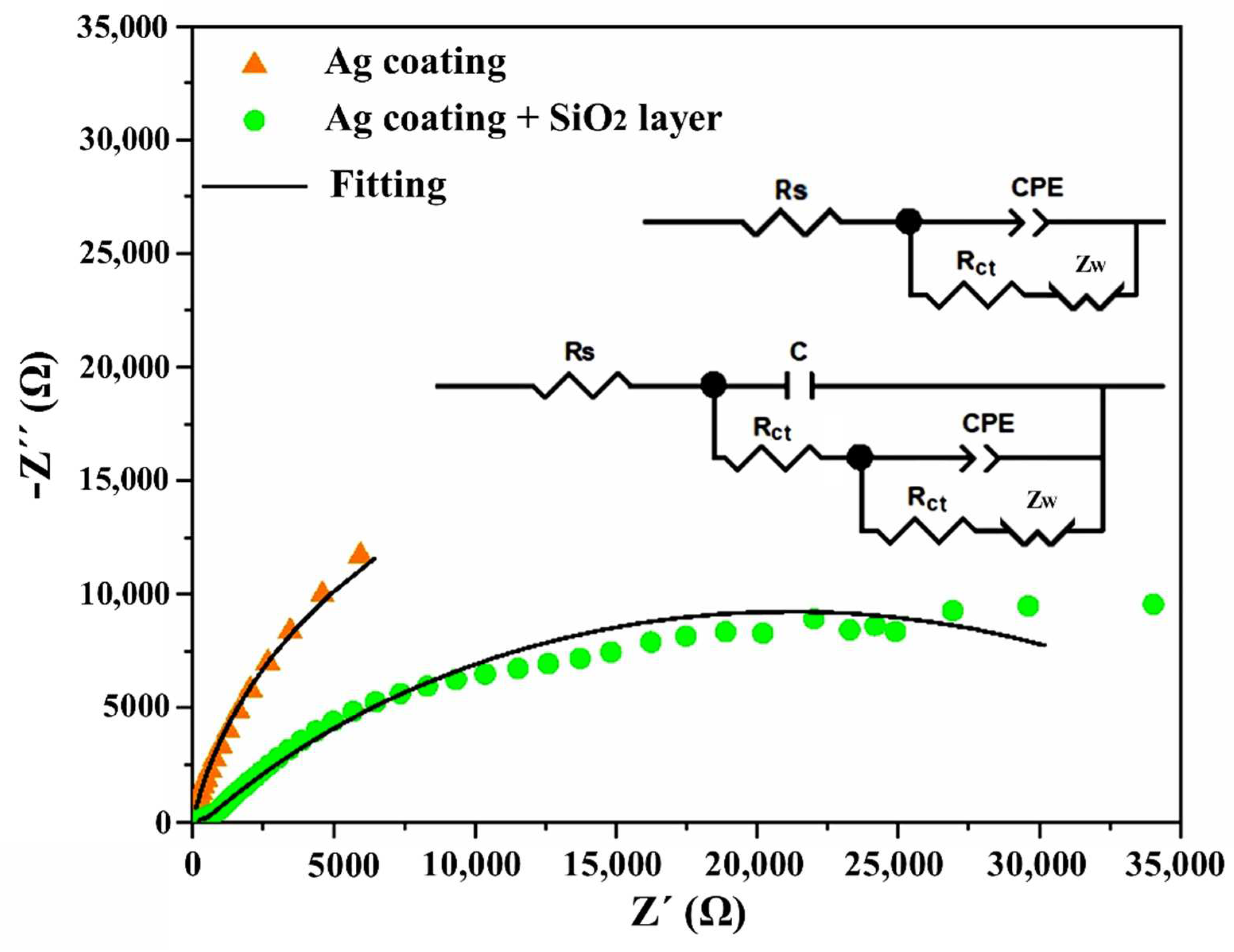
| Sample | βa | βc | Ecorr (mV) | Icorr (µA/cm2) | Epitt (V) | Corrosion Rate (mmpy) |
|---|---|---|---|---|---|---|
| Ag Coating | 174.167 ± 36 | 120.3 ± 32 | 63.25 ± 37.33 | 1.24 ± 0.59 | 0.13 ± 0.02 | 0.0208 ± 0.01 |
| Ag Coating + SiO2 layer | 185.5 ± 26 | 150.3 ± 29 | 8.42 ± 12.78 | 0.54 ± 0.13 | 0.21 ± 0.06 | 0.0091 ± 0.003 |
| Sample | Rs (Ω) | C (F) | Rtc (Ω) | CPE-T (F.s(n−1)) | CPE-P (F.s(n−1)) | Rtc (Ω) |
|---|---|---|---|---|---|---|
| Ag Coating | 25.52 ± 10.13 | - | - | 0.000544 ± 0.0003 | 0.8437 ± 0.059 | 30,937.5 ± 13,408 |
| Ag Coating + SiO2 layer | 210.2 ± 8.20 | 7.24 × 10−8 | 205.12 ± 11.71 | 4.81 × 10−5 ± 1.2 × 10−5 | 0.0061 ± 0.002 | 41,527.93 ± 1572 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magdaleno López, C.; Pérez Bueno, J.d.J.; Cabello Mendez, J.A.; Hernández Leos, R.; Mendoza López, M.L.; Sosa Domínguez, A.; Meas Vong, Y. Deterioration of Novel Silver Coated Mirrors on Polycarbonate Used for Concentrated Solar Power. Sustainability 2022, 14, 16360. https://doi.org/10.3390/su142416360
Magdaleno López C, Pérez Bueno JdJ, Cabello Mendez JA, Hernández Leos R, Mendoza López ML, Sosa Domínguez A, Meas Vong Y. Deterioration of Novel Silver Coated Mirrors on Polycarbonate Used for Concentrated Solar Power. Sustainability. 2022; 14(24):16360. https://doi.org/10.3390/su142416360
Chicago/Turabian StyleMagdaleno López, Coraquetzali, José de Jesús Pérez Bueno, José Antonio Cabello Mendez, Rosalba Hernández Leos, Maria Luisa Mendoza López, Adrián Sosa Domínguez, and Yunny Meas Vong. 2022. "Deterioration of Novel Silver Coated Mirrors on Polycarbonate Used for Concentrated Solar Power" Sustainability 14, no. 24: 16360. https://doi.org/10.3390/su142416360
APA StyleMagdaleno López, C., Pérez Bueno, J. d. J., Cabello Mendez, J. A., Hernández Leos, R., Mendoza López, M. L., Sosa Domínguez, A., & Meas Vong, Y. (2022). Deterioration of Novel Silver Coated Mirrors on Polycarbonate Used for Concentrated Solar Power. Sustainability, 14(24), 16360. https://doi.org/10.3390/su142416360








