Evaluating Different Soil Amendments as Bioremediation Strategy for Wetland Soil Contaminated by Crude Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mesocosm Soil and Experimental Design
2.2. Soil and FWAD Physicochemical Properties Determination
2.3. Soil Biological Properties Determination
2.4. Hydrocarbons Analysis
2.5. Metal(Loid)s Analysis
2.6. Ecotoxicity Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Soil Characterisation
3.2. Soil Respiration and Its Relationship with Hydrocarbons Degradation
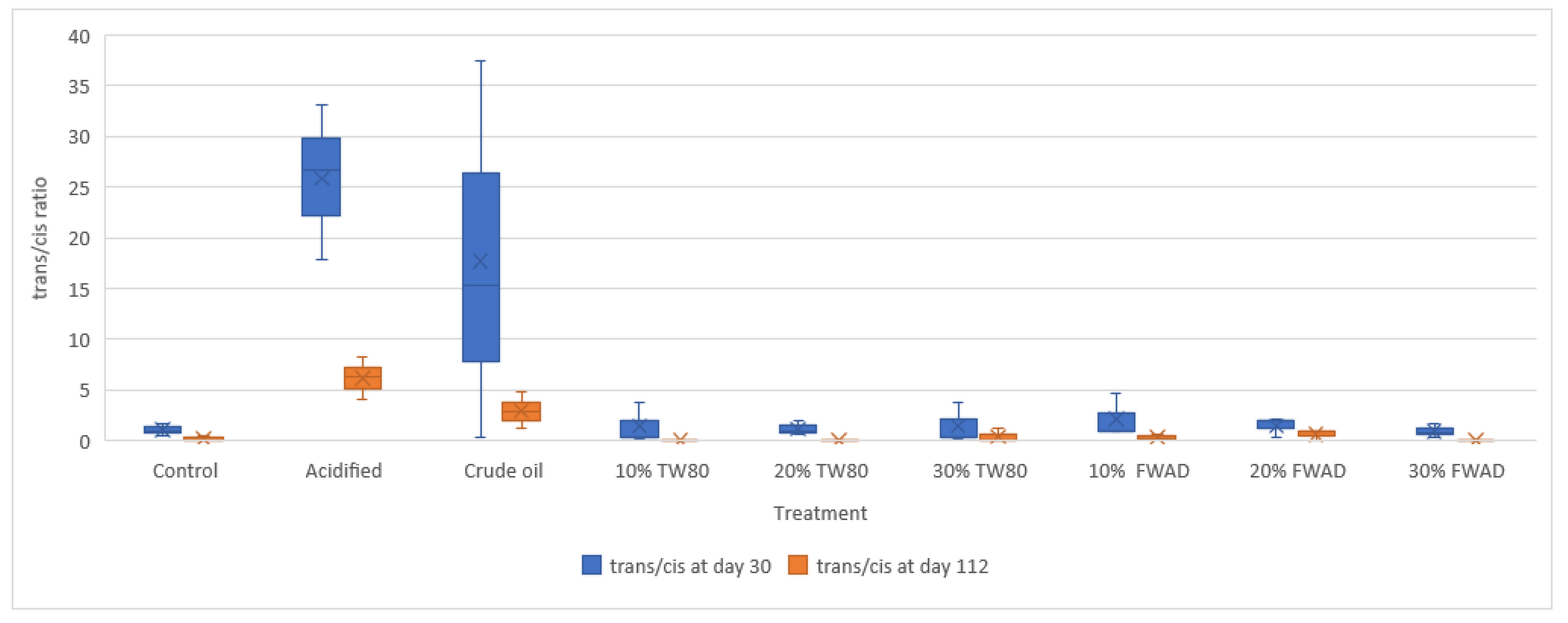
3.3. Soil Microbial Community Dynamics, and Environmental Stress
3.4. Crude Oil Degradation
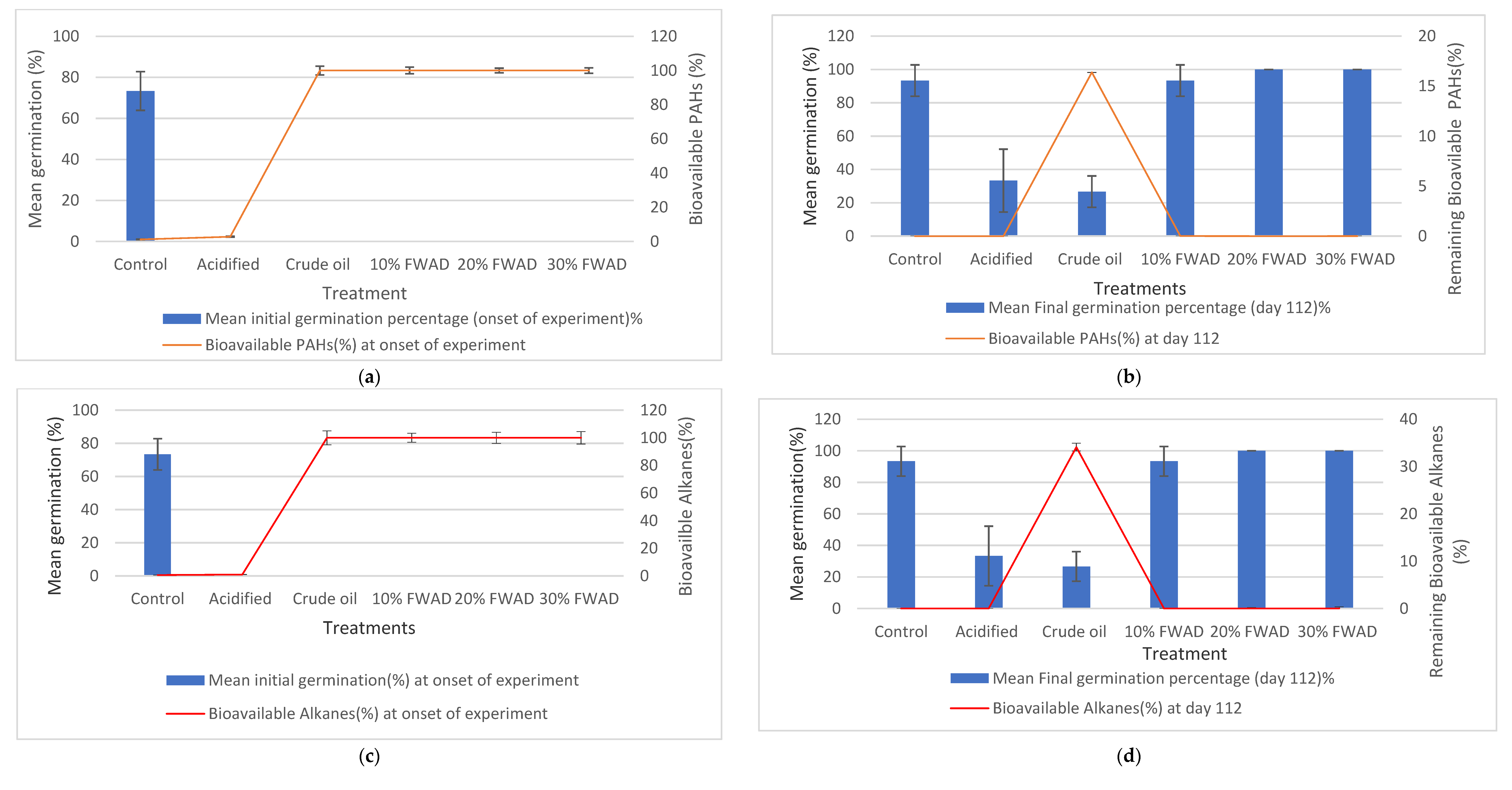
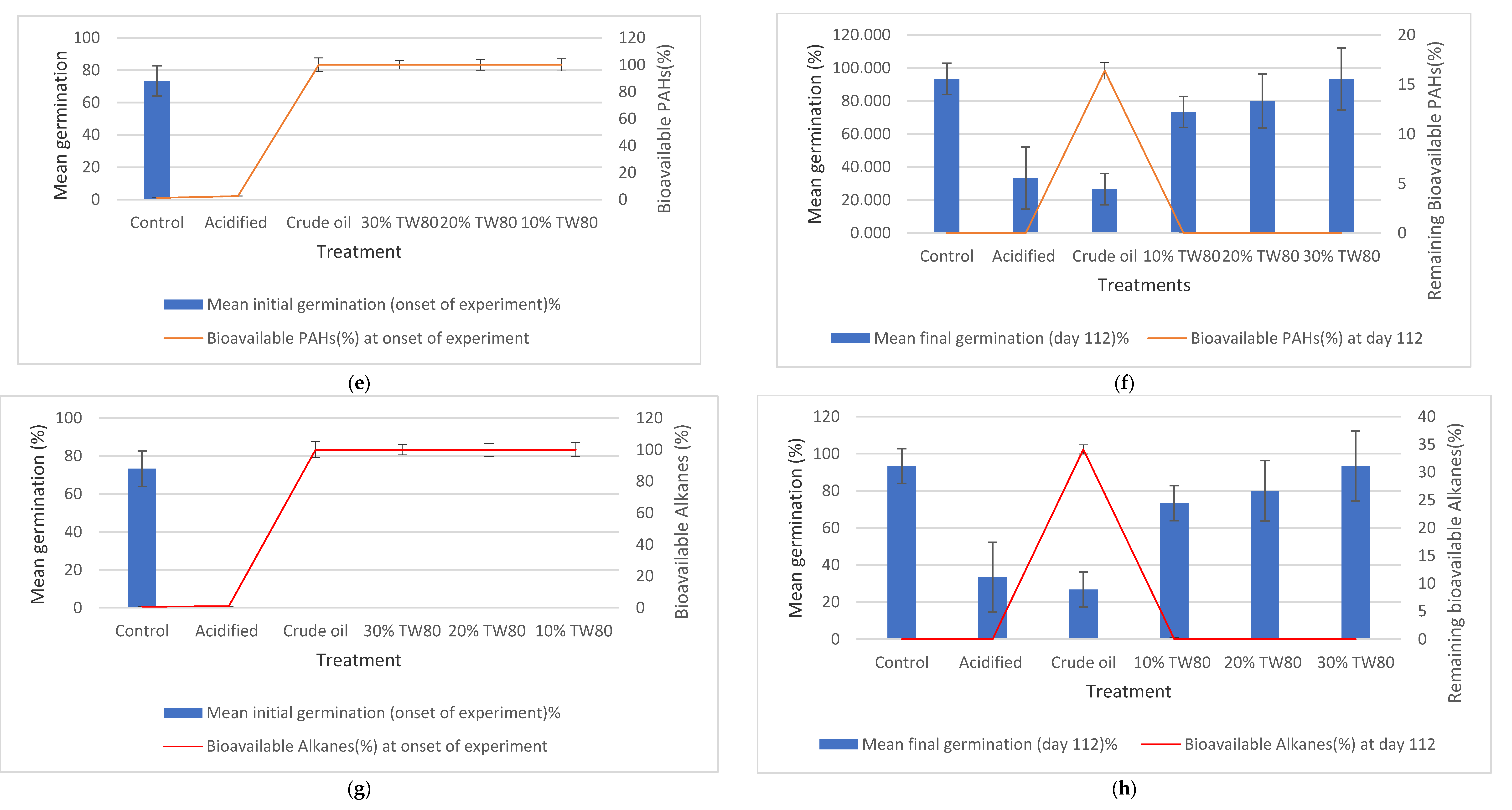
3.5. Remediation Endpoint
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drake, H.L.; Horn, M.A.; Wüst, P.K. Intermediary ecosystem metabolism as a main driver of methanogenesis in acidic wetland soil. Environ. Microbiol. Rep. 2009, 1, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Morse, J.L.; Ardón, M.; Bernhardt, E.S. Greenhouse gas fluxes in South-Eastern U.S. coastal plain wetlands under contrasting land uses. Ecol. Appl. 2012, 22, 264–280. [Google Scholar] [CrossRef] [PubMed]
- Nwankwoala, H.O.; Okujagu, D.C. A Review of Wetlands and Coastal Resources of the Niger Delta: Potentials, Challenges and Prospects. Environ. Ecosyst. Sci. 2014, 5, 37–46. [Google Scholar]
- Johnston, S.G.; Burton, E.D.; Aaso, T.; Tuckerman, G. Sulphur, iron and carbon cycling following hydrological restoration of acidic freshwater wetlands. Chem. Geol. 2014, 371, 9–26. [Google Scholar] [CrossRef]
- Ohimain, E.I. Environmental Impacts of oil mining activities in the Niger Delta Mangrove Ecosystem. In Proceedings of the 8th International Congress on Mine Water & the Environment, Johannesburg, South Africa; Nel, P.J.L., Ed.; International Mine Water Association: Granada, Spain, 2003; pp. 503–517. [Google Scholar]
- Konne, B.R. Inadequate monitoring and enforcement in the Nigerian oil industry: The case of shell and ogoniland. Cornell Int. Law J. 2014, 47, 181–204. [Google Scholar]
- Jeffries, D.S.; Brydges, T.G.; Dillon, P.J.; Keller, W. Monitoring the results of Canada / USA acid rain control programs: Some lake responses. Environ. Monit. Assess. 2003, 88, 3–19. [Google Scholar] [CrossRef]
- Singh, S.; Chakraborty, S. Performance of organic substrate amended constructed wetland treating acid mine drainage (AMD) of North-Eastern India. J. Hazard. Mater. 2020, 397, 122–130. [Google Scholar] [CrossRef]
- Ruley, J.A.; Amoding, A.; Tumuhairwe, J.B.; Basamba, T.A.; Opolot, E. Enhancing the phytoremediation of hydrocarbon-contaminated soils in the Sudd Wetlands, South Sudan, using organic manure. Appl. Environ. Soil Sci. 2020, 2020, 4614286. [Google Scholar] [CrossRef]
- Osuji, L.C.; Idung, I.D.; Ojinnaka, C.M. Preliminary investigation on Mgbede-20 oil-polluted site in Niger Delta, Nigeria. Chem. Biodivers. 2006, 3, 568–577. [Google Scholar] [CrossRef]
- Zhu, X.; Venosa, A.; Suidan, M.; Lee, K. Guidelines for the Bioremediation of Oil-Contaminated Salt Marshes. EPA, July 2004. pp. 1–61. Available online: http://edocs.dlis.state.fl.us/fldocs/oilspill/federal/LPS68040.pdf (accessed on 21 August 2021).
- Nwaichi, E.O.; Uzazobona, M.A. Estimation of the CO2 Level due to Gas Flaring in the Niger Delta. Res. J. Environ. Sci. 2011, 5, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Osuji, C.; Adesiyan, S.O.; Obute, G.C. Post-impact assessment of oil pollution in agbada west plain of niger delta, nigeria: Field reconnaissance and total extractable hydrocarbon. Chem. Biodivers. 2004, 1, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Sam, K.; Coulon, F.; Prpich, G. Working towards an integrated land contamination management framework for Nigeria. Sci. Total Environ. 2016, 571, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Bonte, M.; Gill, R.; Dawick, J.; Boogaard, P.J. Heavy hydrocarbon fate and transport in the environment. Q. J. Eng. Geol. Hydrogeol. 2017, 50, 333–346. [Google Scholar] [CrossRef]
- Robichaud, K.; Lebeau, M.; Martineau, S.; Amyot, M. Bioremediation of engine-oil contaminated soil using local residual organic matter. PeerJ 2019, 7, e7389. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Liu, L.; Yang, C.; Kang, W.; Yan, Z.; Zhu, Y.; Wang, J.; Zhang, H. Removal kinetics of petroleum hydrocarbons from low-permeable soil by sand mixing and thermal enhancement of soil vapor extraction. Chemosphere 2019, 236, 124319. [Google Scholar] [CrossRef]
- Ngene, S.; Tota-maharaj, K. Environmental technologies for remediation of contaminated lands in the Niger Delta region of Nigeria: Opportunities for ecosystem services to host. In Proceedings of the Environmental Design and Management International Conference, Ile-Ife, Nigeria, 2–22 May 2019; pp. 1–12. [Google Scholar]
- Okoye, A.U.; Chikere, C.B.; Okpokwasili, G.C. Isolation and Characterization of Hexadecane Degrading Bacteria from Oil-polluted soil in Gio Community, Niger Delta, Nigeria. Sci. Afr. 2020, 9, e00340. [Google Scholar] [CrossRef]
- Scanferla, P.; Ferrari, G.; Pellay, R.; Volpi-Ghirardini, A.; Zanetto, G.; Libralato, G. An innovative stabilization/solidification treatment for contaminated soil remediation: Demonstration project results. J. Soils Sediments 2009, 9, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Ossai, I.C.; Hamid, F.S.; Hassan, A. Micronised keratinous wastes as co-substrates, and source of nutrients and microorganisms for trichoremediation of petroleum hydrocarbon polluted soil. Biocatal. Agric. Biotechnol. 2002, 43, 102346. [Google Scholar] [CrossRef]
- Cipullo, S.; Negrin, I.; Claveau, L.; Snapir, B.; Tardif, S.; Pulleyblank, C.; Prpich, G.; Campo, P.; Coulon, F. Linking bioavailability and toxicity changes of complex chemicals mixture to support decision making for remediation endpoint of contaminated soils. Sci. Total Environ. 2019, 650, 2150–2163. [Google Scholar] [CrossRef] [Green Version]
- Abdulyekeen, K.A.; Umar, A.A.; Patah, M.F.A.; Daud, W.M.A.W. Torrefaction of biomass: Production of enhanced solid biofuel from municipal solid waste and other types of biomass. Renew. Sustain. Energy Rev. 2021, 150, 111436. [Google Scholar] [CrossRef]
- Aghalibe, C.; Igwe, J.; Obike, A. Studies on the removal of petroleum hydrocarbons (PHCs) from a crude oil impacted soil amended with cow dung, poultry manure and npk fertilizer. Chemrj. Org. 2017, 2, 22–30. [Google Scholar]
- Lee, C.T.; Hashim, H.; Ho, C.S.; Fan, Y.V.; Klemeš, J.J. Sustaining the low-carbon emission development in Asia and beyond: Sustainable energy, water, transportation and low-carbon emission technology. J. Clean. Prod. 2017, 146, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ngene, S.; Tota-Maharaj, K. Effectiveness of Sand Filtration and Activated Carbon in Oilfield Wastewater Treatment. Int. J. Chem. Eng. Res. 2020, 7, 13–23. [Google Scholar] [CrossRef]
- Smidt, E.; Tintner, J.; Böhm, K.; Binner, E. Transformation of Biogenic Waste Materials through Anaerobic Digestion and subsequent composting of the residues. A case study. Dyn. Soil Dyn. Plant 2011, 5, 63–69. [Google Scholar]
- Peng, W.; Pivato, A. Sustainable Management of Digestate from the Organic Fraction of Municipal Solid Waste and Food Waste Under the Concepts of Back to Earth Alternatives and Circular Economy. Waste Biomass Valorization 2019, 10, 465–481. [Google Scholar] [CrossRef]
- Fernández-Bayo, J.D.; Achmon, Y.; Harrold, D.R.; McCurry, D.G.; Hernandez, K.; Dahlquist-Willard, R.M.; Simmons, C.W. Assessment of Two Solid Anaerobic Digestate Soil Amendments for Effects on Soil Quality and Biosolarization Efficacy. J. Agric. Food Chem. 2017, 65, 3434–3442. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef] [Green Version]
- Vaneeckhaute, C.; Meers, E.; Michels, E.; Christiaens, P.; Tack, F.M.G. Fate of macronutrients in water treatment of digestate using vibrating reversed osmosis. Water Air Soil Pollut. 2012, 223, 1593–1603. [Google Scholar] [CrossRef] [Green Version]
- Gielnik, A.; Pechaud, Y.; Huguenot, D.; Cébron, A.; Esposito, G.; van Hullebusch, E.D. Bacterial seeding potential of digestate in bioremediation of diesel contaminated soil. Int. Biodeterior. Biodegrad. 2019, 143, 104715. [Google Scholar] [CrossRef]
- Bustamante, M.; Durán, N.; Diez, M.C. Biosurfactants are useful tools for the bioremediation of contaminated soil: A review. J. Soil Sci. Plant Nutr. 2012, 12, 667–687. [Google Scholar] [CrossRef] [Green Version]
- Gielnik, A.; Pechaud, Y.; Huguenot, D.; Esposito, G.; Guibaud, G.; van Hullebusch, E.D. Potential Use of Waste-to-Bioenergy By-Products in Bioremediation of Total Petroleum Hydrocarbons (TPH)-Contaminated Soils. In Environmental Soil Remediation and Rehabilitation; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 978-3-030-40347-8. [Google Scholar]
- Silva, F.B.; Pereira, F.L.; Le-Hyaric, M. A Comparison of the physicochemical properties and fatty acid composition of Indaiá (Attalea dubia) and Babassu (Orbignya phalerata) oils. Sci. World J. 2012, 2012, 532374. [Google Scholar] [CrossRef] [Green Version]
- Andrew, R. Using quality anaerobic digestate to benefit crops Nutrients in digestate are valuable. Soil Crops 2012, 9, 1–12. [Google Scholar]
- Yu, A.I.; Geletukha, G.G.; Kucheruk, P.P. Digestate Potential to Substitute Mineral Fertilizers: Engineering Approaches. J. Eng. Sci. 2022, 9, 1–10. [Google Scholar]
- Opatokun, S.A.; Strezov, V.; Kan, T. Product based evaluation of pyrolysis of food waste and its digestate. Energy 2015, 92, 349–354. [Google Scholar] [CrossRef]
- Sung, K.; Kim, K.S.; Park, S.; Sung, K.; Kim, K.S.; Park, S. Enhancing degradation of total petroleum hydrocarbons and uptake of heavy metals in a wetland microcosm planted with phragmites communis by humic acids addition. Int. J. Phytoremediation 2013, 15, 536–549. [Google Scholar] [CrossRef]
- Wang, X.; Sun, L.; Wang, H.; Wu, H.; Chen, S.; Zheng, X. Surfactant-enhanced bioremediation of DDTs and PAHs in contaminated farmland soil. Environ. Technol. Taylor Fr. 2018, 39, 1733–1744. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Yang, C.; Lai, C.; Zhang, C.; Liu, Y. Tween 80 surfactant-enhanced bioremediation: Toward a solution to the soil contamination by hydrophobic organic compounds. Crit. Rev. Biotechnol. 2018, 38, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Bishop, P.L. Influence of nonionic surfactant on attached biofilm formation and phenanthrene bioavailability during simulated surfactant enhanced bioremediation. Environ. Sci. Technol. 2007, 41, 7107–7113. [Google Scholar] [CrossRef]
- Ceschia, E.; Harjani, J.R.; Liang, C.; Ghoshouni, Z.; Andrea, T.; Brown, R.S.; Jessop, P.G. Switchable anionic surfactants for the remediation of oil-contaminated sand by soil washing. RSC Adv. 2014, 4, 4638–4645. [Google Scholar] [CrossRef]
- Nduka, J.K.C.; Orisakwe, O.E.; Ezenweke, L.O.; Ezenwa, T.E.; Chendo, M.N.; Ezeabasili, N.G. Acid rain phenomenon in Niger Delta region of Nigeria: Economic, biodiversity, and public health concern. Sci. World J. 2008, 8, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Onu, P.U.; Quan, X.; Ling, X. Acid rain: An analysis on the cause, impacts and abatement measures Niger Delta perspective, Nigeria. Int. J. Sci. Eng. Res. 2014, 5, 1214–1219. [Google Scholar]
- Chidinma Peace Okafor, N.L.; Chikere, C.B.; Akaranta, O.; Ntushelo, K. Indigenous microbial strains as bioresource for remediation of chronically polluted Niger Delta soils. Sci. Afr. 2021, 11, e00682. [Google Scholar]
- Ugochukwu, U.C.; Ochonogor, A.; Jidere, C.M.; Agu, C.; Nkoloagu, F.; Ewoh, J.; Okwu-delunzu, V.U. Exposure risks to polycyclic aromatic hydrocarbons by humans and livestock (cattle ) due to hydrocarbon spill from petroleum products in Niger-delta wetland’, Environment International. Elsevier 2018, 115, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Fubara-Manuel, I.; Igoni, A.H.; Jumbo, R.B. Performance of irrigated maize in a crude-oil polluted soil remediated by three nutrients in Nigeria’s Niger Delta. Am. J. Eng. Res. 2017, 6, 180–185. [Google Scholar]
- Nwankwo, C.A. Using Compost to Reduce Oil Contamination in Soils. Chindo Anulika Nwankwo Submitted in Accordance with the Requirements for the Degree of Doctor of Philosophy; The University of Leeds School of Civil Engineering: Leeds, UK, 2014. [Google Scholar]
- Trinchera, A.; Baratella, V. Use of a non-ionicwater surfactant in lettuce fertigation for optimizingwater use, improving nutrient use efficiency, and increasing crop quality. Water 2018, 10, 613. [Google Scholar] [CrossRef] [Green Version]
- British Standard (BS), 7755: Section 3.1; Soil Quality—Determination Dry Matter and Water Content on a Mass Basis by a Gravimetric Method. British Standards Institution Publications: London, UK, 1994.
- British Standard, EN 13039; Determination of the Organic Matter Content and Ash. British Standards Institution Publications: London, UK, 2000.
- British Standard BS 7755 Section 5.5; Water Retention Characteristics. British Standards Institution Publications: London, UK, 1999.
- British Standard BS 11277; Determination of Particle Size Distribution in Mineral Soil Material. British Standards Institution Publications: London, UK, 2009.
- International Organization for Standardization. ISO Standard No 11277:2009; Soil Quality Determination of Particle Size Distribution in Mineral Soil Material: Method by Sieving and Sedimentation. International Organization for Standardization: Geneva, Switzerland, 2009.
- British Standard, 7755: Section 3.6; Determination of Phosphorus. British Standards Institution Publications: London, UK, 1995.
- British Standard, 3882: (Annexes D, E & G); Determination of Potassium and Magnesium. British Standards Institution Publications: London, UK, 1994.
- British Standard, 7755 Section 3.8; Determination of Organic and Total Carbon after Dry Combustion. British Standards Institution Publications: London, UK, 1995.
- He, L.; Xu, X. Mapping soil microbial residence time at the global scale. Glob. Change Biol. 2021, 27, 6484–6497. [Google Scholar] [CrossRef]
- Pawlett, M.; Ritz, K.; Dorey, R.A.; Rocks, S.; Ramsden, J.; Harris, J.A. The impact of zero-valent iron nanoparticles upon soil microbial communities is context dependent. Environ. Sci. Pollut. Res. 2013, 20, 1041–1049. [Google Scholar] [CrossRef] [Green Version]
- Ritz, K.; Harris, M.; Pawlett, M.; Stone, D. Catabolic profiles as an indicator of soil microbial functional diversity. In Environment Agency Science Report, SC040063/R; Environment Agency: Bristol, UK, 2006. [Google Scholar]
- Frostegård, A.; Bååth, E. Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl. Environ. Microbiol. 1993, 59, 3605–3617. [Google Scholar] [CrossRef] [Green Version]
- Dowling, N.J.E.; White, D.C. Phospholipid ester linked fatty acid biomarkers of acetate-oxidising sulphate reducers and other sulphate forming bacteria. J. Gen. Microbiol. 1986, 132, 1815–1825. [Google Scholar]
- Quideau, S.A.; McIntosh, A.C.S.; Norris, C.E.; Lloret, E.; Swallow, M.J.B.; Hannam, K. Extraction and analysis of microbial Phospholipid fatty acids in soils. J. Vis. Exp. 2016, 114, 54360. [Google Scholar] [CrossRef]
- Varjani, S.; Upasani, V.N. Influence of abiotic factors, natural attenuation, bioaugmentation and nutrient supplementation on bioremediation of petroleum crude contaminated agricultural soil. J. Environ. Manag. 2019, 245, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Risdon, G.C.; Pollard, S.J.T.; Brassington, K.J.; McEwan, J.N.; Paton, G.I.; Semple, K.T.; Coulon, F. Development of an analytical procedure for weathered hydrocarbon contaminated soils within a UK risk-based framework. Anal. Chem. 2008, 80, 7090–7096. [Google Scholar] [CrossRef] [PubMed]
- British Standard, BS 7755: Section 3.13; Determination of Elements Soluble in Aqua Regia. British Standards Institution Publications: London, UK, 1998.
- US EPA Method 3051; Determination of Elements Soluble in Aqua Regia. U.S. Environmental Protection Agency: Washington, DC, USA, 2007.
- Baek, K.; Kim, H.; Oh, H.; Yoon, B.; Kim, J.; Lee, I. Effects of Crude Oil, Oil Components, and Bioremediation on Plant Growth. J. Environ. Sci. Health 2004, 39, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska-Kordybach, B.; Smreczak, B. Habitat function of agricultural soils as affected by heavy metals and polycyclic aromatic hydrocarbons contamination. Environ. Int. 2003, 28, 719–728. [Google Scholar] [CrossRef]
- Nyagumbo, I.; Mkuhlani, S.; Pisa, C.; Kamalongo, D.; Dias, D.; Mekuria, M. Maize yield effects of conservation agriculture based maize–legume cropping systems in contrasting agro-ecologies of Malawi and Mozambique. Nutr. Cycl. Agroecosystems 2016, 105, 275–290. [Google Scholar] [CrossRef]
- Masoni, A.; Mariotti, M.; Ercoli, L. Maize growth and nutrient uptake as affected by root zone volume. Ital. J. Agron. 2002, 1, 95–102. [Google Scholar]
- Chen, X.; Chen, H.Y.H. Plant mixture balances terrestrial ecosystem C:N:P stoichiometry. Nat. Commun. 2021, 12, 4562. [Google Scholar] [CrossRef]
- US EPA 5401R; A guide for corrective action plan reviewers, land and emergency management. United States Environmental Protection Agency: Washington, DC, USA, 1994.
- Griffiths, B.S.; Spilles, A.; Bonkowski, M. C:N:P stoichiometry and nutrient limitation of the soil microbial biomass in a grazed grassland site under experimental P limitation or excess. Ecol. Process. 2012, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Chintala, R.; Mollinedo, J.; Schumacher, T.E.; Malo, D.D.; Julson, J.L. Effect of biochar on chemical properties of acidic soil. Arch. Agron. Soil Sci. 2014, 60, 393–404. [Google Scholar] [CrossRef]
- Ning, D.; Liang, Y.; Liu, Z.; Xiao, J.; Duan, A. Impacts of steel-slag-based silicate fertilizer on soil acidity and silicon availability and metals-immobilization in a paddy soil. PLoS ONE 2016, 11, e0168163. [Google Scholar] [CrossRef] [Green Version]
- Abu, G.O.; Dike, P.O. A study of natural attenuation processes involved in a microcosm model of a crude oil-impacted wetland sediment in the Niger Delta. Bioresour. Technol. 2008, 99, 4761–4767. [Google Scholar] [CrossRef] [PubMed]
- Idzi, A.A.; Saleh, S.A.; Igboanusi, P. Chemical composition analysis of soil from selected oil producing communities in the Niger delta region of Nigeria. Int. J. Basic Appl. Chem. Sci. 2013, 3, 84–92. [Google Scholar]
- Kaur, A.; Chaudhary, A.; Kaur, A.; Choudhary, R.; Kaushik, R. Phospholipid fatty acid—A bioindicator of environment monitoring and assessment in soil ecosystem. Curr. Sci. 2005, 89, 1103–1112. [Google Scholar]
- Sándor, Z. Comparison of effects exerted by bio—Fertilizers, npk fertilizers, and cultivation methods on soil respiration in c hernozem soil. Rev. Cienc. Vida 2020, 32, 8–18. [Google Scholar]
- Henryson, K.; Sundberg, C.; Kätterer, T.; Hansson, P.A. Accounting for long-term soil fertility effects when assessing the climate impact of crop cultivation. Agric. Syst. 2018, 164, 185–192. [Google Scholar] [CrossRef]
- St.Clair, S.B.; Lynch, J.P. The opening of Pandora’s Box: Climate change impacts on soil fertility and crop nutrition in developing countries. Plant Soil 2010, 335, 101–115. [Google Scholar] [CrossRef]
- Song, X.; Zhu, Y.; Chen, W. Dynamics of the soil respiration response to soil reclamation in a coastal wetland. Sci. Rep. 2021, 11, 2911. [Google Scholar] [CrossRef]
- Jiang, Y.; Brassington, K.J.; Prpich, G.; Paton, G.I.; Semple, K.T.; Pollard, S.J.T.; Coulon, F. Insights into the biodegradation of weathered hydrocarbons in contaminated soils by bioaugmentation and nutrient stimulation. Chemosphere 2016, 161, 300–307. [Google Scholar] [CrossRef] [Green Version]
- Lazaroaie, M.M. Multiple responses of gram-positive and gram-negative bacteria to mixture of hydrocarbons. Braz. J. Microbiol. 2010, 41, 649–667. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Q.; Wang, C.; Zhang, H.; Zhao, Y.; Zhang, L.; Zhou, Q. Characteristic analysis of phospholipid fatty acids (PLFAs) in typical nutrient polluted lake sediment in Wuhan. Int. J. Sediment Res. 2021, 36, 221–228. [Google Scholar] [CrossRef]
- Dunfield, K.E. Lipid-Based Community Analysis. In Soil Sampling and Methods of Analysis; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2007; p. 587. [Google Scholar]
- Lewe, N.; Hermans, S.; Lear, G.; Kelly, L.T.; Thomson-laing, G.; Weisbrod, B.; Deslippe, J.R. Phospholipid fatty acid (PLFA) analysis as a tool to estimate absolute abundances from compositional 16S rRNA bacterial metabarcoding data. J. Microbiol. Methods 2021, 188, 106–271. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Schauer, F.; Heipieper, H.J. The trans/cis ratio of unsaturated fatty acids is not applicable as biomarker for environmental stress in case of long-term contaminated habitats. Appl. Microbiol. Biotechnol. 2010, 87, 365–371. [Google Scholar] [CrossRef]
- Frostegård, Å.; Tunlid, A.; Bååth, E. Soil Biology & Biochemistry Use and misuse of PLFA measurements in soils. Soil Biol. Biochem. 2011, 43, 1621–1625. [Google Scholar] [CrossRef]
- Trögl, J.; Pavlorková, J.; Packová, P.; Seják, J.; Kuráň, P.; Popelka, J.; Pacina, J. Indication of importance of including soil microbial characteristics into biotope valuation method. Sustainability 2016, 8, 253. [Google Scholar] [CrossRef]
- Feng, L.; Jiang, X.; Huang, Y.; Wen, D.; Fu, T. Petroleum hydrocarbon-contaminated soil bioremediation assisted by isolated bacterial consortium and sophorolipid. In Environmental Pollution, 273; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; p. 116476. [Google Scholar]
- Wei, Z.; Wang, J.J.; Gaston, L.A.; Li, J.; Fultz, L.M.; DeLaune, R.D.; Dodla, S.K. Remediation of crude oil-contaminated coastal marsh soil: Integrated effect of biochar, rhamnolipid biosurfactant and nitrogen application. J. Hazard. Mater. 2020, 396, 122595. [Google Scholar] [CrossRef]
- Wartell, B.; Boufadel, M.; Rodriguez-Freire, L. An effort to understand and improve the anaerobic biodegradation of petroleum hydrocarbons: A literature review. Int. Biodeterior. Biodegrad. 2021, 157, 105156. [Google Scholar] [CrossRef]
- Gielnik, A.; Pechaud, Y.; Huguenot, D.; Cébron, A.; Esposito, G.; van-Hullebusch, E.D. Functional potential of sewage sludge digestate microbes to degrade aliphatic hydrocarbons during bioremediation of a petroleum hydrocarbons contaminated soil. J. Environ. Manag. 2021, 280, 381–397. [Google Scholar] [CrossRef]
- Jin, C.E.; Kim, M.N. Change of bacterial community in oil-polluted soil after enrichment cultivation with low-molecular-weight polyethylene. Int. Biodeterior. Biodegrad. 2017, 118, 27–33. [Google Scholar] [CrossRef]
- Powell, S.M.; Ferguson, S.H.; Bowman, J.P.; Snape, I. Using real-time PCR to assess changes in the hydrocarbon-degrading microbial community in Antarctic soil during bioremediation. Microb. Ecol. 2006, 52, 523–532. [Google Scholar] [CrossRef]
- Iqbal, M.K.; Shafiq, T.; Ahmed, K. Characterization of bulking agents and its effects on physical properties of compost. Bioresour. Technol. 2010, 101, 1913–1919. [Google Scholar] [CrossRef]
- Huang, L.; Ye, J.; Jiang, K.; Wang, Y.; Li, Y. ‘Oil contamination drives the transformation of soil microbial communities: Co-occurrence pattern, metabolic enzymes and culturable hydrocarbon-degrading bacteria. Ecotoxicol. Environ. Saf. 2021, 225, 112–140. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.; Noël, C.; Dufour, L.; Ozier-Lafontaine, H.; Welcker, C.; Desfontaines, L. Mineral nutrition and growth of tropical maize as affected by soil acidity. Plant Soil 2003, 252, 215–226. [Google Scholar] [CrossRef]
- Khaled, H.; Fawy, H. Effect of different Levels of humic acids on the nutrient content, plant growth, and soil properties under conditions of salinity. Soil Water Res. 2011, 6, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, F.; Zhan, Y.; Zhu, L. Shifts in microbial community structure during in situ surfactant-enhanced bioremediation of polycyclic aromatic hydrocarbon-contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 14451–14461. [Google Scholar] [CrossRef] [PubMed]
- Nwankwegu, A.S.; Orji, M.U.; Onwosi, C.O. Chemosphere Studies on organic and in-organic biostimulants in bioremediation of diesel-contaminated arable soil. Chemosphere 2016, 162, 148–156. [Google Scholar] [CrossRef] [PubMed]



| Mesocosm Conditions | Abbreviations |
|---|---|
| Pristine soil (freshly collected from field) | Control |
| Pristine soil acidified at pH 5.8 (using HNO3) | Acidified |
| Pristine soil acidified at pH 5.8 and spiked at 50,000 mg/kg with crude oil | Crude oil |
| Pristine soil acidified at pH 5.8 and spiked at 50,000 mg/kg with crude oil + 10% (w/w) FW digestate | 10% FWAD |
| Pristine soil acidified at pH 5.8 and spiked at 50,000 mg/kg with crude oil + 20% (w/w) FW digestate | 20% FWAD |
| Pristine soil acidified at pH 5.8 and spiked at 50,000 mg/kg with crude oil + 30% (w/w) FW digestate | 30% FWAD |
| Pristine soil acidified at pH 5.8 and spiked at 50,000 mg/kg with crude oil + 10% (w/w) Tween 80 | 10% TW80 |
| Pristine soil acidified at pH 5.8 and spiked at 50,000 mg/kg with crude oil + 20% (w/w) Tween 80 | 20% TW80 |
| Pristine soil acidified at pH 5.8 and spiked at 50,000 mg/kg with crude oil + 30% (w/w) Tween 80 | 30% TW80 |
| Soil Physicochemical Characteristics | |
|---|---|
| Soil Moisture content (%) | 13.75 |
| Loss on ignition (%) | 3.66 |
| Dry matter content (%) | 86.25 |
| Water holding capacity (%) | 54.54 |
| TOC (%) | 3.09 |
| Org C (%) | 2.25 |
| TN (%) | 0.12 |
| TP (mg/kg) | 5.58 |
| TK (mg/kg) | 236.00 |
| Soil Particle size distribution | |
| Sand (%) | 46.67 |
| Silt (%) | 45.89 |
| Clay (%) | 7.44 |
| FWAD characteristics | |
| TOC (%) | 17.22 |
| Org C (%) | 4.97 |
| TN (%) | 0.98 |
| TP (mg/kg) | 300.25 |
| TK (mg/kg) | 8107.50 |
| C:N: P | 250:13:1 |
| Mesocosm | Treatment | K (mg/kg) | C: N: P | Bacteria Count (×105 CFU/g) |
|---|---|---|---|---|
| FWAD | 10% FWAD | 1310.00 ± 2.1 | 128:9:1 | 20 ± 0.48 |
| 20% FWAD | 1694.17 ± 2.3 | 167:10:1 | 10 ± 0.4 | |
| 30% FWAD | 1806.67 ± 2.9 | 180:9:1 | 30 ± 0.47 | |
| TW80 | 10% TW80 | 224.75 ± 1.6 | 60:2:1 | 1 ± 0.32 |
| 20% TW80 | 151.58 ± 1.98 | 65:2:1 | 3 ± 0.48 | |
| 30% TW80 | 141.50 ± 1.7 | 75:3:1 | 4 ± 0.48 | |
| Controls | Control | 236.00 ± 2.2 | 60:2:1 | 102 ± 0.8 |
| Acidified | 243.83 ± 3.1 | 58:4:1 | 2 ± 0.4 | |
| Crude oil | 157.08 ± 1.6 | 60:2:1 | 7 ± 0.48 |
| Mesocosms | Treatment | Slope equation | R2 | Degradation Rates (mgCO2/mg PAHs/Day) |
|---|---|---|---|---|
| FWAD | 10% FWAD | y = −0.2741x + 513.83 | 0.98 | −0.27 |
| 20% FWAD | y = −0.3282x + 688.55 | 0.97 | −0.33 | |
| 30% FWAD | y = −0.45x + 819.33 | 0.97 | −0.45 | |
| TW80 | 10% TW80 | y = −0.3672x + 721.46 | 0.92 | −0.37 |
| 20% TW80 | y = −0.412x + 726.83 | 0.97 | −0.41 | |
| 30% TW80 | y = −0.5206x + 867.58 | 0.98 | −0.52 | |
| Control | Acidified HCs | y = −0.2433x + 469.52 | 0.90 | −0.24 |
| Mesocosms | Treatment | Slope Equation | R2 | Degradation Rates (mgCO2/mg Alkanes/Day) |
|---|---|---|---|---|
| FWAD | 10% FWAD | y= −0.2x + 744.6 | 0.97 | −0.2 |
| 20% FWAD | y= −0.31x + 1182.3 | 0.97 | −0.31 | |
| 30% FWAD | y= −0.416x + 1363.7 | 0.91 | −0.42 | |
| TW80 | 10% TW80 | y= −0.18x + 789.1 | 0.95 | −0.18 |
| 20% TW80 | y= −0.32x + 1022.4 | 0.94 | −0.32 | |
| 30% TW80 | y= −0.34x + 1421.1 | 0.96 | −0.34 | |
| Control | Acidified HCs | y= −0.15x + 752.4 | 0.96 | −0.15 |
| Metal (Loid) (mg/kg) | Control | Acidified | Crude Oil | FWAD |
|---|---|---|---|---|
| Mo | 0.93 ± 0.08 | 1.27 ± 0.13 | 1.21 ± 0.07 | 1.20 ± 0.49 |
| Cr | 45.24 ± 1.23 | 59.56 ± 2.94 | 50.87 ± 1.50 | 36.05 ± 2.17 |
| Ni | 29.65 ± 1.24 | 42.60 ± 0.93 | 35.25 ± 0.79 | 23.79 ± 1.58 |
| As | 14.83 ± 0.98 | 20.21 ± 0.74 | 17.18 ± 0.78 | 10.13 ± 0.52 |
| Cd | 0.59 ± 0.09 | 0.83 ± 0.09 | 0.73 ± 0.07 | 0.72 ± 0.10 |
| Pb | 17.00 ± 0.91 | 23.31 ± 0.45 | 21.41 ± 0.8 | 15.62 ± 0.94 |
| Hg | 0.29 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.02 | 0.14 ± 0.04 |
| Treatment | Spearman Coefficient (ɤ) | Prob > |p| | Correlation Strength |
|---|---|---|---|
| Control | 0.8104 | <0.0001 | ++++++++ |
| Acidified | 0.87 | <0.0001 | +++++++++ |
| Crude oil | 0.8805 | <0.0001 | +++++++++ |
| 10% TW80 | 0.8395 | <0.0001 | ++++++++ |
| 20% TW80 | 0.8732 | <0.0001 | +++++++++ |
| 30% TW80 | 0.8949 | <0.0001 | +++++++++ |
| 10% FWAD | 0.8588 | <0.0001 | +++++++++ |
| 20% FWAD | 0.8358 | <0.0001 | ++++++++ |
| 30% FWAD | 0.8327 | <0.0001 | ++++++++ |
| Alkane Group | Initial Alkanes | Percentage Degradation (%) on Day 112 | ||||||
|---|---|---|---|---|---|---|---|---|
| C11–C18 | (mg/kg) | Crude Oil | 10% FWAD | 20% FWAD | 30% FWAD | 10% TW80 | 20% TW80 | 30% TW80 |
| Undecane | 2339.0 | 93.7 | 99.7 | 99.8 | 99.8 | 99.7 | 99.7 | 99.8 |
| Dodecane | 1226.2 | 59.8 | 99.3 | 99.5 | 99.7 | 99.3 | 99.4 | 99.6 |
| Tridecane | 1710.5 | 74.8 | 99.7 | 99.7 | 99.8 | 99.6 | 99.7 | 99.8 |
| Tetradecane | 1658.9 | 68.5 | 99.6 | 99.6 | 99.7 | 99.5 | 99.5 | 99.6 |
| Pentadecane | 1669.2 | 67.4 | 99.5 | 99.5 | 99.5 | 99.3 | 99.4 | 99.6 |
| Hexadecane | 1597.1 | 62.8 | 99.2 | 99.2 | 99.3 | 98.2 | 99.2 | 99.3 |
| Heptadecane | 1648.6 | 75.1 | 98.7 | 98.7 | 99.3 | 98.7 | 98.2 | 98.8 |
| Octadecane | 1576.5 | 65.2 | 98.2 | 98.2 | 98.5 | 98.4 | 98.4 | 98.5 |
| Overall % degradation | 70.9 | 99.2 | 99.3 | 99.5 | 99.1 | 99.2 | 99.4 | |
| Alkane Group | Initial Alkanes | Percentage Degradation (%) on Day 112 | ||||||
|---|---|---|---|---|---|---|---|---|
| C19–C37 | (mg/kg) | Crude Oil | 10% FWAD | 20% FWAD | 30% FWAD | 10% TW80 | 20% TW80 | 30% TW80 |
| Pristane | 1100.0 | 80.0 | 90.8 | 91.7 | 96.4 | 91.8 | 94.9 | 96.3 |
| Phytane | 1151.8 | 82.5 | 91.0 | 92.2 | 96.4 | 90.4 | 95.5 | 96.6 |
| Nonadecane | 1296.9 | 75.8 | 91.9 | 94.6 | 96.1 | 91.4 | 93.8 | 96.7 |
| Eicosane | 1416.3 | 75.5 | 91.7 | 92.3 | 95.6 | 91.3 | 94.3 | 97.2 |
| Heneicosane | 1714.9 | 81.2 | 93.0 | 93.6 | 95.3 | 89.5 | 94.5 | 96.5 |
| Docosane | 1808.8 | 75.4 | 90.4 | 93.2 | 96.6 | 90.6 | 94.3 | 95.0 |
| Tricosane | 1798.9 | 79.4 | 90.2 | 92.4 | 96.1 | 90.0 | 94.4 | 95.0 |
| Tetracosane | 1775.7 | 77.7 | 89.8 | 92.6 | 93.1 | 89.3 | 95.0 | 96.5 |
| Pentacosane | 1743.9 | 79.3 | 89.6 | 93.0 | 94.5 | 89.1 | 94.2 | 95.9 |
| Hexacosane | 1890.3 | 77.3 | 88.7 | 93.2 | 94.2 | 86.5 | 94.4 | 94.9 |
| Heptacosane | 1070.9 | 69.7 | 71.8 | 87.8 | 90.5 | 68.9 | 90.5 | 92.3 |
| Octacosane | 1936.8 | 79.5 | 83.1 | 91.7 | 94.1 | 80.0 | 89.8 | 94.9 |
| Nonacosane | 1953.8 | 78.4 | 83.5 | 87.7 | 89.7 | 74.5 | 89.3 | 94.9 |
| Triacontane | 1612.5 | 67.7 | 74.8 | 79.2 | 87.3 | 67.3 | 86.2 | 93.8 |
| Hentriacontane | 1569.9 | 66.9 | 73.9 | 75.3 | 81.0 | 65.0 | 77.0 | 84.7 |
| Dotriacontane | 1313.9 | 45.2 | 60.6 | 62.6 | 81.8 | 55.4 | 61.6 | 81.0 |
| Tritriacontane | 1058.0 | 22.3 | 51.1 | 52.8 | 72.7 | 43.5 | 51.6 | 78.0 |
| Tetratriacontane | 1183.6 | 38.6 | 56.5 | 58.7 | 81.2 | 44.6 | 48.5 | 65.0 |
| Pentatriacontane | 1245.7 | 35.6 | 58.6 | 60.0 | 81.1 | 45.7 | 50.3 | 55.9 |
| Hexatriacontane | 1176.4 | 32.3 | 56.2 | 57.7 | 78.9 | 42.3 | 43.9 | 44.9 |
| Heptatriacontane | 1254.6 | 20.4 | 59.3 | 61.7 | 68.4 | 44.3 | 46.6 | 48.1 |
| Overall % degradation | 63.8 | 77.9 | 81.1 | 88.6 | 72.9 | 80.0 | 85.4 | |
| PAH Group | Initial PAHs | Percentage Degradation (%) on Day 112 | ||||||
|---|---|---|---|---|---|---|---|---|
| C10–C18 | (mg/kg) | Crude Oil | 10% FWAD | 20% FWAD | 30% FWAD | 10% TW80 | 20% TW80 | 30% TW80 |
| Naphthalene | 224.1 | 91.4 | 99.9 | 99.9 | 99.9 | 99.9 | 99.9 | 99.9 |
| Fluorene | 458.5 | 91.6 | 99.1 | 99.9 | 99.9 | 99.9 | 99.9 | 99.9 |
| Phenanthrene | 931.1 | 95.5 | 99.9 | 99.9 | 99.9 | 99.9 | 99.9 | 99.9 |
| Anthracene | 297.2 | 96.7 | 99.7 | 99.9 | 99.9 | 99.3 | 99.1 | 99.9 |
| Pyrene | 67.9 | 87.9 | 98.5 | 99.9 | 99.9 | 97.0 | 98.1 | 99.9 |
| Benz(a)anthracene | 212.3 | 90.4 | 98.6 | 99.9 | 99.9 | 98.1 | 98.9 | 99.5 |
| Chrysene | 356.6 | 88.8 | 98.6 | 99.9 | 99.9 | 98.0 | 99.4 | 99.0 |
| Overall % degradation | 91.8 | 99.2 | 99.9 | 99.9 | 98.9 | 99.1 | 99.7 | |
| PAH Group | Initial PAHs | Percentage Degradation (%) on Day 112 | ||||||
|---|---|---|---|---|---|---|---|---|
| C19–C22 | (mg/kg) | Crude Oil | 10% FWAD | 20% FWAD | 30% FWAD | 10% TW80 | 20% TW80 | 30% TW80 |
| Benzo[b]fluoranthene | 334.8 | 93.0 | 99.1 | 99.9 | 99.9 | 99.0 | 99.9 | 99.9 |
| Benzo[k]fluoranthene | 186 | 93.5 | 99.3 | 99.6 | 99.7 | 99.0 | 99.2 | 99.9 |
| Benz(a)pyrene | 176.7 | 87.3 | 99.1 | 99.7 | 99.9 | 98.4 | 99.4 | 99.9 |
| Benzo(ghi)perylene | 818.4 | 89.4 | 99.1 | 99.7 | 99.7 | 98.1 | 99.1 | 99.6 |
| Benzo[b)triphenylene | 604.5 | 89.8 | 99.3 | 99.4 | 99.7 | 98.0 | 99.1 | 99.7 |
| Indeno(123)[cd]pyrene | 344.1 | 86.5 | 98.5 | 99.2 | 99.8 | 97.9 | 98.9 | 99.1 |
| Overall % degradation | 89.9 | 99.1 | 99.6 | 99.8 | 98.4 | 99.3 | 99.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jumbo, R.B.; Coulon, F.; Cowley, T.; Azuazu, I.; Atai, E.; Bortone, I.; Jiang, Y. Evaluating Different Soil Amendments as Bioremediation Strategy for Wetland Soil Contaminated by Crude Oil. Sustainability 2022, 14, 16568. https://doi.org/10.3390/su142416568
Jumbo RB, Coulon F, Cowley T, Azuazu I, Atai E, Bortone I, Jiang Y. Evaluating Different Soil Amendments as Bioremediation Strategy for Wetland Soil Contaminated by Crude Oil. Sustainability. 2022; 14(24):16568. https://doi.org/10.3390/su142416568
Chicago/Turabian StyleJumbo, Raphael Butler, Frédéric Coulon, Tamazon Cowley, Ikeabiama Azuazu, Emmanuel Atai, Imma Bortone, and Ying Jiang. 2022. "Evaluating Different Soil Amendments as Bioremediation Strategy for Wetland Soil Contaminated by Crude Oil" Sustainability 14, no. 24: 16568. https://doi.org/10.3390/su142416568









