Global Advancements and Current Challenges of Electric Vehicle Batteries and Their Prospects: A Comprehensive Review
Abstract
:1. Introduction
2. Prospects of Electric Vehicle Battery
3. Challenges of the Electric Vehicle Battery
3.1. Technological Challenges
3.2. Financial Challenges
4. Progress in Technological and Financial Challenges
4.1. Progress in Energy Density Enhancement
4.2. Progress in Optimization of Fast Charging
4.3. End of Life Issue
4.4. Progress for Financial Challenges
5. Potential Suggestions and Ongoing Research to Overcome the Challenges
5.1. Research on Technological Challenges
5.1.1. Energy Density Improvement
5.1.2. Fast Charging
5.1.3. End of Life
5.2. Suggestions for Financial Challenges
5.2.1. Quality Control
5.2.2. Electrode Processing
5.2.3. Shortening the Formation Period
6. Conclusions
- One of the major challenges of EVs is the limited driving range. Increasing the energy density of the batteries can solve the problem; however, it has the drawback of increased battery weight and cost of the vehicle. Porous cathode materials, hybrid electrode materials, increasing cell output voltage, and laminated structure battery cells can solve this problem.
- Slow charging capacity of batteries creates a range anxiety problem among BEV consumers that can be solved with fast charging. To support fast charging with sufficient charging capacity, further developments in battery cells, electrode materials, power system, charging piles, etc. are needed. Hierarchically porous carbon anode, boron-doped graphene as an anode material, and Li4Ti5O12 (LTO) can be the most promising material to be used in the anode of lithium batteries for fast charging. The establishment and distribution of ultrapowered charging stations is important to achieve maximum EV market penetration.
- EoL of EV batteries is another concern from the environmental perspective. A well-defined EoL plan for battery cells used in BEVs is required. Remanufacturing, repurposing, and recycling are some options, but each of these approaches has several drawbacks including environmental and health impacts.
- The battery used in a BEV accounts for almost half of the vehicle’s price. There is a need to reduce the battery cost to make EVs more affordable and to compete in the market with ICE vehicles. Methods of cost reduction based on stakeholder interviews and modeling exercises should be introduced.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kosai, S.; Zakaria, S.; Che, H.S.; Hasanuzzaman; Rahim, N.A.; Tan, C.; Ahmad, R.D.R.; Abbas, A.R.; Nakano, K.; Yamasue, E.; et al. Estimation of Greenhouse Gas Emissions of Petrol, Biodiesel and Battery Electric Vehicles in Malaysia Based on Life Cycle Approach. Sustainability 2022, 14, 5783. [Google Scholar] [CrossRef]
- Morfeldt, J.; Kurland, S.D.; Johansson, D.J. Carbon footprint impacts of banning cars with internal combustion engines. Transp. Res. Part D Transp. Environ. 2021, 95, 102807. [Google Scholar] [CrossRef]
- IEA. Tracking Transport 2020; International Energy Agency (IEA): Paris, France, 2020. Available online: https://www.iea.org/reports/tracking-transport-2020 (accessed on 27 July 2022).
- Muzir, N.A.Q.; Mojumder, R.H.; Hasanuzzaman, M.; Selvaraj, J. Challenges of Electric Vehicles and Their Prospects in Malaysia: A Comprehensive Review. Sustainability 2022, 14, 8320. [Google Scholar] [CrossRef]
- Sun, B.; Gu, T.; Xie, M.; Wang, P.; Gao, S.; Zhang, X. Strategy Design and Performance Analysis of an Electromechanical Flywheel Hybrid Scheme for Electric Vehicles. Sustainability 2022, 14, 11017. [Google Scholar] [CrossRef]
- Cao, J.; Chen, X.; Qiu, R.; Hou, S. Electric vehicle industry sustainable development with a stakeholder engagement system. Technol. Soc. 2021, 67, 101771. [Google Scholar] [CrossRef]
- Gönül, Ö.; Duman, A.C.; Güler, Ö. Electric vehicles and charging infrastructure in Turkey: An overview. Renew. Sustain. Energy Rev. 2021, 143, 110913. [Google Scholar] [CrossRef]
- Khalid, M.R.; Alam, M.S.; Sarwar, A.; Asghar, M.J. A Comprehensive review on electric vehicles charging infrastructures and their impacts on power-quality of the utility grid. ETransportation 2019, 1, 100006. [Google Scholar] [CrossRef]
- Lebrouhi, B.; Khattari, Y.; Lamrani, B.; Maaroufi, M.; Zeraouli, Y.; Kousksou, T. Key challenges for a large-scale development of battery electric vehicles: A comprehensive review. J. Energy Storage 2021, 44, 103273. [Google Scholar] [CrossRef]
- Macioszek, E. E-mobility infrastructure in the Górnośląsko-Zagłębiowska Metropolis, Poland, and potential for development. In Proceedings of the 5th World Congress on New Technologies (NewTech’19), Lisbon, Portugal, 18–20 August 2019; p. ICERT 108. [Google Scholar]
- Macioszek, E. Electric Vehicles—Problems and Issues. In Smart and Green Solutions for Transport Systems; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Gandoman, F.H.; Ahmed, E.M.; Ali, Z.M.; Berecibar, M.; Zobaa, A.F.; Abdel Aleem, S.H. Reliability evaluation of lithium-ion batteries for E-mobility applications from practical and technical perspectives: A case study. Sustainability 2021, 13, 11688. [Google Scholar] [CrossRef]
- Manzetti, S.; Mariasiu, F. Electric vehicle battery technologies: From present state to future systems. Renew. Sustain. Energy Rev. 2015, 51, 1004–1012. [Google Scholar] [CrossRef]
- Catenacci, M.; Fiorese, G.; Verdolini, E.; Bosetti, V. Going electric: Expert survey on the future of battery technologies for electric vehicles. In Innovation under Uncertainty; Edward Elgar Publishing: Cheltenham, UK, 2015. [Google Scholar]
- Dannier, A. Overview of Main Electric Subsystems of Zero-Emission Vehicles. Propuls. Syst. 2019, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Rydh, C.J.; Karlström, M. Life cycle inventory of recycling portable nickel–cadmium batteries. Resour. Conserv. Recycl. 2002, 34, 289–309. [Google Scholar] [CrossRef]
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-ion batteries: Current status and future perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Gao, Y.; Pan, Z.; Sun, J.; Liu, Z.; Wang, J. High-Energy Batteries: Beyond Lithium-Ion and Their Long Road to Commercialisation. Nano-Micro Lett. 2022, 14, 1–49. [Google Scholar] [CrossRef]
- Younesi, R.; Veith, G.M.; Johansson, P.; Edström, K.; Vegge, T. Lithium salts for advanced lithium batteries: Li–metal, Li–O2, and Li–S. Energy Environ. Sci. 2015, 8, 1905–1922. [Google Scholar] [CrossRef] [Green Version]
- Duan, J.; Tang, X.; Dai, H.; Yang, Y.; Wu, W.; Wei, X.; Huang, Y. Building safe lithium-ion batteries for electric vehicles: A review. Electrochem. Energy Rev. 2020, 3, 1–42. [Google Scholar] [CrossRef] [Green Version]
- Stampatori, D.; Raimondi, P.P.; Noussan, M. Li-Ion Batteries: A Review of a Key Technology for Transport Decarbonization. Energies 2020, 13, 2638. [Google Scholar] [CrossRef]
- El Kharbachi, A.; Zavorotynska, O.; Latroche, M.; Cuevas, F.; Yartys, V.; Fichtner, M. Exploits, advances and challenges benefiting beyond Li-ion battery technologies. J. Alloys Compd. 2020, 817, 153261. [Google Scholar] [CrossRef]
- Jin, L.; Zheng, J.; Wu, Q.; Shellikeri, A.; Yturriaga, S.; Gong, R.; Huang, J.; Zheng, J.P. Exploiting a hybrid lithium ion power source with a high energy density over 30 Wh/kg. Mater. Today Energy 2018, 7, 51–57. [Google Scholar] [CrossRef]
- Singh, M.; Kaiser, J.; Hahn, H. Effect of Porosity on the Thick Electrodes for High Energy Density Lithium Ion Batteries for Stationary Applications. Batteries 2016, 2, 35. [Google Scholar] [CrossRef]
- Vu, A.; Qian, Y.; Stein, A. Porous Electrode Materials for Lithium-Ion Batteries—How to Prepare Them and What Makes Them Special. Adv. Energy Mater. 2012, 2, 1056–1085. [Google Scholar] [CrossRef]
- Chen, L.; Fan, X.; Hu, E.; Ji, X.; Chen, J.; Hou, S.; Deng, T.; Li, J.; Su, D.; Yang, X.; et al. Achieving High Energy Density through Increasing the Output Voltage: A Highly Reversible 5.3 V Battery. Chem 2019, 5, 896–912. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhu, Y.; Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 2019, 4, 540–550. [Google Scholar] [CrossRef]
- Nag, B.; De, D. The Indian automobile industry: Technology enablers preparing for the future. In New Frontiers of the Automobile Industry; Springer: Berlin/Heidelberg, Germany, 2020; pp. 301–321. [Google Scholar]
- Chen, M.; Ma, X.; Chen, B.; Arsenault, R.; Karlson, P.; Simon, N.; Wang, Y. Recycling End-of-Life Electric Vehicle Lithium-Ion Batteries. Joule 2019, 3, 2622–2646. [Google Scholar] [CrossRef]
- Ramoni, M.O.; Zhang, H.-C. End-of-life (EOL) issues and options for electric vehicle batteries. Clean Technol. Environ. Policy 2013, 15, 881–891. [Google Scholar] [CrossRef]
- Burch, I.; Gilchrist, J. Survey of Global Activity to Phase out Internal Combustion Engine Vehicles; Hancock, A., Waaland, G., Eds.; The Climate Center: Santa Rosa, CA, USA, 2018. [Google Scholar]
- Asghar, R.; Rehman, F.; Ullah, Z.; Qamar, A.; Ullah, K.; Iqbal, K.; Aman, A.; Nawaz, A.A. Electric vehicles and key adaptation challenges and prospects in Pakistan: A comprehensive review. J. Clean. Prod. 2021, 278, 123375. [Google Scholar] [CrossRef]
- Wicki, M.; Brückmann, G.; Quoss, F.; Bernauer, T. What do we really know about the acceptance of battery electric vehicles?—Turns out, not much. Transp. Rev. 2023, 43, 62–87. [Google Scholar] [CrossRef]
- Liu, W.; Placke, T.; Chau, K. Overview of batteries and battery management for electric vehicles. Energy Rep. 2022, 8, 4058–4084. [Google Scholar] [CrossRef]
- Un-Noor, F.; Padmanaban, S.; Mihet-Popa, L.; Mollah, M.N.; Hossain, E. A Comprehensive Study of Key Electric Vehicle (EV) Components, Technologies, Challenges, Impacts, and Future Direction of Development. Energies 2017, 10, 1217. [Google Scholar] [CrossRef] [Green Version]
- Vynakov, O.; Savolova, E.; Skrynnyk, A. Modern Electric Cars of Tesla Motors Company. Autom. Technol. Bus. Process. 2016, 8. [Google Scholar] [CrossRef]
- Ferrari, L. How Long Does It Take to Charge an Electric Vehicle? 2020. Available online: https://www.evconnect.com/blog/how-long-does-it-takes-to-charge-an-electric-car (accessed on 27 July 2022).
- Kamal, T.; Karabacak, M.; Hassan, S.Z.; Fernández-Ramírez, L.M.; Riaz, M.H.; Riaz, M.T.; Khan, M.A.; Khan, L. Energy Management and Switching Control of PHEV Charging Stations in a Hybrid Smart Micro-Grid System. Electronics 2018, 7, 156. [Google Scholar] [CrossRef] [Green Version]
- Holland, A. Li-Ion Battery Recycling: 2020–2040. Available online: https://www.idtechex.com/en/research-report/li-ion-battery-recycling-2020-2040/751 (accessed on 27 July 2022).
- Ferrara, C.; Ruffo, R.; Quartarone, E.; Mustarelli, P. Circular Economy and the Fate of Lithium Batteries: Second Life and Recycling. Adv. Energy Sustain. Res. 2021, 2, 2100047. [Google Scholar] [CrossRef]
- Frith, J. EV Battery Prices Risk Reversing Downward Trend as Metals Surge. 2021. Available online: https://www.bloomberg.com/news/newsletters/2021-09-14/ev-battery-prices-risk-reversing-downward-trend-as-metals-surge (accessed on 27 July 2022).
- Haram, M.H.S.M.; Lee, J.W.; Ramasamy, G.; Ngu, E.E.; Thiagarajah, S.P.; Lee, Y.H. Feasibility of utilising second life EV batteries: Applications, lifespan, economics, environmental impact, assessment, and challenges. Alex. Eng. J. 2021, 60, 4517–4536. [Google Scholar] [CrossRef]
- Gabbar, H.A.; Othman, A.M.; Abdussami, M.R. Review of Battery Management Systems (BMS) Development and Industrial Standards. Technologies 2021, 9, 28. [Google Scholar] [CrossRef]
- Yang, Y.; Tan, Z.; Ren, Y. Research on Factors That Influence the Fast Charging Behavior of Private Battery Electric Vehicles. Sustainability 2020, 12, 3439. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Shang, F.; Salameh, M.; Krishnamurthy, M. Challenges and Advancements in Fast Charging Solutions for EVs: A Technological Review. In Proceedings of the 2018 IEEE Transportation Electrification Conference and Expo (ITEC), Long Beach, CA, USA, 13–15 June 2018. [Google Scholar]
- Skeete, J.-P.; Wells, P.; Dong, X.; Heidrich, O.; Harper, G. Beyond the Event horizon: Battery waste, recycling, and sustainability in the United Kingdom electric vehicle transition. Energy Res. Soc. Sci. 2020, 69, 101581. [Google Scholar] [CrossRef]
- Mojumder, M.R.H.; Antara, F.A.; Hasanuzzaman, M.; Alamri, B.; Alsharef, M. Electric Vehicle-to-Grid (V2G) Technologies: Impact on the Power Grid and Battery. Sustainability 2022, 14, 13856. [Google Scholar] [CrossRef]
- Botsford, C.; Szczepanek, A. Fast Charging vs. Slow Charging: Pros and cons for the New Age of Electric Vehicles. In Proceedings of the EVS24 International Battery, Hybrid and Fuel Cell Electric Vehicle Symposium, Stavanger, Norway, 13–16 May 2009. [Google Scholar]
- He, W.; Guo, W.; Wu, H.; Lin, L.; Liu, Q.; Han, X.; Xie, Q.; Liu, P.; Zheng, H.; Wang, L.; et al. Challenges and Recent Advances in High Capacity Li-Rich Cathode Materials for High Energy Density Lithium-Ion Batteries. Adv. Mater. 2021, 33, 2005937. [Google Scholar] [CrossRef]
- Collin, R.; Miao, Y.; Yokochi, A.; Enjeti, P.; von Jouanne, A. Advanced Electric Vehicle Fast-Charging Technologies. Energies 2019, 12, 1839. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Wang, L.; Chen, Z.; He, X. Challenges of Fast Charging for Electric Vehicles and the Role of Red Phosphorous as Anode Material: Review. Energies 2019, 12, 3897. [Google Scholar] [CrossRef]
- Ma, Y.; Qiu, K. Recovery of lead from lead paste in spent lead acid battery by hydrometallurgical desulfurization and vacuum thermal reduction. Waste Manag. 2015, 40, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wu, Y.; Hou, P.; Liang, S.; Qu, S.; Xu, M.; Zuo, T. Environmental impact and economic assessment of secondary lead production: Comparison of main spent lead-acid battery recycling processes in China. J. Clean. Prod. 2017, 144, 142–148. [Google Scholar] [CrossRef]
- Brown, T.W.; Bischof-Niemz, T.; Blok, K.; Breyer, C.; Lund, H.; Mathiesen, B.V. Response to ‘Burden of proof: A comprehensive review of the feasibility of 100% renewable-electricity systems’. Renew. Sustain. Energy Rev. 2018, 92, 834–847. [Google Scholar] [CrossRef]
- Zakiyya, H.; Distya, Y.D.; Ellen, R. A Review of Spent Lead-Acid Battery Recycling Technology in Indonesia: Comparison and Recommendation of Environment-friendly Process. IOP Conf. Ser. Mater. Sci. Eng. 2018, 288, 012074. [Google Scholar] [CrossRef]
- Lee, T.J.; Cheng, T.T.; Juang, H.K.; Chen, S.Y.; Fey, G.T.K.; Jaw, H.K. Relationship of cathode pore-size distribution and rated capacity in Li/MnO2 cells. J. Power Source 1993, 44, 709–712. [Google Scholar] [CrossRef]
- Gao, J.; Abruña, H.D. Key Parameters Governing the Energy Density of Rechargeable Li/S Batteries. J. Phys. Chem. Lett. 2014, 5, 882–885. [Google Scholar] [CrossRef]
- Ke, F.-S.; Huang, L.; Cai, J.-S.; Sun, S.-G. Electroplating synthesis and electrochemical properties of macroporous Sn–Cu alloy electrode for lithium-ion batteries. Electrochim. Acta 2007, 52, 6741–6747. [Google Scholar] [CrossRef]
- Bae, K.Y.; Cho, S.H.; Kim, B.H.; Son, B.D.; Yoon, W.Y. Energy-Density Improvement in Li-Ion Rechargeable Batteries Based on LiCoO2 + LiV3O8 and Graphite + Li-Metal Hybrid Electrodes. Materials 2019, 12, 2025. [Google Scholar] [CrossRef] [Green Version]
- Worthmann, C. Nissan Leaf Battery (Replacement, Lifespan, & More). Available online: https://climatebiz.com/nissan-leaf-battery/ (accessed on 27 July 2022).
- Tomaszewska, A.; Chu, Z.; Feng, X.; O’kane, S.; Liu, X.; Chen, J.; Ji, C.; Endler, E.; Li, R.; Liu, L. Lithium-ion battery fast charging: A review. ETransportation 2019, 1, 100011. [Google Scholar] [CrossRef]
- Froboese, L.; van der Sichel, J.F.; Loellhoeffel, T.; Helmers, L.; Kwade, A. Effect of Microstructure on the Ionic Conductivity of an All Solid-State Battery Electrode. J. Electrochem. Soc. 2019, 166, A318–A328. [Google Scholar] [CrossRef]
- Gannett, C.N.; Melecio-Zambrano, L.; Theibault, M.J.; Peterson, B.M.; Fors, B.P.; Abruña, H.D. Organic electrode materials for fast-rate, high-power battery applications. Mater. Rep. Energy 2021, 1, 100008. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Ming, J.; Cao, Z.; Wahyudi, W.; Li, M.; Kumar, P.; Wu, Y.; Hwang, J.-Y.; Hedhili, M.N.; Cavallo, L.; Sun, Y.-K.; et al. New Insights on Graphite Anode Stability in Rechargeable Batteries: Li Ion Coordination Structures Prevail over Solid Electrolyte Interphases. ACS Energy Lett. 2018, 3, 335–340. [Google Scholar] [CrossRef]
- Guo, B.; Wang, X.; Fulvio, P.F.; Chi, M.; Mahurin, S.M.; Sun, X.-G.; Dai, S. Soft-Templated Mesoporous Carbon-Carbon Nanotube Composites for High Performance Lithium-ion Batteries. Adv. Mater. 2011, 23, 4661–4666. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, L.; Cai, L.; Liu, H.; Fu, R.; Zhang, M.; Wu, D. Strong contribution of pore morphology to the high-rate electrochemical performance of lithium-ion batteries. Chem. Commun. 2016, 52, 803–806. [Google Scholar] [CrossRef]
- Yu, C.; Chen, M.; Li, X.; Zhao, C.; He, L.; Qiu, J. Hierarchically porous carbon architectures embedded with hollow nanocapsules for high-performance lithium storage. J. Mater. Chem. A 2015, 3, 5054–5059. [Google Scholar] [CrossRef]
- Wang, H.; Yu, W.; Mao, N.; Shi, J.; Liu, W. Effect of surface modification on high-surface-area carbon nanosheets anode in sodium ion battery. Microporous Mesoporous Mater. 2016, 227, 1–8. [Google Scholar] [CrossRef]
- Sahoo, M.; Sreena, K.P.; Vinayan, B.P.; Ramaprabhu, S. Green synthesis of boron doped graphene and its application as high performance anode material in Li ion battery. Mater. Res. Bull. 2015, 61, 383–390. [Google Scholar] [CrossRef]
- Huang, X.; Deng, J.; Qi, Y.; Liu, D.; Wu, Y.; Gao, W.; Zhong, W.; Zhang, F.; Bao, S.; Xu, M. A highly-effective nitrogen-doped porous carbon sponge electrode for advanced K–Se batteries. Inorg. Chem. Front. 2020, 7, 1182–1189. [Google Scholar] [CrossRef]
- Pohjalainen, E.; Rauhala, T.; Valkeapää, M.; Kallioinen, J.; Kallio, T. Effect of Li4Ti5O12 Particle Size on the Performance of Lithium Ion Battery Electrodes at High C-Rates and Low Temperatures. J. Phys. Chem. C 2015, 119, 2277–2283. [Google Scholar] [CrossRef]
- Shen, L.; Uchaker, E.; Zhang, X.; Cao, G. Hydrogenated Li4Ti5O12 Nanowire Arrays for High Rate Lithium Ion Batteries. Adv. Mater. 2012, 24, 6502–6506. [Google Scholar] [CrossRef]
- Zhu, G.-N.; Liu, H.-J.; Zhuang, J.-H.; Wang, C.-X.; Wang, Y.-G.; Xia, Y.-Y. Carbon-coated nano-sized Li4Ti5O12 nanoporous micro-sphere as anode material for high-rate lithium-ion batteries. Energy Environ. Sci. 2011, 4, 4016–4022. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Gu, L.; Guo, Y.-G.; Li, H.; He, X.-Q.; Tsukimoto, S.; Ikuhara, Y.; Wan, L.-J. Rutile-TiO2 Nanocoating for a High-Rate Li4Ti5O12 Anode of a Lithium-Ion Battery. J. Am. Chem. Soc. 2012, 134, 7874–7879. [Google Scholar] [CrossRef]
- Li, N.; Chen, Z.; Ren, W.; Li, F.; Cheng, H.-M. Flexible graphene-based lithium ion batteries with ultrafast charge and discharge rates. Proc. Natl. Acad. Sci. USA 2012, 109, 17360–17365. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.; Kim, H.; Cho, J. MoS2 Nanoplates Consisting of Disordered Graphene-like Layers for High Rate Lithium Battery Anode Materials. Nano Lett. 2011, 11, 4826–4830. [Google Scholar] [CrossRef]
- Luo, J.; Liu, J.; Zeng, Z.; Ng, C.F.; Ma, L.; Zhang, H.; Lin, J.; Shen, Z.; Fan, H.J. Three-Dimensional Graphene Foam Supported Fe3O4 Lithium Battery Anodes with Long Cycle Life and High Rate Capability. Nano Lett. 2013, 13, 6136–6143. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tan, B.; Wu, Y. Mesoporous Co3O4 Nanowire Arrays for Lithium Ion Batteries with High Capacity and Rate Capability. Nano Lett. 2008, 8, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chan, G.; Choi, J.W.; Ryu, I.; Yao, Y.; McDowell, M.T.; Lee, S.W.; Jackson, A.; Yang, Y.; Hu, L.; et al. Stable cycling of double-walled silicon nanotube battery anodes through solid–electrolyte interphase control. Nat. Nanotechnol. 2012, 7, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.M.; Mauger, A.; Zaghib, K.; Groult, H. Comparative Issues of Cathode Materials for Li-Ion Batteries. Inorganics 2014, 2, 132–154. [Google Scholar] [CrossRef] [Green Version]
- Byles, B.W.; Palapati, N.K.R.; Subramanian, A.; Pomerantseva, E. The role of electronic and ionic conductivities in the rate performance of tunnel structured manganese oxides in Li-ion batteries. APL Mater. 2016, 4, 046108. [Google Scholar] [CrossRef]
- Nisar, U.; Amin, R.; Essehli, R.; Shakoor, R.; Kahraman, R.; Kim, D.K.; Khaleel, M.A.; Belharouak, I. Extreme fast charging characteristics of zirconia modified LiNi0.5Mn1.5O4 cathode for lithium ion batteries. J. Power Source 2018, 396, 774–781. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, S.; Chang, Z.; Yang, Y.; Wu, Y. Nanoporous LiNi1/3Co1/3Mn1/3O2 as an ultra-fast charge cathode material for aqueous rechargeable lithium batteries. Chem. Commun. 2013, 49, 9209–9211. [Google Scholar] [CrossRef]
- Okubo, M.; Hosono, E.; Kim, J.; Enomoto, M.; Kojima, N.; Kudo, T.; Zhou, H.; Honma, I. Nanosize effect on high-rate Li-ion intercalation in LiCoO2 electrode. J. Am. Chem. Soc. 2007, 129, 7444–7452. [Google Scholar] [CrossRef]
- Lee, S.; Cho, Y.; Song, H.-K.; Lee, K.T.; Cho, J. Carbon-Coated Single-Crystal LiMn2O4 Nanoparticle Clusters as Cathode Material for High-Energy and High-Power Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2012, 51, 8748–8752. [Google Scholar] [CrossRef]
- Nokami, T.; Matsuo, T.; Inatomi, Y.; Hojo, N.; Tsukagoshi, T.; Yoshizawa, H.; Shimizu, A.; Kuramoto, H.; Komae, K.; Tsuyama, H.; et al. Polymer-Bound Pyrene-4,5,9,10-tetraone for Fast-Charge and -Discharge Lithium-Ion Batteries with High Capacity. J. Am. Chem. Soc. 2012, 134, 19694–19700. [Google Scholar] [CrossRef]
- Billaud, J.; Bouville, F.; Magrini, T.; Villevieille, C.; Studart, A.R. Magnetically aligned graphite electrodes for high-rate performance Li-ion batteries. Nat. Energy 2016, 1, 16097. [Google Scholar] [CrossRef]
- Jungst, R.G. Recycling of electric vehicle batteries. In Industrial Chemistry Library; Pistoia, G., Wiaux, J.P., Wolsky, S.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 295–327. [Google Scholar]
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Everson, M.P.; Wallington, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Nagpure, S.C.; Downing, R.G.; Bhushan, B.; Babu, S.S.; Cao, L. Neutron depth profiling technique for studying aging in Li-ion batteries. Electrochim. Acta 2011, 56, 4735–4743. [Google Scholar] [CrossRef]
- Ramoni, M.O.; Zhang, H.-C. An entropy-based metric for product remanufacturability. J. Remanuf. 2012, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Ündey, C.; Ertunç, S.; Mistretta, T.; Looze, B. Applied advanced process analytics in biopharmaceutical manufacturing: Challenges and prospects in real-time monitoring and control. J. Process Control 2010, 20, 1009–1018. [Google Scholar] [CrossRef]
- Li, J.; Du, Z.; Ruther, R.E.; An, S.J.; David, L.A.; Hays, K.; Wood, M.; Phillip, N.D.; Sheng, Y.; Mao, C.; et al. Toward Low-Cost, High-Energy Density, and High-Power Density Lithium-Ion Batteries. JOM 2017, 69, 1484–1496. [Google Scholar] [CrossRef] [Green Version]
- Hsu, D.Y.-C. Global EV Market Up 105% YoY in January. In EE Times Asia; 2022; Available online: https://www.eetasia.com/global-ev-market-up-105-yoy-in-january/ (accessed on 27 July 2022).
- Doyle, M.; Fuller, T.F.; Newman, J. Modeling of Galvanostatic Charge and Discharge of the Lithium/Polymer/Insertion Cell. J. Electrochem. Soc. 1993, 140, 1526–1533. [Google Scholar] [CrossRef]
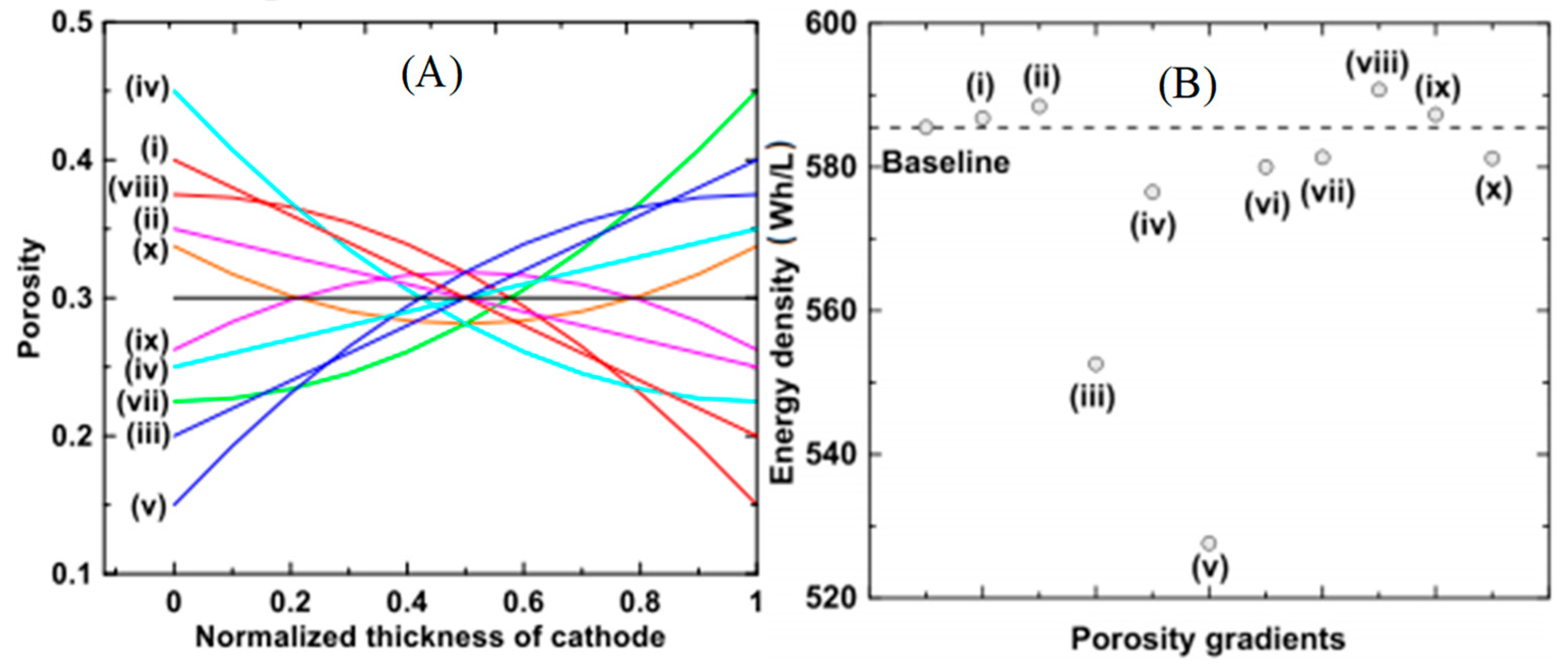
- Dai, Y.; Srinivasan, V. On Graded Electrode Porosity as a Design Tool for Improving the Energy Density of Batteries. J. Electrochem. Soc. 2015, 163, A406–A416. [Google Scholar] [CrossRef]
- Du, Z.; Wood, D.L.; Daniel, C.; Kalnaus, S.; Li, J. Understanding limiting factors in thick electrode performance as applied to high energy density Li-ion batteries. J. Appl. Electrochem. 2017, 47, 405–415. [Google Scholar] [CrossRef]
- Tjaden, B.; Cooper, S.J.; Brett, D.J.L.; Kramer, D.; Shearing, P.R. On the origin and application of the Bruggeman correlation for analysing transport phenomena in electrochemical systems. Curr. Opin. Chem. Eng. 2016, 12, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Ogihara, N.; Itou, Y.; Sasaki, T.; Takeuchi, Y. Impedance Spectroscopy Characterization of Porous Electrodes under Different Electrode Thickness Using a Symmetric Cell for High-Performance Lithium-Ion Batteries. J. Phys. Chem. C 2015, 119, 4612–4619. [Google Scholar] [CrossRef]
- Bae, C.-J.; Erdonmez, C.K.; Halloran, J.W.; Chiang, Y.-M. Design of Battery Electrodes with Dual-Scale Porosity to Minimize Tortuosity and Maximize Performance. Adv. Mater. 2013, 25, 1254–1258. [Google Scholar] [CrossRef]
- Hu, Y.S.; Adelhelm, P.; Smarsly, B.M.; Hore, S.; Antonietti, M.; Maier, J. Synthesis of Hierarchically Porous Carbon Monoliths with Highly Ordered Microstructure and Their Application in Rechargeable Lithium Batteries with High-Rate Capability. Adv. Funct. Mater. 2007, 17, 1873–1878. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, M.; Lei, Y. Nanoarchitectured Array Electrodes for Rechargeable Lithium- and Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1502514. [Google Scholar] [CrossRef]
- Srinivasan, V.; Newman, J. Discharge Model for the Lithium Iron-Phosphate Electrode. J. Electrochem. Soc. 2004, 151, A1517–A1529. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Huang, C.; Grant, P.S. Layer-by-layer printing of multi-layered heterostructures using Li4Ti5O12 and Si for high power Li-ion storage. Nano Energy 2019, 61, 96–103. [Google Scholar] [CrossRef]
- Huang, C.; Dontigny, M.; Zaghib, K.; Grant, P.S. Low-tortuosity and graded lithium ion battery cathodes by ice templating. J. Mater. Chem. A 2019, 7, 21421–21431. [Google Scholar] [CrossRef] [Green Version]
- Tjaden, B.; Brett, D.J.L.; Shearing, P.R. Tortuosity in electrochemical devices: A review of calculation approaches. Int. Mater. Rev. 2018, 63, 47–67. [Google Scholar] [CrossRef] [Green Version]
- Sander, J.S.; Erb, R.M.; Li, L.; Gurijala, A.; Chiang, Y.M. High-performance battery electrodes via magnetic templating. Nat. Energy 2016, 1, 16099. [Google Scholar] [CrossRef]
- Mangang, M.; Seifert, H.; Pfleging, W. Influence of laser pulse duration on the electrochemical performance of laser structured LiFePO4 composite electrodes. J. Power Source 2016, 304, 24–32. [Google Scholar] [CrossRef]
- Sun, K.; Wei, T.-S.; Ahn, B.Y.; Seo, J.Y.; Dillon, S.J.; Lewis, J.A. 3D Printing of Interdigitated Li-Ion Microbattery Architectures. Adv. Mater. 2013, 25, 4539–4543. [Google Scholar] [CrossRef] [Green Version]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Buqa, H.; Goers, D.; Holzapfel, M.; Spahr, M.E.; Novák, P. High Rate Capability of Graphite Negative Electrodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2005, 152, A474. [Google Scholar] [CrossRef]
- Ellis, B.L.; Lee, K.T.; Nazar, L.F. Positive Electrode Materials for Li-Ion and Li-Batteries. Chem. Mater. 2010, 22, 691–714. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, Z.; Dahn, J.R. Staging Phase Transitions in LixCoO2. J. Electrochem. Soc. 2002, 149, A1604. [Google Scholar] [CrossRef]
- Xia, H.; Meng, Y.; Lu, L.; Ceder, G. Electrochemical Behavior and Li Diffusion Study of LiCoO2 Thin Film Electrodes Prepared by PLD. 2007. Available online: https://www.researchgate.net/publication/37996446_Electrochemical_Behavior_and_Li_Diffusion_Study_of_LiCoO_Thin_Film_Electrodes_Prepared_by_PLD (accessed on 27 July 2022).
- Choi, Y.-M.; Pyun, S.-I.; Moon, S.-I. Effects of cation mixing on the electrochemical lithium intercalation reaction into porous Li 1−δNi1−yCoyO2 electrodes. Solid State Ion. 1996, 89, 43–52. [Google Scholar] [CrossRef]
- Vogler, C.; Hemmer, R.; Arnold, G.; Trépo, A.; Wohlfahrt-Mehrens, M. Lithium nickel oxide Li(Ni0.75Al0.17Co0.08)O2 as cathode material for lithium ion batteries. Ionics 1999, 5, 421–425. [Google Scholar] [CrossRef]
- Noh, H.-J.; Youn, S.; Yoon, C.S.; Sun, Y.-K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Source 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Gallagher, K.G.; Goebel, S.; Greszler, T.; Mathias, M.; Oelerich, W.; Eroglu, D.; Srinivasan, V. Quantifying the promise of lithium–air batteries for electric vehicles. Energy Environ. Sci. 2014, 7, 1555–1563. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Shkrob, I.A.; Abraham, D.P. Transition Metal Dissolution, Ion Migration, Electrocatalytic Reduction and Capacity Loss in Lithium-Ion Full Cells. J. Electrochem. Soc. 2017, 164, A389–A399. [Google Scholar] [CrossRef]
- Lin, F.; Markus, I.M.; Nordlund, D.; Weng, T.-C.; Asta, M.D.; Xin, H.L.; Doeff, M.M. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 2014, 5, 3529. [Google Scholar] [CrossRef] [Green Version]
- Kasnatscheew, J.; Rodehorst, U.; Streipert, B.; Wiemers-Meyer, S.; Jakelski, R.; Wagner, R.; Laskovic, I.C.; Winter, M. Learning from Overpotentials in Lithium Ion Batteries: A Case Study on the LiNi1/3Co1/3Mn1/3O2(NCM) Cathode. J. Electrochem. Soc. 2016, 163, A2943–A2950. [Google Scholar] [CrossRef]
- Rong, H.; Xu, M.; Zhu, Y.; Xie, B.; Lin, H.; Liao, Y.; Xing, L.; Li, W. A novel imidazole-based electrolyte additive for improved electrochemical performance of high voltage nickel-rich cathode coupled with graphite anode lithium ion battery. J. Power Source 2016, 332, 312–321. [Google Scholar] [CrossRef]
- Liang, L.; Jiang, F.; Cao, Y.; Hu, G.; Du, K.; Peng, Z. One strategy to enhance electrochemical properties of Ni-based cathode materials under high cut-off voltage for Li-ion batteries. J. Power Source 2016, 328, 422–432. [Google Scholar] [CrossRef]
- Mohanty, D.; Dahlberg, K.; King, D.M.; David, L.A.; Sefat, A.S.; Wood, D.L.; Daniel, C.; Dhar, S.; Mahajan, V.; Lee, M.; et al. Modification of Ni-Rich FCG NMC and NCA Cathodes by Atomic Layer Deposition: Preventing Surface Phase Transitions for High-Voltage Lithium-Ion Batteries. Sci. Rep. 2016, 6, 26532. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.-Y.; Manthiram, A. Surface-modified concentration-gradient Ni-rich layered oxide cathodes for high-energy lithium-ion batteries. J. Power Source 2015, 282, 429–436. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Wang, X.; Cai, X.; Xing, L.; Xu, M.; Liao, Y.; Li, X.; Li, W. Constructing a Protective Interface Film on Layered Lithium-Rich Cathode Using an Electrolyte Additive with Special Molecule Structure. ACS Appl. Mater. Interfaces 2016, 8, 30116–30125. [Google Scholar] [CrossRef]
- Cao, Z.; Li, Y.; Shi, M.; Zhu, G.; Zhang, R.; Li, X.; Yue, H.; Yang, S. Improvement of the Cycling Performance and Thermal Stability of Lithium-Ion Batteries by Coating Cathode Materials with Al2O3 Nano Layer. J. Electrochem. Soc. 2017, 164, A475–A481. [Google Scholar] [CrossRef]
- Croy, J.R.; Abouimrane, A.; Zhang, Z. Next-generation lithium-ion batteries: The promise of near-term advancements. MRS Bull. 2014, 39, 407–415. [Google Scholar] [CrossRef]
- Liao, J.-Y.; Oh, S.-M.; Manthiram, A. Core/Double-Shell Type Gradient Ni-Rich LiNi0.76Co0.10Mn0.14O2 with High Capacity and Long Cycle Life for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 24543–24549. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Myung, S.-T.; Park, B.-C.; Prakash, J.; Belharouak, I.; Amine, K. High-energy cathode material for long-life and safe lithium batteries. Nat. Mater. 2009, 8, 320–324. [Google Scholar] [CrossRef]
- Lim, B.-B.; Yoon, S.-J.; Park, K.-J.; Yoon, C.S.; Kim, S.-J.; Lee, J.J.; Sun, Y.-K. Advanced Concentration Gradient Cathode Material with Two-Slope for High-Energy and Safe Lithium Batteries. Adv. Funct. Mater. 2015, 25, 4673–4680. [Google Scholar] [CrossRef]
- Kim, U.-H.; Lee, E.-J.; Yoon, C.S.; Myung, S.-T.; Sun, Y.-K. Compositionally Graded Cathode Material with Long-Term Cycling Stability for Electric Vehicles Application. Adv. Energy Mater. 2016, 6, 1601417. [Google Scholar] [CrossRef]
- Lim, B.-B.; Myung, S.-T.; Yoon, C.S.; Sun, Y.-K. Comparative Study of Ni-Rich Layered Cathodes for Rechargeable Lithium Batteries: Li[Ni0.85Co0.11Al0.04]O2 and Li[Ni0.84Co0.06Mn0.09Al0.01]O2 with Two-Step Full Concentration Gradients. ACS Energy Lett. 2016, 1, 283–289. [Google Scholar] [CrossRef]
- Wang, J.; Du, C.; Xu, X.; He, X.; Yin, G.; Ma, Y.; Zuo, P.; Cheng, X.; Gao, Y. Lithium Phosphorus Oxynitride Coated Concentration Gradient Li[Ni0.73Co0.12Mn0.15]O2 Cathode Material with Enhanced Electrochemical Properties. Electrochim. Acta 2016, 192, 340–345. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Kang, S.-H.; Johnson, C.S.; Vaughey, J.T.; Benedek, R.; Hackney, S.A. Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 2007, 17, 3112–3125. [Google Scholar] [CrossRef]
- Martha, S.K.; Nanda, J.; Veith, G.M.; Dudney, N.J. Electrochemical and rate performance study of high-voltage lithium-rich composition: Li1.2Mn0.525Ni0.175Co0.1O2. J. Power Source 2012, 199, 220–226. [Google Scholar] [CrossRef]
- Mohanty, D.; Kalnaus, S.; Meisner, R.A.; Rhodes, K.J.; Li, J.; Payzant, E.A.; Wood, D.L.; Daniel, C. Structural transformation of a lithium-rich Li1.2Co0.1Mn0.55Ni0.15O2 cathode during high voltage cycling resolved by in situ X-ray diffraction. J. Power Source 2013, 229, 239–248. [Google Scholar] [CrossRef]
- Mohanty, D.; Li, J.; Abraham, D.P.; Huq, A.; Payzant, E.A.; Wood, D.L.; Daniel, C. Unraveling the Voltage-Fade Mechanism in High-Energy-Density Lithium-Ion Batteries: Origin of the Tetrahedral Cations for Spinel Conversion. Chem. Mater. 2014, 26, 6272–6280. [Google Scholar] [CrossRef]
- Birrozzi, A.; Laszczynski, N.; Hekmatfar, M.; von Zamory, J.; Giffin, G.A.; Passerini, S. Beneficial effect of propane sultone and tris(trimethylsilyl) borate as electrolyte additives on the cycling stability of the lithium rich nickel manganese cobalt (NMC) oxide. J. Power Source 2016, 325, 525–533. [Google Scholar] [CrossRef]
- Mohanty, D.; Sefat, A.S.; Payzant, E.A.; Li, J.; Wood, D.L.; Daniel, C. Unconventional irreversible structural changes in a high-voltage Li–Mn-rich oxide for lithium-ion battery cathodes. J. Power Source 2015, 283, 423–428. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Q.Q.; Zhang, H.Z.; Li, G.R.; Ye, S.H.; Wang, C.W.; Gao, X.P. Surface modification of Li-rich layered Li(Li0.17Ni0.25Mn0.58)O2 oxide with Li–Mn–PO4 as the cathode for lithium-ion batteries. J. Mater. Chem. A 2013, 1, 5262–5268. [Google Scholar] [CrossRef]
- Zhang, X.; Belharouak, I.; Li, L.; Lei, Y.; Elam, J.W.; Nie, A.; Chen, X.; Yassar, R.S.; Axelbaum, R.L. Structural and Electrochemical Study of Al2O3 and TiO2 Coated Li1.2Ni0.13Mn0.54Co0.13O2 Cathode Material Using ALD. Adv. Energy Mater. 2013, 3, 1299–1307. [Google Scholar] [CrossRef]
- Mohanty, D.; Kalnaus, S.; Meisner, R.A.; Safat, A.S.; Li, J.; Payzant, E.A.; Rhodes, K.; Wood, I.I.I.D.L.; Daniel, C. Structural transformation in a Li1.2Co0.1Mn0.55Ni0.15O2 lithium-ion battery cathode during high-voltage hold. RSC Adv. 2013, 3, 7479–7485. [Google Scholar] [CrossRef]
- Ito, A.; Li, D.; Sato, Y.; Arao, M.; Watanabe, M.; Hatano, M.; Horie, H.; Ohsawa, Y. Cyclic deterioration and its improvement for Li-rich layered cathode material Li[Ni0.17Li0.2Co0.07Mn0.56]O2. J. Power Source 2010, 195, 567–573. [Google Scholar] [CrossRef]
- Martha, S.K.; Nanda, J.; Kim, Y.; Unocic, R.R.; Pannala, S.; Dudney, N.J. Solid electrolyte coated high voltage layered–layered lithium-rich composite cathode: Li1.2Mn0.525Ni0.175Co0.1O2. J. Mater. Chem. A 2013, 1, 5587–5595. [Google Scholar] [CrossRef]
- Lin, J.; Mu, D.; Jin, Y.; Wu, B.; Ma, Y.; Wu, F. Li-rich layered composite Li[Li0.2Ni0.2Mn0.6]O2 synthesized by a novel approach as cathode material for lithium ion battery. J. Power Source 2013, 230, 76–80. [Google Scholar] [CrossRef]
- Croy, J.R.; Park, J.S.; Shin, Y.; Yonemoto, B.T.; Balasubramanian, M.; Long, B.R.; Ren, Y.; Thackeray, M.M. Prospects for spinel-stabilized, high-capacity lithium-ion battery cathodes. J. Power Source 2016, 334, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.-P.; Yang, H.-X. Multi-electron reaction materials for high energy density batteries. Energy Environ. Sci. 2010, 3, 174–189. [Google Scholar] [CrossRef]
- Zhang, S.S. Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems, and solutions. J. Power Source 2013, 231, 153–162. [Google Scholar] [CrossRef]
- Liang, Y.; Tao, Z.; Chen, J. Organic Electrode Materials for Rechargeable Lithium Batteries. Adv. Energy Mater. 2012, 2, 742–769. [Google Scholar] [CrossRef]
- Masquelier, C.; Croguennec, L. Polyanionic (Phosphates, Silicates, Sulfates) Frameworks as Electrode Materials for Rechargeable Li (or Na) Batteries. Chem. Rev. 2013, 113, 6552–6591. [Google Scholar] [CrossRef]
- Courtney, I.A.; Dahn, J.R. Electrochemical and In Situ X-Ray Diffraction Studies of the Reaction of Lithium with Tin Oxide Composites. J. Electrochem. Soc. 1997, 144, 2045–2052. [Google Scholar] [CrossRef]
- McDowell, M.T.; Lee, S.W.; Ryu, I.; Wu, H.; Nix, W.D.; Choi, J.W.; Cui, Y. Novel Size and Surface Oxide Effects in Silicon Nanowires as Lithium Battery Anodes. Nano Lett. 2011, 11, 4018–4025. [Google Scholar] [CrossRef]
- Besenhard, J.O.; Yang, J.; Winter, M. Will advanced lithium-alloy anodes have a chance in lithium-ion batteries? J. Power Source 1997, 68, 87–90. [Google Scholar] [CrossRef]
- Courtney, I.A.; McKinnon, W.R.; Dahn, J.R. On the Aggregation of Tin in SnO Composite Glasses Caused by the Reversible Reaction with Lithium. J. Electrochem. Soc. 1999, 146, 59–68. [Google Scholar] [CrossRef]
- Dimov, N.; Kugino, S.; Yoshio, M. Mixed silicon–graphite composites as anode material for lithium ion batteries: Influence of preparation conditions on the properties of the material. J. Power Source 2004, 136, 108–114. [Google Scholar] [CrossRef]
- Karulkar, M.; Blaser, R.; Kudla, B. Automotive assessment of carbon–silicon composite anodes and methods of fabrication. J. Power Source 2015, 273, 1194–1201. [Google Scholar] [CrossRef]
- Eom, J.Y.; Park, J.W.; Kwon, H.S.; Rajendran, S. Electrochemical Insertion of Lithium into Multiwalled Carbon Nanotube/Silicon Composites Produced by Ballmilling. J. Electrochem. Soc. 2006, 153, A1678. [Google Scholar] [CrossRef]
- Kim, N.; Oh, C.; Kim, J.; Kim, J.-S.; Jeong, E.D.; Bae, J.-S.; Hong, T.E.; Lee, J.K. High-Performance Li-Ion Battery Anodes Based on Silicon-Graphene Self-Assemblies. J. Electrochem. Soc. 2016, 164, A6075–A6083. [Google Scholar] [CrossRef]
- Wang, J.; Xu, T.; Huang, X.; Li, H.; Ma, T. Recent progress of silicon composites as anode materials for secondary batteries. RSC Adv. 2016, 6, 87778–87790. [Google Scholar] [CrossRef]
- Etacheri, V.; Haik, O.; Goffer, Y.; Roberts, G.A.; Stefan, I.C.; Fasching, R.; Aurbach, D. Effect of Fluoroethylene Carbonate (FEC) on the Performance and Surface Chemistry of Si-Nanowire Li-Ion Battery Anodes. Langmuir 2012, 28, 965–976. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Haering, D.; Solchenbach, S.; Marino, C.; Tsiouvaras, N.; Stinner, C.; Gasteiger, H.A. Consumption of Fluoroethylene Carbonate (FEC) on Si-C Composite Electrodes for Li-Ion Batteries. J. Electrochem. Soc. 2016, 163, A1705–A1716. [Google Scholar] [CrossRef]
- Byrd, I.; Chen, H.; Webber, T.; Li, J.; Wu, J. Self-assembled asymmetric membrane containing micron-size germanium for high capacity lithium ion batteries. RSC Adv. 2015, 5, 92878–92884. [Google Scholar] [CrossRef]
- Tian, H.; Xin, F.; Wang, X.; He, W.; Han, W. High capacity group-IV elements (Si, Ge, Sn) based anodes for lithium-ion batteries. J. Mater. 2015, 1, 153–169. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, X.-T. Introduction to Chemical Vapour Deposition. In Engineering Materials and Processes 2010; Springer: London, UK, 2010. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, X. Electrospun carbon nanofibers containing silicon particles as an energy-storage medium. Carbon 2009, 47, 3219–3226. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, L.; Toprakci, O.; Liang, Y.; Alcoutlabi, M. Electrospun Nanofiber-Based Anodes, Cathodes, and Separators for Advanced Lithium-Ion Batteries. Polym. Rev. 2011, 51, 239–264. [Google Scholar] [CrossRef]
- Agubra, V.; Fergus, J. Lithium Ion Battery Anode Aging Mechanisms. Materials 2013, 6, 1310–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anseán, D.; González, M.; Viera, J.C.; García, V.M.; Blanco, C.; Valledor, M. Fast charging technique for high power lithium iron phosphate batteries: A cycle life analysis. J. Power Source 2013, 239, 9–15. [Google Scholar] [CrossRef]
- Somerville, L.; Bareño, J.; Trask, S.; Jennings, P.; McGordon, A.; Lyness, C.; Bloom, I. The effect of charging rate on the graphite electrode of commercial lithium-ion cells: A post-mortem study. J. Power Source 2016, 335, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Notten, P.; Veld, J.O.H.; van Beek, J. Boostcharging Li-ion batteries: A challenging new charging concept. J. Power Source 2005, 145, 89–94. [Google Scholar] [CrossRef]
- Cipollone, R.; di Battista, D.; Marchionni, M.; Villante, C. Model based Design and Optimization of a Fuel Cell Electric Vehicle. Energy Procedia 2014, 45, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Genovese, A.; Ortenzi, F.; Villante, C. On the energy efficiency of quick DC vehicle battery charging. World Electr. Veh. J. 2015, 7, 570–576. [Google Scholar] [CrossRef] [Green Version]
- Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutz, M. Development of a recycling process for Li-ion batteries. J. Power Source 2012, 207, 173–182. [Google Scholar] [CrossRef]
- Gaines, L.; Richa, K.; Spangenberger, J. Key issues for Li-ion battery recycling. MRS Energy Sustain. 2018, 5, E14. [Google Scholar] [CrossRef] [Green Version]
- Yan, T.; Zhong, S.; Zhou, M.; Guo, X.; Hu, J.; Wang, F.; Zeng, F.; Zuo, S. High-efficiency method for recycling lithium from spent LiFePO4 cathode. Nanotechnol. Rev. 2020, 9, 1586–1593. [Google Scholar] [CrossRef]
- Li, H.; Dai, J.; Wang, A.; Zhao, S.; Ye, H.; Zhang, J. Recycling and Treatment of Waste Batteries. IOP Conf. Ser. Mater. Sci. Eng. 2019, 612, 052020. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Manthiram, A. Materials Challenges and Opportunities of Lithium Ion Batteries. J. Phys. Chem. Lett. 2011, 2, 176–184. [Google Scholar] [CrossRef]
- Gao, F.; Tang, Z. Kinetic behavior of LiFePO4/C cathode material for lithium-ion batteries. Electrochim. Acta 2008, 53, 5071–5075. [Google Scholar] [CrossRef]
- Wang, G.X.; Needham, S.; Yao, J.; Wang, J.Z.; Liu, R.S.; Liu, H.K. A study on LiFePO4 and its doped derivatives as cathode materials for lithium-ion batteries. J. Power Source 2006, 159, 282–286. [Google Scholar] [CrossRef]
- Olapiriyakul, S.; Caudill, R.J. A framework for risk management and end-of-life (EOL) analysis for nanotechnology products: A case study in lithium-ion batteries. In Proceedings of the 2008 IEEE International Symposium on Electronics and the Environment, San Francisco, CA, USA, 19–22 May 2008. [Google Scholar]
- Dupre, N.; Cuisinier, M.; Guyomard, D. Electrode/Electrolyte Interface Studies in Lithium Batteries Using NMR. Interface Mag. 2011, 20, 61–67. [Google Scholar] [CrossRef]
- Ramoni, M.; Zhang, H. Remanufacturing processes of electric vehicle battery. In Proceedings of the 2012 IEEE International Symposium on Sustainable Systems and Technology (ISSST), Boston, MA, USA, 16–18 May 2012. [Google Scholar]
- Panitz, J.-C.; Novák, P. Raman microscopy as a quality control tool for electrodes of lithium-ion batteries. J. Power Source 2001, 97–98, 174–180. [Google Scholar] [CrossRef]
- Shekhar, H.; Sharma, S.; Patel, U.; Sawant, V. Exploring Cost-Reduction Strategies for Electric Vehicle (EV) Batteries; Indian Council for Research on International Economic Relations: New Delhi, India, 2020.
- Ruther, R.E.; Callender, A.F.; Zhou, H.; Martha, S.K.; Nanda, J. Raman Microscopy of Lithium-Manganese-Rich Transition Metal Oxide Cathodes. J. Electrochem. Soc. 2014, 162, A98–A102. [Google Scholar] [CrossRef]
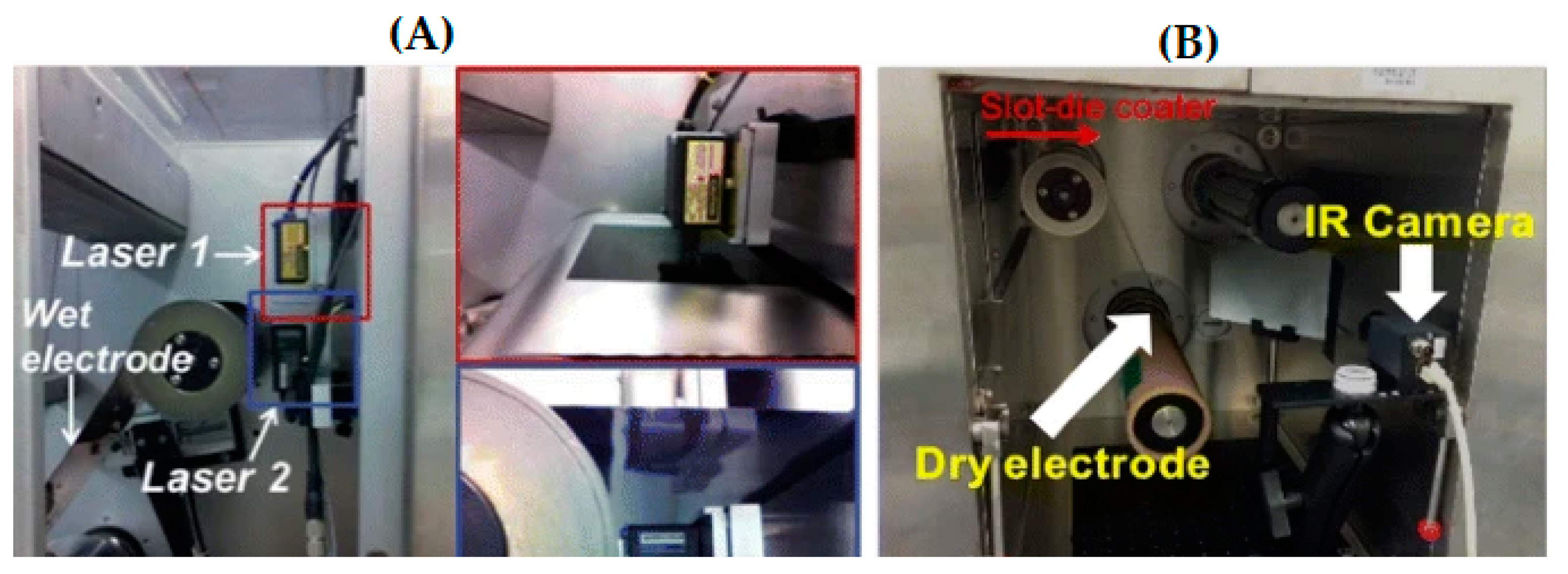
- Mohanty, D.; Li, J.; Born, R.; Maxey, L.C.; Dinwiddie, R.B.; Daniel, C.; Wood, I.I.I.D.L. Non-destructive evaluation of slot-die-coated lithium secondary battery electrodes by in-line laser caliper and IR thermography methods. Anal. Methods 2014, 6, 674–683. [Google Scholar] [CrossRef]
- Mohanty, D.; Hockaday, E.; Li, J.; Hensley, D.K.; Daniel, C.; Wood, D.L. Effect of electrode manufacturing defects on electrochemical performance of lithium-ion batteries: Cognizance of the battery failure sources. J. Power Source 2016, 312, 70–79. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Armstrong, B.L.; Kiggans, J.; Daniel, C.; Wood, D.L. Lithium Ion Cell Performance Enhancement Using Aqueous LiFePO4Cathode Dispersions and Polyethyleneimine Dispersant. J. Electrochem. Soc. 2012, 160, A201–A206. [Google Scholar] [CrossRef] [Green Version]
- Wood, D.L., III; Li, J.; Daniel, C. Prospects for reducing the processing cost of lithium ion batteries. J. Power Source 2015, 275, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Wood, D.L.; Quass, J.D.; Li, J.; Ahmed, S.; Ventola, D.; Daniel, C. Technical and economic analysis of solvent-based lithium-ion electrode drying with water and NMP. Dry. Technol. 2018, 36, 234–244. [Google Scholar] [CrossRef]
- Zackrisson, M.; Avellán, L.; Orlenius, J. Life cycle assessment of lithium-ion batteries for plug-in hybrid electric vehicles—Critical issues. J. Clean. Prod. 2010, 18, 1519–1529. [Google Scholar] [CrossRef]
- Li, J.; Rulison, C.; Kiggans, J.; Daniel, C.; Wood, D.L. Superior Performance of LiFePO4 Aqueous Dispersions via Corona Treatment and Surface Energy Optimization. J. Electrochem. Soc. 2012, 159, A1152–A1157. [Google Scholar] [CrossRef]
- Du, Z.; Rollag, K.M.; Li, J.; An, S.J.; Wood, M.; Sheng, Y.; Mukherjee, P.P.; Daniel, C.; Wood, D.L. Enabling aqueous processing for crack-free thick electrodes. J. Power Source 2017, 354, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Daniel, C.; An, S.J.; Wood, D. Evaluation Residual Moisture in Lithium-Ion Battery Electrodes and Its Effect on Electrode Performance. MRS Adv. 2016, 1, 1029–1035. [Google Scholar] [CrossRef] [Green Version]
- Li, C.-C.; Lee, J.-T.; Peng, X.-W. Improvements of Dispersion Homogeneity and Cell Performance of Aqueous-Processed LiCoO2 Cathodes by Using Dispersant of PAA–NH4. J. Electrochem. Soc. 2006, 153, A809. [Google Scholar] [CrossRef]
- Li, C.-C.; Peng, X.-W.; Lee, J.-T.; Wang, F.-M. Using Poly(4-Styrene Sulfonic Acid) to Improve the Dispersion Homogeneity of Aqueous-Processed LiFePO4 Cathodes. J. Electrochem. Soc. 2010, 157, A517. [Google Scholar] [CrossRef]
- Kim, K.M.; Jeon, W.S.; Chung, I.J.; Chang, S.H. Effect of mixing sequences on the electrode characteristics of lithium-ion rechargeable batteries. J. Power Source 1999, 83, 108–113. [Google Scholar] [CrossRef]
- Li, J.; Armstrong, B.L.; Daniel, C.; Kiggans, J.; Wood, D.L. Optimization of multicomponent aqueous suspensions of lithium iron phosphate (LiFePO4) nanoparticles and carbon black for lithium-ion battery cathodes. J. Colloid Interface Sci. 2013, 405, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Pappas, S.P. Radiation Curing: Science and Technology; Springer: Boston, MA, USA, 1992; p. 1. [Google Scholar]
- Du, Z.; Janke, C.J.; Li, J.; Daniel, C.; Wood, D.L. Electron Beam Curing of Composite Positive Electrode for Li-Ion Battery. J. Electrochem. Soc. 2016, 163, A2776–A2780. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and Interphases in Li-Ion Batteries and Beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Alpas, A.T. Micromechanisms of solid electrolyte interphase formation on electrochemically cycled graphite electrodes in lithium-ion cells. Carbon 2012, 50, 5359–5371. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Riahi, A.R.; Alpas, A.T. Role of Voltage Scan Rate on Degradation of Graphite Electrodes Electrochemically Cycled vs. Li/Li+. MRS Online Proc. Libr. 2011, 1388, 3. [Google Scholar] [CrossRef]
- Lee, H.-H.; Wang, Y.-Y.; Wan, C.-C.; Yang, M.-H.; Wu, H.-C.; Shieh, D.-T. A fast formation process for lithium batteries. J. Power Source 2004, 134, 118–123. [Google Scholar] [CrossRef]
- Colclasure, A.M.; Smith, K.A.; Kee, R.J. Modeling detailed chemistry and transport for solid-electrolyte-interface (SEI) films in Li–ion batteries. Electrochim. Acta 2011, 58, 33–43. [Google Scholar] [CrossRef]
- Collins, J.; Gourdin, G.; Foster, M.; Qu, D. Carbon surface functionalities and SEI formation during Li intercalation. Carbon 2015, 92, 193–244. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, C.; Wan, C.; Tsuchida, E. Effects of catalytic oxidation on the electrochemical performance of common natural graphite as an anode material for lithium ion batteries. Electrochem. Commun. 2000, 2, 272–275. [Google Scholar] [CrossRef]
- Wu, Y.P.; Jiang, C.; Wan, C.; Holze, R. Anode materials for lithium ion batteries by oxidative treatment of common natural graphite. Solid State Ion. 2003, 156, 283–290. [Google Scholar] [CrossRef]
- Ye, J.C.; Charnvanichborikarn, S.; Worsley, M.A.; Kucheyev, S.O.; Wood, B.C.; Wang, Y.M. Enhanced electrochemical performance of ion-beam-treated 3D graphene aerogels for lithium ion batteries. Carbon 2015, 85, 269–278. [Google Scholar] [CrossRef]
- Spahr, M.E.; Wilhelm, H.; Joho, F.; Panitz, J.-C.; Wambach, J.; Novák, P.; Dupont-Pavlovsky, N. Purely Hexagonal Graphite and the Influence of Surface Modifications on Its Electrochemical Lithium Insertion Properties. J. Electrochem. Soc. 2002, 149, A960. [Google Scholar] [CrossRef]
- Ein-Eli, Y.; Koch, V.R. Chemical Oxidation: A Route to Enhanced Capacity in Li-Ion Graphite Anodes. J. Electrochem. Soc. 1997, 144, 2968–2973. [Google Scholar] [CrossRef]




| Battery Types | Specific Energy (Wh/Kg) | Energy/Volume (Wh/L) | Power/Weight (W/Kg) | Self-Discharge Coefficient (% per 24 h) | Recharging Cycles |
|---|---|---|---|---|---|
| Pb-acid | 40 | 70 | 180 | 1 | 500 |
| Ni-Cd | 60 | 100 | 150 | 5 | 1350 |
| Ni-MH | 70 | 250 | 1000 | 2 | 1350 |
| Li-ion | 200 | 270 | 1800 | 1 | 1000 |
| Battery Capacity (kWh) | Range in One Full Charge (km) | Empty to Full Charging Time (h) | |||||
|---|---|---|---|---|---|---|---|
| Charger Power (kW) | |||||||
| Slow | Fast | Rapid | |||||
| 3.7 | 7 | 22 | 43–50 | 250 | |||
| Mitsubishi Outlander PHEV | 13.8 | 24 | 4 | 4 | 4 | 0.67 | Not usable |
| Nissan Leaf | 40 | 143 | 11 | 6 | 6 | 1 | Not usable |
| Tesla model S | 75 | 238 | 21 | 11 | 5 | 2 | 1 |
| Recycling Technology | Major Drawbacks |
| Pyrometallurgy [52] |
|
| Hydrometallurgy [51] |
|
| Direct Recycling [53] |
|
| Technological/Financial Challenges | Actions or Progress to Overcome the Challenge | Limitations |
|---|---|---|
| Energy density | Developing electrode materials holding more charge in a fixed volume enhance energy density. Using effective porous electrode material to accommodate solid reaction products. | Increasing energy density needs additional space. Other concerns with energy density are durability and reliability. |
| Fast charging | Improving ionic and electrical conductivity, introducing porous carbon anode to reduce li-ion diffusion pathway along with boron doping in the anode. | Reduces energy efficiency and power fade because of the high current used to accelerate the charging process. |
| End of life | Remanufacturing for reuse, reengineering for stationary energy storage, recycling by separating part by part, and recovering precious metals. | Each different material from the recycling process has a different market size, which will collide with each other, and can cause a sudden collapse in the price range. |
| Financial challenges | Variation in physical and chemical properties of battery component. Changing the manufacturing process from SPC to APC to reduce process variability. | Changing physical and chemical properties can affect other battery properties. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, H.; Roy, B.N.; Hasanuzzaman, M.; Islam, M.S.; Abdel-Khalik, A.S.; Hamad, M.S.; Ahmed, S. Global Advancements and Current Challenges of Electric Vehicle Batteries and Their Prospects: A Comprehensive Review. Sustainability 2022, 14, 16684. https://doi.org/10.3390/su142416684
Roy H, Roy BN, Hasanuzzaman M, Islam MS, Abdel-Khalik AS, Hamad MS, Ahmed S. Global Advancements and Current Challenges of Electric Vehicle Batteries and Their Prospects: A Comprehensive Review. Sustainability. 2022; 14(24):16684. https://doi.org/10.3390/su142416684
Chicago/Turabian StyleRoy, Hridoy, Bimol Nath Roy, Md. Hasanuzzaman, Md. Shahinoor Islam, Ayman S. Abdel-Khalik, Mostaf S. Hamad, and Shehab Ahmed. 2022. "Global Advancements and Current Challenges of Electric Vehicle Batteries and Their Prospects: A Comprehensive Review" Sustainability 14, no. 24: 16684. https://doi.org/10.3390/su142416684
APA StyleRoy, H., Roy, B. N., Hasanuzzaman, M., Islam, M. S., Abdel-Khalik, A. S., Hamad, M. S., & Ahmed, S. (2022). Global Advancements and Current Challenges of Electric Vehicle Batteries and Their Prospects: A Comprehensive Review. Sustainability, 14(24), 16684. https://doi.org/10.3390/su142416684








