Effect of Water Treatment on the Chemical Composition of Drinking Water: A Case of Lovozero, Murmansk Region, Russia
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Svetlov, A.V.; Minenko, V.G.; Samusev, A.L.; Salakhov, E.M. Treating the mine water of Kola MMC’s Severny Mine using electrochemical coagulation. Non-Ferr. Metals. 2019, 11, 52–56. (In Russian) [Google Scholar]
- Dvoichenkova, G.P.; Minenko, V.G.; Pismenny, A.V.; Zyryanov, I.V.; Ostrovskaya, G.K.h. Environmentally safe method of processing and disposal of mineralized circulating tailings water at the concentration plants operated by ALROSA. Min. J. 2011, 1, 97–100. (In Russian) [Google Scholar]
- Zaostrovtsev, V.N.; Minyaeva, I.A.; Sukhanevich, M.M.; Gorev, A.V. Treatment process for mining wastewater. Min. J. 2014, 3, 68–73. (In Russian) [Google Scholar]
- Skorokhodov, V.F.; Kitaeva, A.S.; Nikitin, R.M. Using a combination of coagulation and flotation for wastewater treatment. Ore Dress. 2019, 1, 39–45. (In Russian) [Google Scholar] [CrossRef]
- Horn, R. Marine Chemistry (Water Structure and Hydrosphere Chemistry); Translated from English; Mir: Moscow, Russia, 1972; 399p. (In Russian) [Google Scholar]
- Shvartsev, S.L. Water as the main factor of global evolution. Her. Russ. Acad. Sci. 2013, 83, 78–85. (In Russian) [Google Scholar] [CrossRef]
- Chanturiya, V.; Chanturiya, E.; Minenko, V.A.L. Samusev Enlarged tests of the electrochemical method of water treatment for the enrichment of tantalum-niobium ores. Obogashchenie Rud. 2017, 3, 27–35. (In Russian) [Google Scholar] [CrossRef]
- GOST 56237-2014; Drinking Water. Sampling at Water Treatment Plants and from Pipeline Distribution Systems. National Academic Press: Washington, DC, USA, 2006. (In Russian)
- Water Supply and Sewerage System in the Lovozero Community, Lovozero District, Murmansk Region, for 2014–2018 and for the Period up to 2028; Energo Consult LLC: Abu Dhabi, United Arab Emirates, 2014; 186p. (In Russian)
- Mazukhina, S.I. Formation of Surface and Underground Water of the Khibiny Mountain Range; Publishing House of the KSC RAS: Apatity, Russia, 2012; 173p. (In Russian) [Google Scholar]
- Mazukhina, S.I.; Maksimova, V.V.; Chudnenko, K.V.; Masloboev, V.A.; Sandimirov, S.S.; Drogobuzhskaya, S.V.; Tereshchenko, P.S.; Pozhilenko, V.I.; Gudkov, A.V. Water Quality in the Arctic Zone of the Russian Federation: Physical and Chemical Modeling of Water Formation, Forms of Element Migration, Impact on the Human Body: Monograph; Publishing House of the Federal Research Center of the Russian Academy of Sciences: Apatity, Russia, 2020; 158p, ISBN 978-5-91137-437-2. (In Russian) [Google Scholar]
- Chudnenko, K.V. Termodinamicheskoe Modelirovanie v Geokhimii: Teoriya, Algoritmy, Programmnoe Obespechenie, Prilozheniya; Akademicheskoe izd-vo “Geo”: Novosibirsk, Russia, 2010; 287p. (In Russian) [Google Scholar]
- Mazukhina, S.; Krasavtseva, E.; Makarov, D.; Maksimova, V. Thermodynamic Modeling of Hypergene Processes in Loparite Ore Concentration Tailings. Minerals 2021, 11, 996. [Google Scholar] [CrossRef]
- Sandimirov, S.S.; Pozhilenko, V.I.; Mazukhina, S.I.; Drogobuzhskaya, S.V.; Shirokaya, A.A.; Tereshchenko, P.S. Chemical composition of natural waters of the Lovozero massif, Russia. Model. Earth Syst. Environ. 2022, 8, 4307–4315. [Google Scholar] [CrossRef]
- Berman, R.G. Internally-consistent thermodynamic data for minerals in the systems: Na2O-K2O-CaO-MgO-FeO-Fe2O3-Al2O3-SiO2-TiO2-H2O-CO2. J. Petrol. 1988, 29, 445–522. [Google Scholar] [CrossRef]
- Rid, R. Gases and Liquids Properties: Handbook; Rid, R., Prausnic, D., Shervud, T., Eds.; Himija: Leningrad, Russia, 1982; 592p. [Google Scholar]
- Robie, R.A.; Hemingway, B.S. Thermodynamic Properties of Minerals and Related Substancies of 298.15 K and 1 Bar Pressure and at Higher Temperatures; US Geological Survey: Washington, DC, USA, 1995; p. 461. [Google Scholar]
- Johnson, J.W.; Oelkers, E.H.; Helgeson, H.C. SUPCRT 92: Software package for calculating the standard molal thermodynamic properties of mineral, gases, aqueous species, and reactions from 1 to 5000 bars and 0 to 1000 °C. Comput. Geosci. 1992, 18, 899–947. [Google Scholar] [CrossRef]
- Holland, T.J.B.; Powell, R. An internally consistent thermodynamic data set for phases of petrological interest. J. Metamorph. Geol. 1998, 16, 309–343. [Google Scholar] [CrossRef]
- Yokokawa, H. Tables of thermodynamic properties of inorganic compounds. J. Natl. Chem. Lab. Ind. 1988, 83, 27–118. [Google Scholar]
- Linnik, P.N.; Zhezherya, V.A. Aluminum in surface water in Ukraine: Content, forms of migration, distribution of abiotic components. Water Resour. 2013, 40, 157–169. (In Russian) [Google Scholar] [CrossRef]
- Wickstrøm, T.; Clarke, N.; Derome, K.; Derome, J.; Røgeberg, E. Comparison study of five analytical methods for the fractionation and subsequent determination of aluminium in natural water samples. J. Environ. Monit. 2000, 2, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Goncharuk, V.; Zuy, O.; Melnik, L.; Mishchuk, N.; Nanieva, A.; Pelishenko, A. Assessment of the physiological adequacy of drinking water by means of biotesting. Chem. Sustain. Dev. 2021, 29, 34–39. [Google Scholar] [CrossRef]
- SanPIN 2.1.4.1116-02; Potable Water. Public Health Requirements for the Quality of Water Packaged in Containers. Quality Control. Ministry of Health of Russia: Moscow, Russia, 2002. Available online: https://meganorm.ru/Index2/1/4294815/4294815037. (accessed on 1 December 2021). (In Russian)
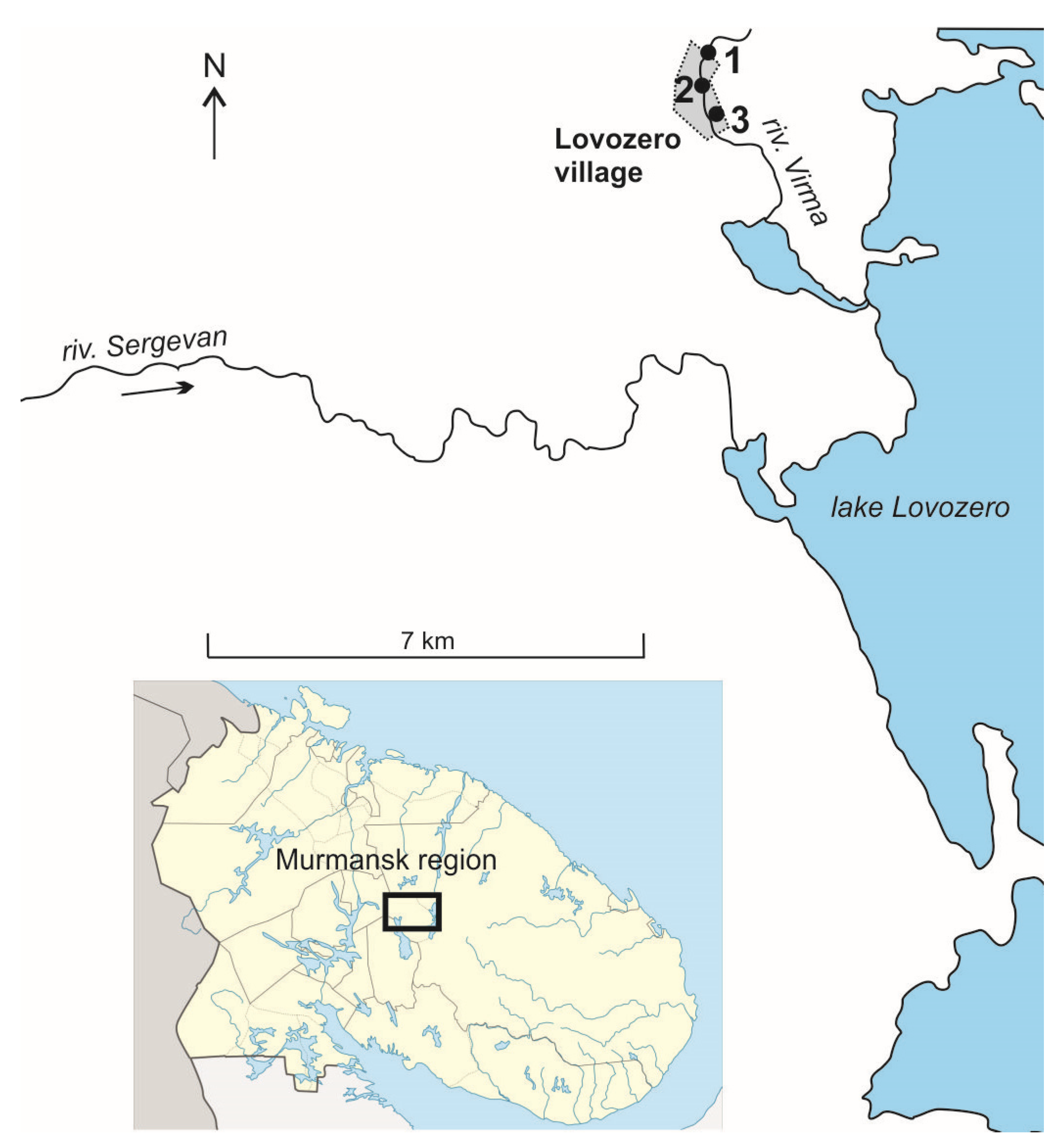


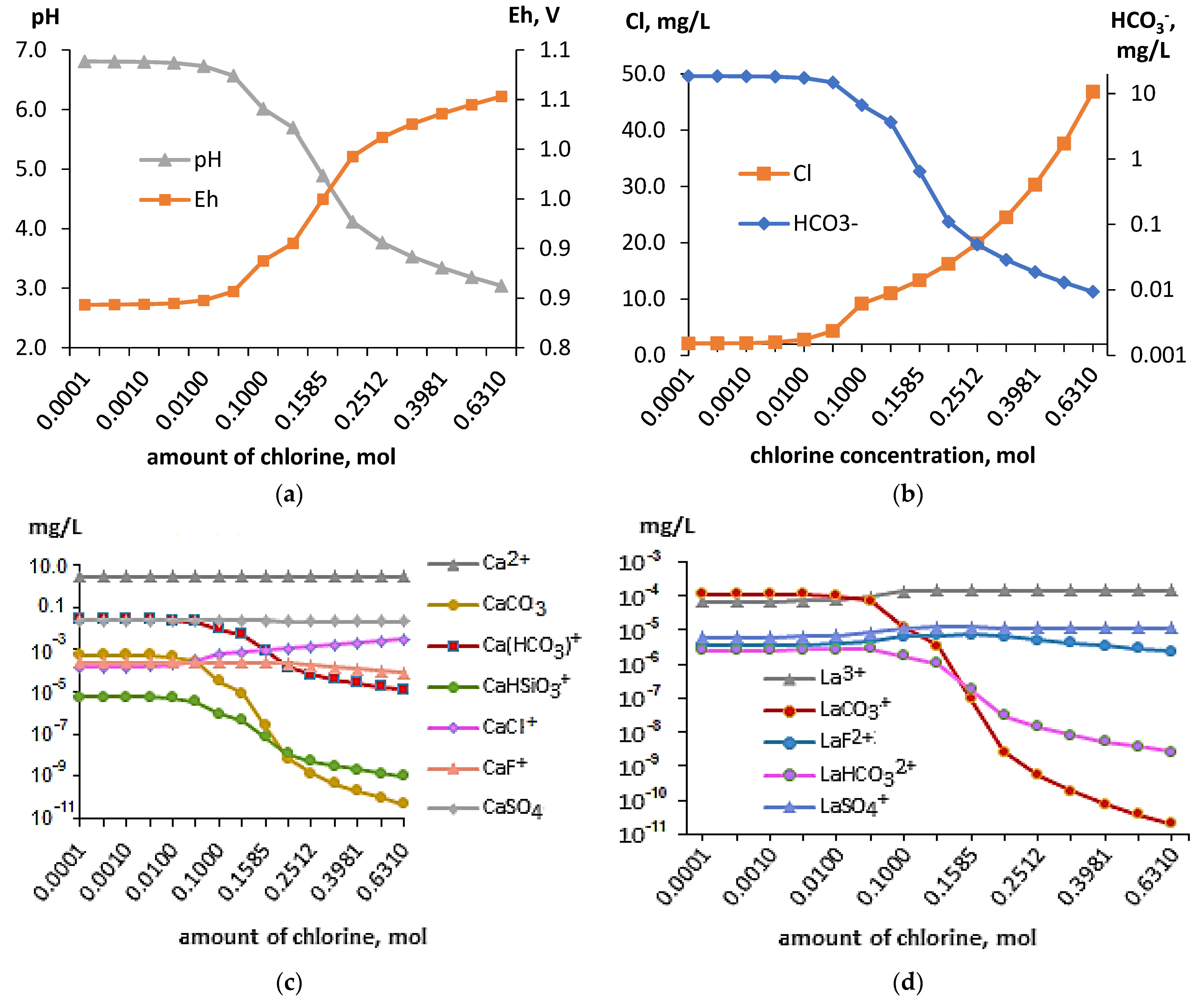


| Indicator | Virma River | Tap Water-Lovozero | Indicator | Virma River | Tap Water | ||||
|---|---|---|---|---|---|---|---|---|---|
| AD | MR | AD | MR | AD | MR | AD | MR | ||
| Eh | 0.828 | 0.770 | Ba | 0.0047 | 4.71 × 10−3 | 0.0028 | 2.83 × 10−3 | ||
| pH | 6.67 | 6.667 | 7.68 | 7.688 | Ba2+ | 4.71 × 10−3 | 2.83 × 10−3 | ||
| Is (ionic strength) | 0.000575 | 0.001381 | BaCO3 | 1.53 × 10−7 | 2.38 × 10−6 | ||||
| Al | 0.057 | 5.71 × 10−2 | 0.176 | 1.76 × 10−1 | BaCl+ | 1.00 × 10−7 | 3.05 × 10−7 | ||
| Al(OH)2+ | 1.17 × 10−3 | 1.17 × 10−4 | BaOH+ | 4.25 × 10−10 | 2.56 × 10−9 | ||||
| Al(OH)2F | 9.16 × 10−2 | 5.37 × 10−3 | Si | 6.31 | 6.31 | 5.46 | 5.46 | ||
| Al(OH)2F2− | 2.03 × 10−4 | 7.18 × 10−6 | SiO2 | 4.48 | 3.86 | ||||
| AlO2− | 1.77 × 10−2 | 1.94 × 10−1 | HSiO3− | 6.29 × 10−3 | 5.77 × 10−2 | ||||
| HAlO2 | 1.40 × 10−2 | 1.43 × 10−2 | H4SiO4 | 14.4 | 12.4 | ||||
| Al(OH)2+ | 2.74 × 10−4 | 2.73 × 10−6 | Sr | 0.0333 | 3.32 × 10−2 | 0.0328 | 3.28 × 10−2 | ||
| Al(OH)3 | 1.22 × 10−2 | 1.24 × 10−2 | Sr2+ | 3.31 × 10−2 | 3.24 × 10−2 | ||||
| Al(OH)4− | 2.42 × 10−2 | 2.65 × 10−1 | SrOH+ | 5.19 × 10−9 | 5.08 × 10−8 | ||||
| Al3+ | 4.63 × 10−6 | 4.73 × 10−9 | SrCO3 | 2.07 × 10−6 | 5.25 × 10−5 | ||||
| Ca | 3.06 | 3.06 | 3.96 | 3.96 | SrHCO3+ | 2.45 × 10−4 | 6.01 × 10−4 | ||
| Ca2+ | 3.04 | 3.91 | SrCl+ | 1.45 × 10−6 | 7.19 × 10−6 | ||||
| CaOH+ | 1.74 × 10−6 | 2.23 × 10−5 | SrF+ | 6.87 × 10−7 | 3.83 × 10−7 | ||||
| CaCO3 | 8.33 × 10−4 | 2.77 × 10−2 | Zn | <0.00001 | <0.00001 | ||||
| Ca(HCO3)+ | 2.50 × 10−2 | 8.05 × 10−2 | Cd | 0.00003 | 2.80 × 10−5 | 0.00003 | 2.88 × 10−5 | ||
| CaHSiO3+ | 7.22 × 10−6 | 7.99 × 10−5 | Cd2+ | 2.78 × 10−5 | 2.79 × 10−5 | ||||
| CaCl+ | 1.62 × 10−4 | 1.06 × 10−3 | CdCl+ | 2.07 × 10−7 | 1.05 × 10−6 | ||||
| CaCl2 | 6.80 × 10−9 | 2.33 × 10−7 | CdO | 1.45 × 10−12 | 1.50 × 10−10 | ||||
| CaF+ | 2.65 × 10−4 | 1.94 × 10−4 | CdOH+ | 8.58 × 10−9 | 8.60 × 10−8 | ||||
| CaSO4 | 2.58 × 10−2 | 3.15 × 10−2 | Ni | <0.00001 | <0.00001 | ||||
| B | 0.0095 | 9.50 × 10−3 | 0.00803 | 8.03 × 10−3 | Pb | 0.00006 | 6.35 × 10−5 | 0.00007 | 7.93 × 10−5 |
| H3BO3 | 5.42 × 10−2 | 4.48 × 10−2 | Pb2+ | 1.57 × 10−5 | 2.51 × 10−6 | ||||
| BO2− | 8.74 × 10−5 | 7.69 × 10−4 | PbOH+ | 5.17 × 10−5 | 8.30 × 10−5 | ||||
| Fe | 0.76 | 7.60 × 10−1 | 0.292 | 2.92 × 10−1 | PbO | 2.36 × 10−9 | 3.91 × 10−8 | ||
| Fe2+ | 7.29 × 10−10 | 3.92 × 10−12 | PbCl+ | 2.83 × 10−8 | 2.31 × 10−8 | ||||
| FeSO4+ | 5.15 × 10−9 | 2.79 × 10−12 | Cu | 0.0003 | 2.86 × 10−4 | 0.0012 | 1.19 × 10−3 | ||
| Fe(OH)3 | 7.03 × 10−2 | 4.16 × 10−2 | Cu+ | 6.73 × 10−16 | 1.95 × 10−14 | ||||
| Fe(OH)4− | 1.77 × 10−3 | 1.11 × 10−2 | Cu2+ | 2.75 × 10−4 | 8.45 × 10−4 | ||||
| FeOH2+ | 3.15 × 10−4 | 1.80 × 10−6 | CuCl+ | 6.19 × 10−8 | 9.70 × 10−7 | ||||
| FeOH+ | 1.51 × 10−12 | 8.11 × 10−14 | CuOH+ | 1.40 × 10−5 | 4.33 × 10−4 | ||||
| FeO+ | 3.94 × 10−1 | 2.25 × 10−2 | CuF+ | 1.50 × 10−7 | 2.65 × 10−7 | ||||
| FeSO4 | 3.32 × 10−12 | 1.69 × 10−14 | CuCl2− | - | 1.09 × 10−15 | ||||
| HFeO2 | 6.61 × 10−1 | 3.91 × 10−1 | HCuO2− | 4.30 × 10−12 | 1.46 × 10−8 | ||||
| FeO2− | 5.77 × 10−4 | 3.63 × 10−3 | P | 3.26 × 10−4 | 3.26 × 10−4 | ||||
| FeCl+ | 4.69 × 10−14 | 1.28 × 10−15 | PO43− | 0.001 | 4.51 × 10−10 | 0.001 | 1.75 × 10−8 | ||
| FeF+ | 3.31 × 10−13 | 1.01 × 10−15 | HPO42− | 2.23 × 10−4 | 7.65 × 10−4 | ||||
| FeF2+ | 1.64 × 10−7 | 5.38 × 10−11 | H2PO4− | 7.96 × 10−4 | 2.48 × 10−4 | ||||
| F− | 2.46 × 10−1 | 1.88 × 10−1 | Co | 0.00007 | 0.00009 | 8.67 × 10−5 | |||
| HF | 7.45 × 10−5 | 5.60 × 10−6 | Co2+ | 9.72 × 10−5 | 8.64 × 10−5 | ||||
| HF2− | 4.33 × 10−10 | 2.48 × 10−11 | CoO | 2.01 × 10−10 | 1.85 × 10−8 | ||||
| K | 0.41 | 4.10 × 10−1 | 0.630 | 6.30 × 10−1 | CoCl+ | 3.38 × 10−8 | 1.53 × 10−7 | ||
| K+ | 1.16 × 10−7 | 6.30 × 10−1 | HCoO2− | 1.86 × 10−16 | 1.82 × 10−13 | ||||
| KCl | 1.16 × 10−7 | 9.34 × 10−7 | CoOH+ | 3.80 × 10−8 | 3.38 × 10−7 | ||||
| KHSO4 | 6.48 × 10−9 | 2.20 × 10−14 | Cl | 2.09 | 11.3 | 11.3 | |||
| KOH | 2.15 × 10−4 | 1.03 × 10−7 | Cl− | 2.09 | 11.3 | ||||
| KSO4− | 4.10 × 10−1 | 3.34 × 10−4 | HCl | 9.59 × 10−8 | 4.87 × 10−8 | ||||
| Mg | 1.57 | 1.57 | 1.92 | 1.92 | Zr | 0.0008 | 7.98 × 10−4 | 0.00029 | 2.95 × 10−4 |
| Mg2+ | 1.56 | 1.90 | HZrO3− | 8.13 × 10−4 | 4.32 × 10−4 | ||||
| MgOH+ | 1.66 × 10−5 | 2.02 × 10−4 | ZrO2 | 3.63 × 10−4 | 1.81 × 10−5 | ||||
| MgCO3 | 2.79 × 10−4 | 8.78 × 10−3 | U | 0.00005 | 4.55 × 10−5 | 0.00003 | 2.87 × 10−5 | ||
| Mg(HCO3)+ | 1.73 × 10−2 | 5.28 × 10−2 | HUO4− | 2.68 × 10−7 | 1.77 × 10−6 | ||||
| MgCl+ | 1.62 × 10−4 | 1.00 × 10−3 | UO22+ | 6.76 × 10−8 | 4.06 × 10−10 | ||||
| MgF+ | 7.88 × 10−4 | 5.46 × 10−4 | UO2OH+ | 1.50 × 10−6 | 9.04 × 10−8 | ||||
| MgSO4 | 2.53 × 10−2 | 2.92 × 10−2 | UO2 | - | - | ||||
| MgHSiO3+ | 9.67 × 10−6 | 1.01 × 10−4 | UO3 | 5.29 × 10−5 | 3.28 × 10−5 | ||||
| Mn | 0.0467 | 4.67 × 10−2 | 0.0161 | 1.61 × 10−2 | Li | 0.00025 | 2.53 × 10−4 | 0.00037 | |
| Mn2+ | 4.66 × 10−2 | 1.61 × 10−2 | Li+ | 2.53 × 10−4 | 3.68 × 10−4 | ||||
| MnOH+ | 4.51 × 10−6 | 1.56 × 10−5 | LiOH | 6.23 × 10−11 | 9.38 × 10−10 | ||||
| MnO | 3.50 × 10−11 | 1.25 × 10−9 | Ce | 0.00023 | 2.26 × 10−4 | 0.00005 | 4.67 × 10−5 | ||
| HMnO2− | - | 1.46 × 10−14 | Ce3+ | 1.75 × 10−4 | 3.54 × 10−5 | ||||
| MnSO4 | 1.97 × 10−4 | 6.45 × 10−5 | CeF2+ | 3.58 × 10−5 | 4.03 × 10−6 | ||||
| MnF+ | 5.98 × 10−6 | 1.18 × 10−6 | CeF2+ | 6.06 × 10−7 | 3.89 × 10−8 | ||||
| MnCl+ | 2.85 × 10−6 | 4.99 × 10−6 | CeF3 | 1.74 × 10−9 | 6.58 × 10−11 | ||||
| CO32− | 3.56 × 10−3 | 1.04 × 10−1 | CeF4− | 2.43 × 10−12 | 5.57 × 10−14 | ||||
| HCO3− | 15.7 | 18.2 | 49.12 | CeHCO32+ | 5.74 × 10−6 | 2.83 × 10−6 | |||
| HCO3− | 4.87 × 10−1 | CeO2H | 3.11 × 10−11 | 6.33 × 10−9 | |||||
| HCO2− | - | CeSO4+ | 2.24 × 10−5 | 4.07 × 10−6 | |||||
| SO42− | 2.03 | 1.99 | 2.19 | 2.14 | CeOH2+ | 1.91 × 10−6 | 3.77 × 10−6 | ||
| HNO3 | 5.55 × 10−12 | 5.49 × 10−11 | La | 0.00015 | 1.54 × 10−4 | 0.00006 | |||
| NO3− | 0.001 | 7.71 × 10−4 | 0.08 | 8.14 × 10−2 | La3+ | 7.65 × 10−5 | 2.77 × 10−6 | ||
| Na+ | 2.62 | 2.62 | 18.64 | 18.6 | LaCO3+ | 9.06 × 10−5 | 7.99 × 10−5 | ||
| NaOH | 9.06 × 10−8 | 6.65 × 10−6 | LaF2+ | 6.60 × 10−6 | 1.32 × 10−7 | ||||
| NaAlO2 | 4.72 × 10−7 | 3.55 × 10−5 | LaO+ | 1.44 × 10−9 | |||||
| NaCl | 6.30 × 10−5 | 2.35 × 10−3 | LaO2H | 2.25 × 10−13 | 8.17 × 10−12 | ||||
| NaF | 5.89 × 10−6 | 2.46 × 10−5 | LaOH2+ | 5.05 × 10−7 | 1.78 × 10−7 | ||||
| NaSO4− | 1.35 × 10−3 | 9.65 × 10−3 | LaSO4+ | 9.89 × 10−6 | 3.20 × 10−7 | ||||
| NaHSiO3 | 7.28 × 10−5 | 4.60 × 10−3 | LaF2+ | 6.68 × 10−8 | |||||
| O2 | 6.10 | 7.74 | Ag | 0.0013 | 1.34 × 10−3 | 0.0014 | |||
| CO2 | 6.61 | 1.66 | Ag+ | 5.54 × 10−8 | 1.43 × 10−9 | ||||
| V | 0.0007 | 7.37 × 10−4 | 0.0007 | 7.39 × 10−4 | AgNO3 | 6.29 × 10−4 | 1.66 × 10−3 | ||
| VO2+ | 2.40 × 10−8 | Ag(HS)2− | - | - | |||||
| VO43− | 3.39 × 10−10 | 8.46 × 10−13 | |||||||
| HVO42− | 1.61 × 10−3 | 1.68 × 10−3 | |||||||
| H3VO4 | 6.68 × 10−5 | 6.55 × 10−7 | |||||||
| Concentration, mg/L | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Al(OH)2+ | AlO2− | HAlO2 | Al(OH)2+ | Al(OH)3 | Al(OH)4− | Al3+ | Cl− | Eh, V | pH |
| 5.22 × 103 5.22 × 10³ | 9.64 × 102 9.64 × 102 | 8.30 × 103 8.30 × 10³ | 1.42 × 104 1.42 × 104 | 3.96 × 103 3.96 × 10³ | 4.50 × 103 4.50 × 10³ | 9.47 × 103 9.47 × 10³ | 6.10× 104 6.10 × 104 | −0.216 | 6.07 |
| 10% Solution, L | Al(OH)2F | HAlO2 | Al(OH)2+ | Al(OH)2+ | Al(OH)3 | AlSO4+ | Al3+ | HCO3− |
|---|---|---|---|---|---|---|---|---|
| 0.0001 | 4.73 × 10−5 | 4.55 × 10−6 | 4.70 × 10−7 | 2.13 × 10−6 | 3.26 × 10−6 | 5.47 × 10−11 | 1.85 × 10−8 | 17.7 |
| 0.0032 | 5.01 × 10−5 | 4.57 × 10−6 | 5.25 × 10−7 | 2.26 × 10−6 | 3.28 × 10−6 | 6.45 × 10−11 | 2.18 × 10−8 | 17.4 |
| 0.0100 | 5.75 × 10−5 | 4.67 × 10−6 | 6.77 × 10−7 | 2.59 × 10−6 | 3.35 × 10−6 | 9.35 × 10−11 | 3.16 × 10−8 | 16.6 |
| 0.0316 | 8.65 × 10−5 | 4.92 × 10−6 | 1.46 × 10−6 | 3.90 × 10−6 | 3.53 × 10−6 | 2.87 × 10−10 | 9.70 × 10−8 | 14.2 |
| 0.1000 | 3.96 × 10−4 | 6.55 × 10−6 | 2.38 × 10−5 | 1.81 × 10−5 | 4.70 × 10−6 | 1.56 × 10−8 | 5.67 × 10−6 | 6.42 |
| 0.3162 | 6.42 × 10−2 | 3.02 × 10−5 | 1.82 × 10−1 | 3.34 × 10−3 | 2.17 × 10−5 | 4.17 × 10−3 | 1.86 | 0.213 |
| 1.0000 | 9.94 × 10−2 | 3.02 × 10−5 | 5.93 × 10−1 | 5.84 × 10−3 | 2.17 × 10−5 | 1.84 × 10−2 | 12.0 | 0.130 |
| 5% Solution Na2CO3, L | Eh | pH | Na+, mg/L | Cl−, mg/L | HCO3−, mg/L |
|---|---|---|---|---|---|
| 0 | 0.997 | 4.03 | 2.62 | 16.5 | 9.23 × 10−2 |
| 1 | 0.857 | 6.58 | 14.0 | 16.5 | 23.1 |
| 2 | 0.804 | 7.54 | 25.3 | 16.5 | 53.0 |
| Element | Natural Water (Virma) | Drinking Water (Diner) | Adequacy [23] | Maximum Allowable Concentrations, SanPIN2.1.4.1116-02 [24] | |
|---|---|---|---|---|---|
| Grade 1 | Top Grade | ||||
| Na | 1.88 | 19.87 | 2–20 | 200 | 20 |
| Mg | 1.28 | 1.31 | 5–65 | 65 | 5–50 |
| Al | 0.057 | 0.29 | - | 0.2 | 0.1 |
| Si | 5.45 | 5.31 | - | 1.02 | 4.83 |
| P | 0.018 | 0.001 | - | - | - |
| K | 0.31 | 0.49 | 2–20 | 20 | 2–20 |
| Ca | 1.54 | 3.07 | 25–130 | 130 | 25–80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazukhina, S.; Drogobuzhskaya, S.; Sandimirov, S.; Masloboev, V. Effect of Water Treatment on the Chemical Composition of Drinking Water: A Case of Lovozero, Murmansk Region, Russia. Sustainability 2022, 14, 16996. https://doi.org/10.3390/su142416996
Mazukhina S, Drogobuzhskaya S, Sandimirov S, Masloboev V. Effect of Water Treatment on the Chemical Composition of Drinking Water: A Case of Lovozero, Murmansk Region, Russia. Sustainability. 2022; 14(24):16996. https://doi.org/10.3390/su142416996
Chicago/Turabian StyleMazukhina, Svetlana, Svetlana Drogobuzhskaya, Sergey Sandimirov, and Vladimir Masloboev. 2022. "Effect of Water Treatment on the Chemical Composition of Drinking Water: A Case of Lovozero, Murmansk Region, Russia" Sustainability 14, no. 24: 16996. https://doi.org/10.3390/su142416996
APA StyleMazukhina, S., Drogobuzhskaya, S., Sandimirov, S., & Masloboev, V. (2022). Effect of Water Treatment on the Chemical Composition of Drinking Water: A Case of Lovozero, Murmansk Region, Russia. Sustainability, 14(24), 16996. https://doi.org/10.3390/su142416996





