Sustainable Environmental-Based ZnO Nanoparticles Derived from Pisonia grandis for Future Biological and Environmental Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical
2.2. Extraction of Plant Components
2.3. Synthesis of ZnO Nanoparticles
2.4. Characterization of Fabricated ZnO Nanoparticles
2.5. Biological Efficiencies
2.5.1. Evaluation of Micro-Morbidity
2.5.2. Evaluation of Cytotoxicity
2.5.3. Evaluation of Photo-Degradation
3. Results and Discussion
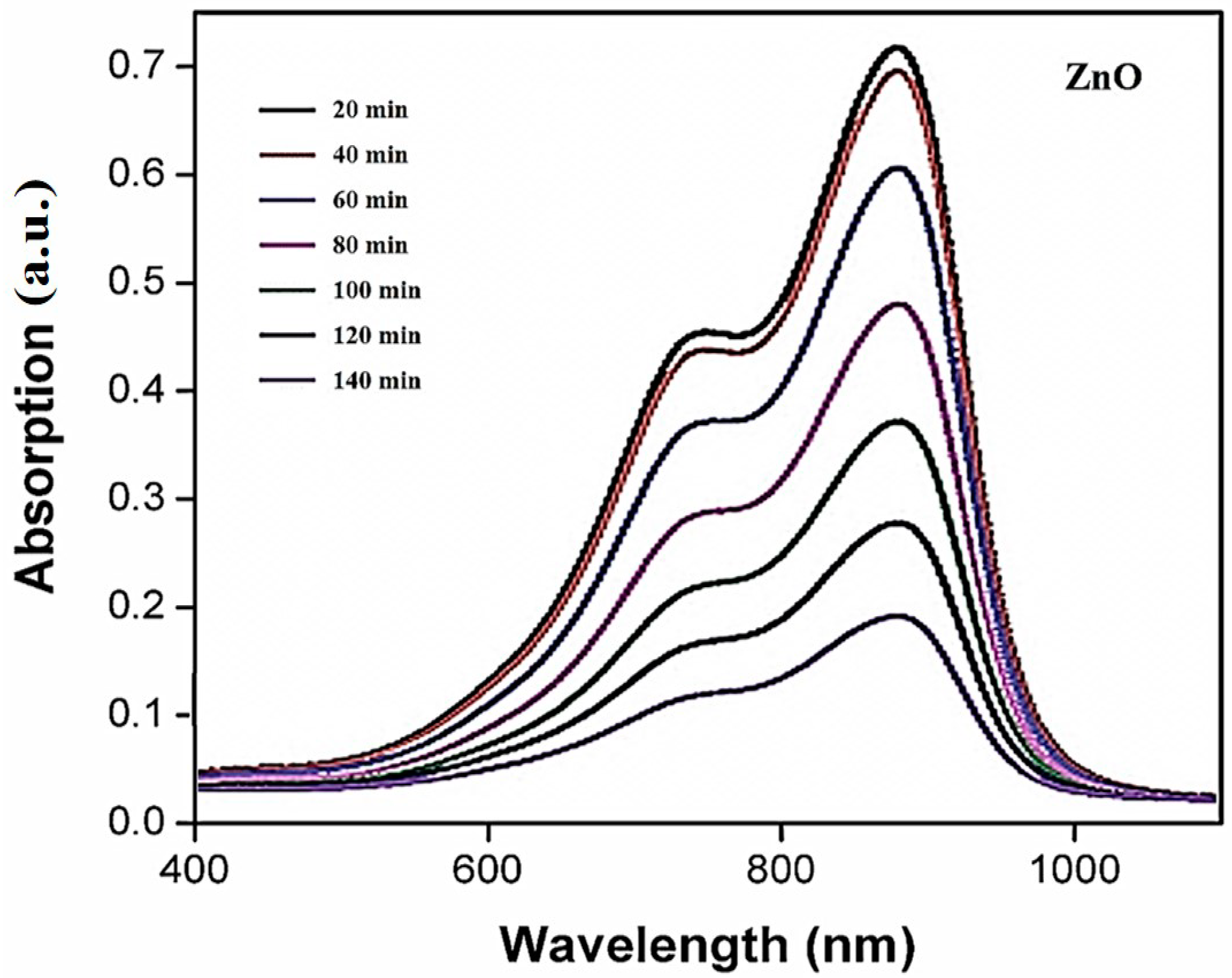
3.1. UV Vis Spectrum
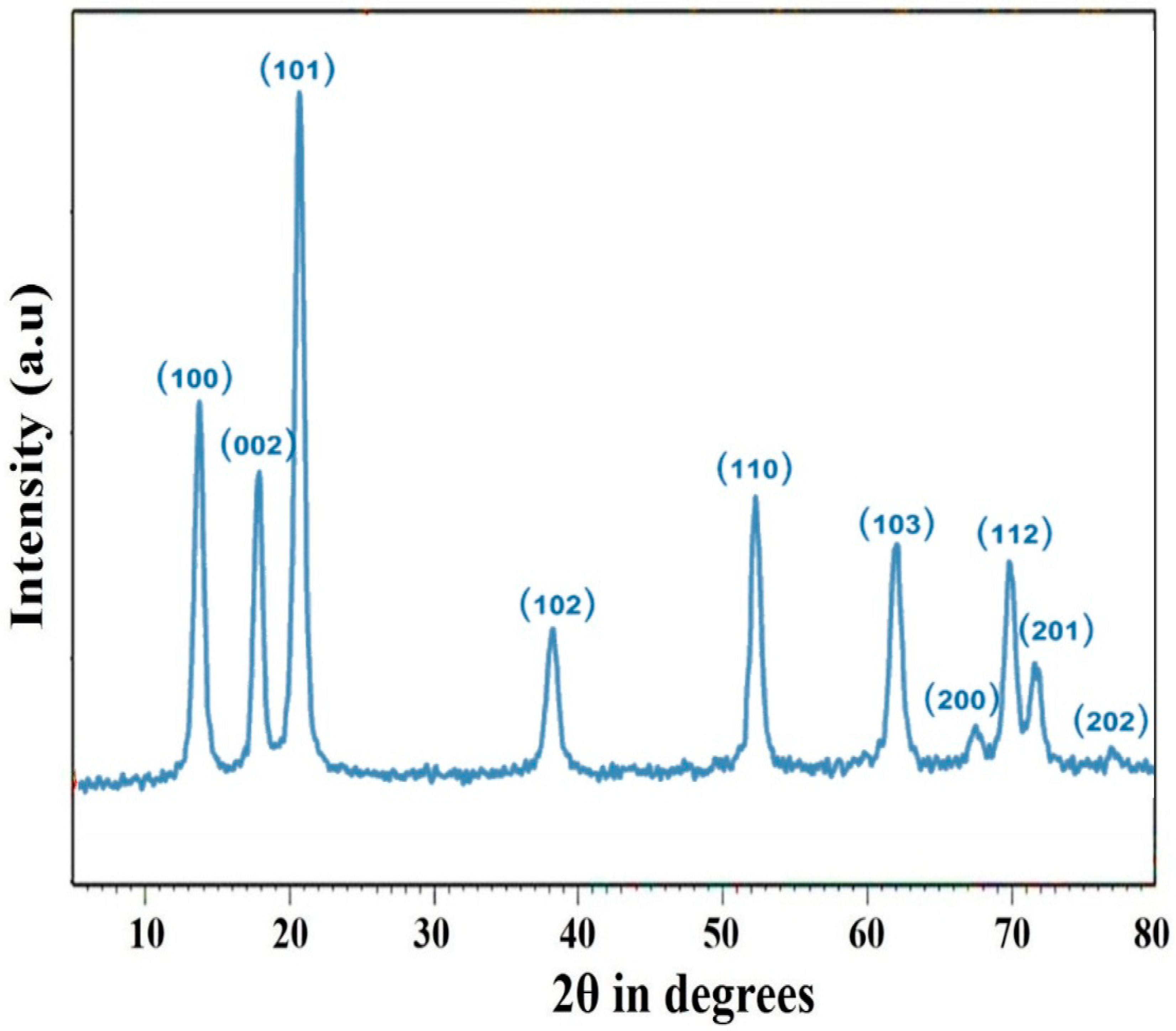
3.2. XRD
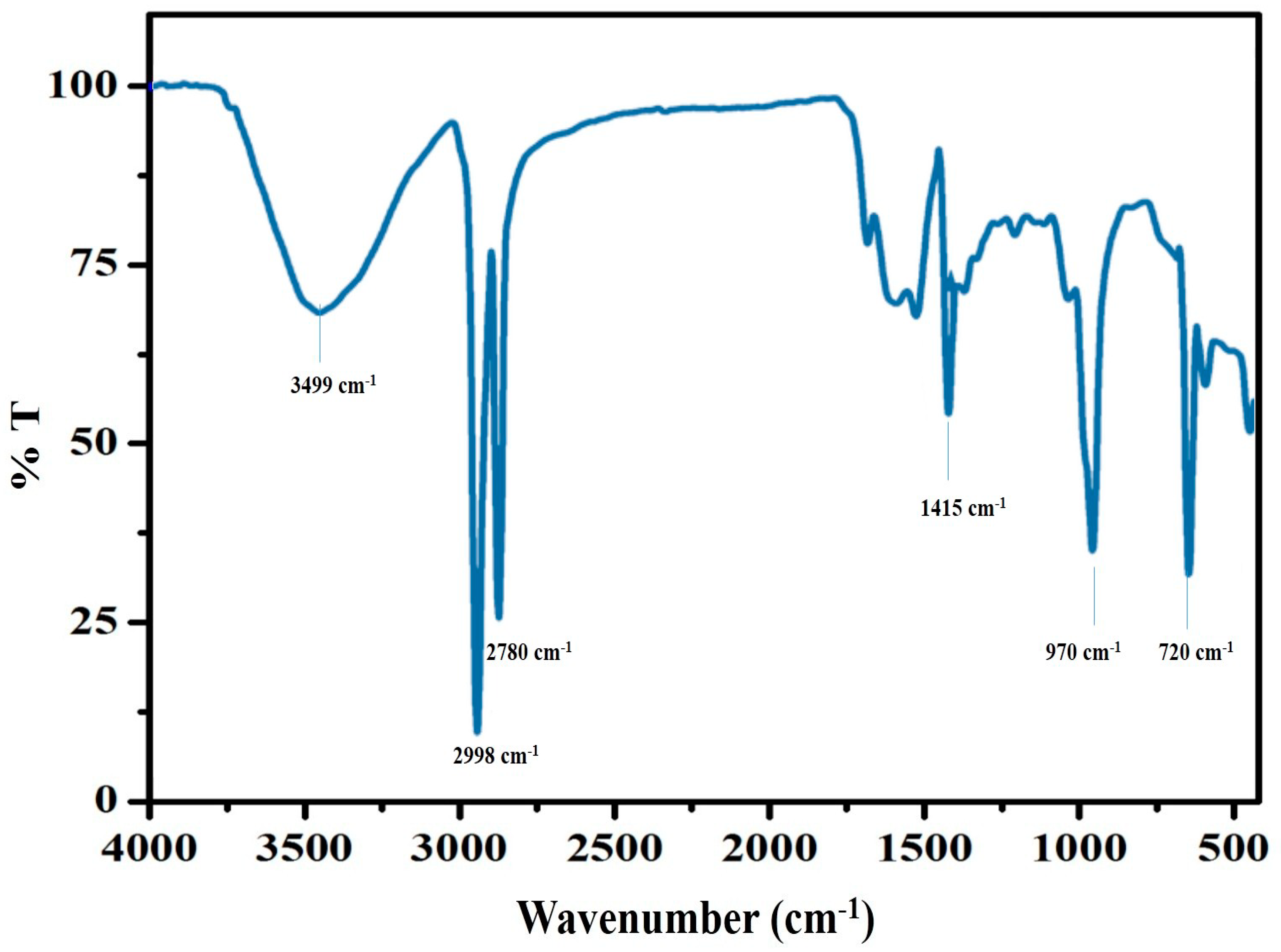
3.3. FT-IR Analysis
3.4. FE-SEM with EDX
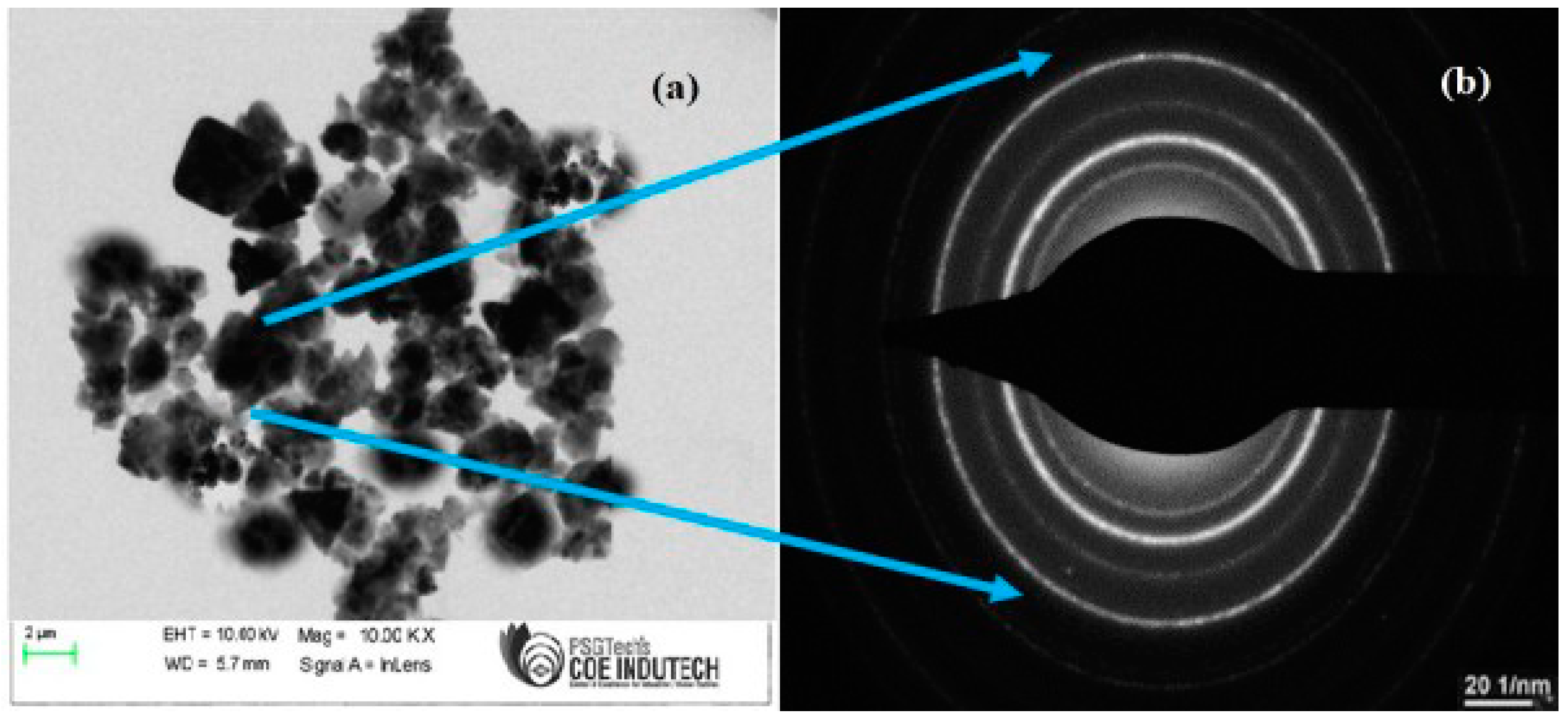
3.5. TEM Analysis
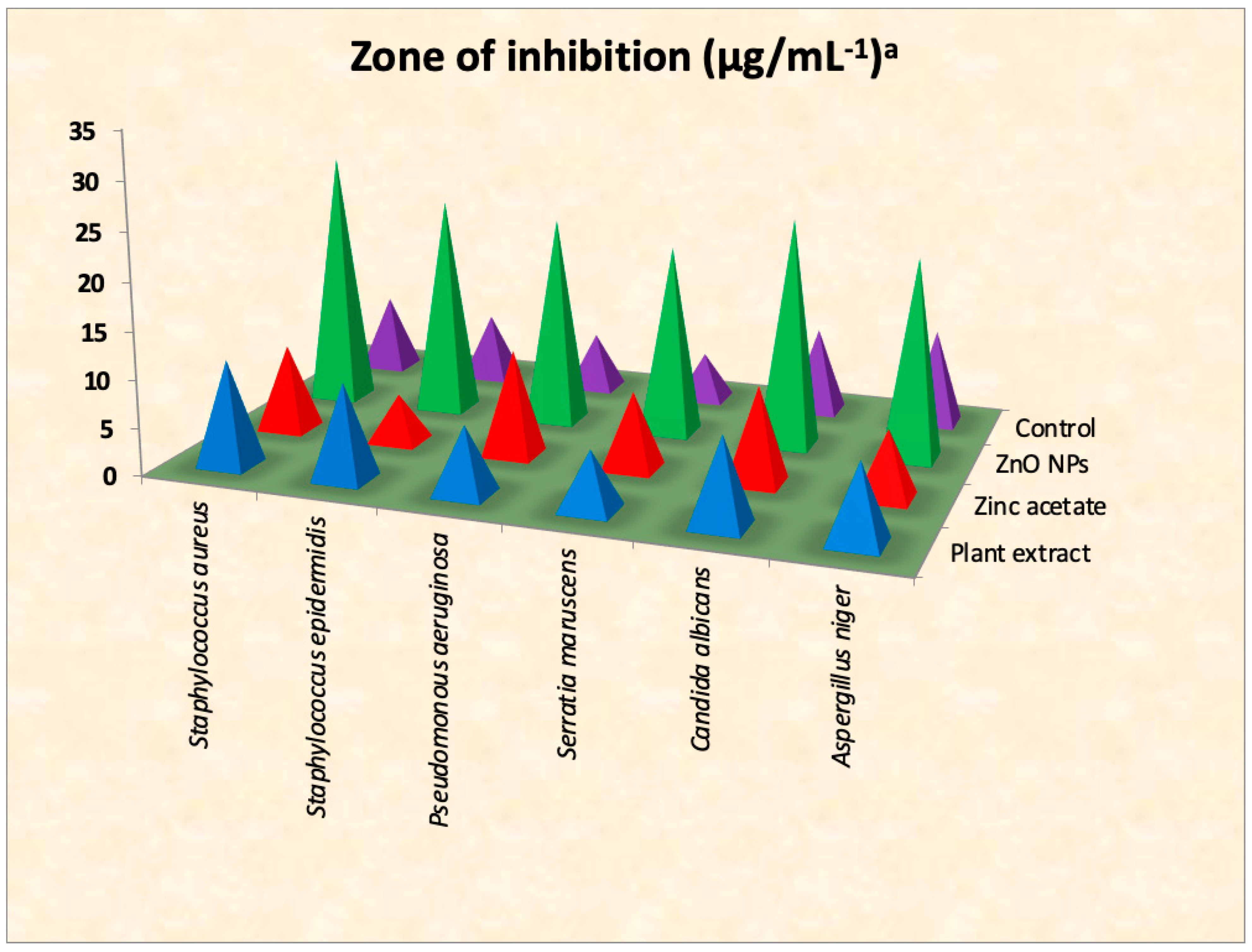
3.6. Antimicrobial Efficacies
3.7. Cytotoxicity Efficiencies
3.8. Photodegrataion Efficiencies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramsden, J. Nanotechnology: An Introduction; William Andrew is an imprint of Elsevier: New York, NY, USA, 2016. [Google Scholar]
- Shah, F.; Hasnain, J.; Sajjad, A.S.; Sumaira, S.; Adnan, K.; Muhammad, T.A.; Muhammad, R.; Faheem, J.; Wajidullah; Noreen, A.; et al. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Aqueous Fruit Extracts of Myristica fragrans: Their Characterizations and Biological and Environmental Applications. ACS Omega 2021, 6, 9709–9722. [Google Scholar] [CrossRef]
- Mihir, H.; Siddhivinayak, B.; Kumar, R. Plant-Mediated Green Synthesis of Iron Nanoparticles. J. Nanopart. 2014, 9, 140614. [Google Scholar] [CrossRef]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” Nanotechnologies: Synthesis of Metal Nanoparticles Using Plants. Acta Nat. 2014, 6, 35–44. [Google Scholar] [CrossRef]
- Minha, N.; Usman, A.; Bushra, K.; Bin, C. Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 2020, 10, 9055. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Nazmi, S.; Yusuf, Z.; Fuat, B. Green synthesized ZnO nanoparticles using Ganoderma lucidum: Characterization and invitro nano-fertilizer effects. J. Alloys Compd. 2022, 918, 165695. [Google Scholar] [CrossRef]
- John Sushma, N.; Mahitha, B.; Mallikarjuna, K.; Deva Prasad, R.B. Bio-inspired ZnO nanoparticles from Ocimum tenuiflorum and their in vitro antioxidant activity. Appl. Phys. 2016, 122, 544. [Google Scholar] [CrossRef]
- Vembu, S.; Vijayakumar, S.; Nilavukkarasi, M.; Vidhya, E.; Punitha, V.N. Phytosynthesis of TiO2 nanoparticles in diverse applications: What is the exact mechanism of action? Sens. Int. 2022, 3, 100161. [Google Scholar] [CrossRef]
- Aravind, M.; Amalanathan, M.; Sony Michael Mary, M. Synthesis of TiO2 nanoparticles by chemical and green synthesis methods and their multifaceted properties. SN Appl. Sci. 2021, 3, 409. [Google Scholar] [CrossRef]
- Vidhya, E.; Vijayakumar, S.; Prathipkumar, S.; Praseetha, P.K. Green way biosynthesis: Characterization, antimicrobial and anticancer activity of ZnO nanoparticles. Gene Rep. 2020, 20, 100688. [Google Scholar] [CrossRef]
- Sasani Ghamsari, M.; Alamdari, S.; Han, W.; Park, H. Impact of nanostructured thin ZnO film in ultraviolet protection. Int. J. Nanomed. 2017, 12, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Ashwini, J.; Aswathy, T.R.; Achuthsankar, S.N. Green synthesis and characterization of zinc oxide nanoparticles using Cayratia pedata leaf extract. Biochem. Biophys. Rep. 2021, 26, 100995. [Google Scholar] [CrossRef]
- Jayachandran, A.; Thankamani, R.A.; Anil, B.R.; Gautham, M.T.; Achuthsankar, S.N. Synthesis and Characterization of Zinc Oxide Nanoparticles Using Acacia caesia Bark Extract and Its Photocatalytic and Antimicrobial Activities. Catalysts 2021, 11, 1507. [Google Scholar] [CrossRef]
- Udayachandran Thampy, U.S.; Rama Krishna, C.; Venkata Reddy, C.; Babu, B.; Reddy, Y.P.; Rao, P.S.; Ravikumar, R.V.S.S.N. Spectral Investigations on Cu2+-Doped ZnO Nanopowders. Appl. Magn. Reson. 2011, 41, 69. [Google Scholar] [CrossRef]
- Abdul Mathin, S.K.; David Raju, M.; Rama Sekhara Reddy, D.; Jayadev, D.J. Synthesis of Zinc Oxide Nano-particles using Anthocephalus Cadamba Plant Extracts and Explores its Anti-Oxidant, Anti-Inflammatory and Anti-Diabetics Activity. Int. J. Mod. Trends Sci. Technol. 2021, 7, 162–169. [Google Scholar]
- Sarangi, S.N.; Nozaki, S.; Sahu, S.N. ZnO Nanorod-Based Non-Enzymatic Optical Glucose Biosensor. J. Biomed. Nanotechnol. 2015, 11, 988–996. [Google Scholar] [CrossRef]
- Lei, Z.; Yang, H.; Xue, W.; Ao, Z.; Xianli, G.; Abu El-Gasim, A.Y.; Haile, M.; Cunshan, Z. Ultrasound-Assisted Synthesis of Potentially Food-GradeNano-Zinc Oxide in Ionic Liquids: A Safe, Green, Efficient Approach and Its Acoustics Mechanism. Foods 2022, 11, 1656. [Google Scholar] [CrossRef]
- Malte, B.; Frank, G.; Annette, T.; Robert, S. Minerals as Model Compounds for Cu/ZnO Catalyst Precursors: Structural and Thermal Properties and IR Spectra of Mineral and Synthetic (Zincian) Malachite, Rosasite and Aurichalcite and a Catalyst Precursor Mixture. Eur. J. Inorg. Chem. 2009, 2009, 1347–1357. [Google Scholar] [CrossRef]
- Al-Fandi, M.; Oweis, R.; Albiss, B.A.; AlZoubi, T.; Al-Akhras, M.-A.; Qutaish, H.; Khwailah, H.; Al-Hattami, S.; Al-Shawwa, E. A prototype Ultraviolet Light Sensor based on ZnO Nanoparticles/Graphene Oxide Nanocomposite Using Low Temperature Hydrothermal Method. IOP Conf. Ser. Mater. Sci. Eng. 2015, 92, 012009. [Google Scholar] [CrossRef]
- Pillai, A.M.; Sivasankarapillai, V.S.; Rahdar, A.; Joseph, J.; Sadeghfar, F.; Anuf, A.R.; Rajesh, K.; Kyzas, G.Z. Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. J. Mol. Struct. 2020, 1211, 128107. [Google Scholar] [CrossRef]
- Keerthana, P.; Vijayakumar, S.; Vidhya, E.; Punitha, V.N.; Nilavukkarasi, M.; Praseetha, P.K. Biogenesis of ZnO nanoparticles for revolutionizing agriculture: A step towards anti -infection and growth promotion in plants. Ind. Crops Prod. 2021, 170, 113762. [Google Scholar] [CrossRef]
- Ahmed, S.; Abdelbaky, T.A.; Abd El-Mageed, T.A.; Ahmad, O.B.; Samy, S.; Mohamed, A.M.H.A. Green Synthesis and Characterization of ZnO Nanoparticles Using Pelargonium odoratissimum (L.) Aqueous Leaf Extract and Their Antioxidant, Antibacterial and Anti-inflammatory Activities. Antioxidants 2022, 11, 1444. [Google Scholar] [CrossRef]
- Sekar, V.; Baskaralingam, V.; Balasubramanian, M.; Malaikkarasu, S. Laurus nobilis leaf extract mediated green synthesis of ZnO nanoparticles: Characterization and biomedical applications. Biomed. Pharm. 2016, 84, 1213–1222. [Google Scholar] [CrossRef]
- Happy, A.; Soumya, M.; Venkat Kumar, S.; Rajeshkumar, S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem. Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef]
- Rexlin, J.; Vijayakumar, S.; Nilavukkarasi, M.; Vidhya, E.; Nahed, S.A.; Maryium, S.; Punitha, V.N.; Praseetha, P.K. Bioengineered ZnO nanoparticles as a nano priming agent in Cyamopsis tetragonoloba (L).Taub. to improve yield and disease resistance. Appl. Nanosci. 2022. 123, 1–9. [CrossRef]
- Rizzato, C.; Torres, J.; Kasamatsu, E.; Camorlinga-Ponce, M.; Bravo, M.M.; Canzian, F.; Kato, I. Potential role of biofilm formation in the development of digestive tract cancer with special reference to Helicobacter pylori infection. Front. Microbiol. 2019, 10, 846. [Google Scholar] [CrossRef]
- Farina, M.; Preeti, B.; Neelam, P. Phytochemical Evaluation, Antimicrobial Activity, and Determination of Bioactive Components from Leaves of Aegle Marmelos. Biomed. Res. Int. 2014, 2014, 497606. [Google Scholar] [CrossRef]
- Mustapha, F.H.; Jail, A.A.; Mohamed, M.; Triwahyono, S.; Hassan, N.S.; Khusnun, N.F.; Hitam, C.N.C.; Rahman, A.F.A.; Firmanshah, L.; Zolkifil, A.S. New insight into self-modified surfaces with defect-rich rutile TiO2 as a visible-light-driven photocatalyst. J. Clean Prod. 2017, 168, 1150–1162. [Google Scholar] [CrossRef]
- Mohammad Sajadi, S.; Kamal, K.; Samir, M.H.; Sarbast, A.M.; Azeez, A.B.; Sarbast, M.H. Green Synthesis of the Ag/Bentonite Nanocomposite Using Euphorbia larica Extract: A Reusable Catalyst for Efficient Reduction of Nitro Compounds and Organic Dyes. Chem. Sel. 2018, 3, 12274–12280. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutharappa Kaliyamoorthy, T.; Subramaniyan, V.; Renganathan, S.; Elavarasan, V.; Ravi, J.; Prabhakaran Kala, P.; Subramaniyan, P.; Vijayakumar, S. Sustainable Environmental-Based ZnO Nanoparticles Derived from Pisonia grandis for Future Biological and Environmental Applications. Sustainability 2022, 14, 17009. https://doi.org/10.3390/su142417009
Sutharappa Kaliyamoorthy T, Subramaniyan V, Renganathan S, Elavarasan V, Ravi J, Prabhakaran Kala P, Subramaniyan P, Vijayakumar S. Sustainable Environmental-Based ZnO Nanoparticles Derived from Pisonia grandis for Future Biological and Environmental Applications. Sustainability. 2022; 14(24):17009. https://doi.org/10.3390/su142417009
Chicago/Turabian StyleSutharappa Kaliyamoorthy, Thiyakarajan, Vijayakumar Subramaniyan, Sangeetha Renganathan, Vidhya Elavarasan, Jagatheesvaran Ravi, Praseetha Prabhakaran Kala, Prathipkumar Subramaniyan, and Sekar Vijayakumar. 2022. "Sustainable Environmental-Based ZnO Nanoparticles Derived from Pisonia grandis for Future Biological and Environmental Applications" Sustainability 14, no. 24: 17009. https://doi.org/10.3390/su142417009
APA StyleSutharappa Kaliyamoorthy, T., Subramaniyan, V., Renganathan, S., Elavarasan, V., Ravi, J., Prabhakaran Kala, P., Subramaniyan, P., & Vijayakumar, S. (2022). Sustainable Environmental-Based ZnO Nanoparticles Derived from Pisonia grandis for Future Biological and Environmental Applications. Sustainability, 14(24), 17009. https://doi.org/10.3390/su142417009








