De-hydrogenation/Rehydrogenation Properties and Reaction Mechanism of AmZn(NH2)n-2nLiH Systems (A = Li, K, Na, and Rb)
Abstract
:1. Introduction
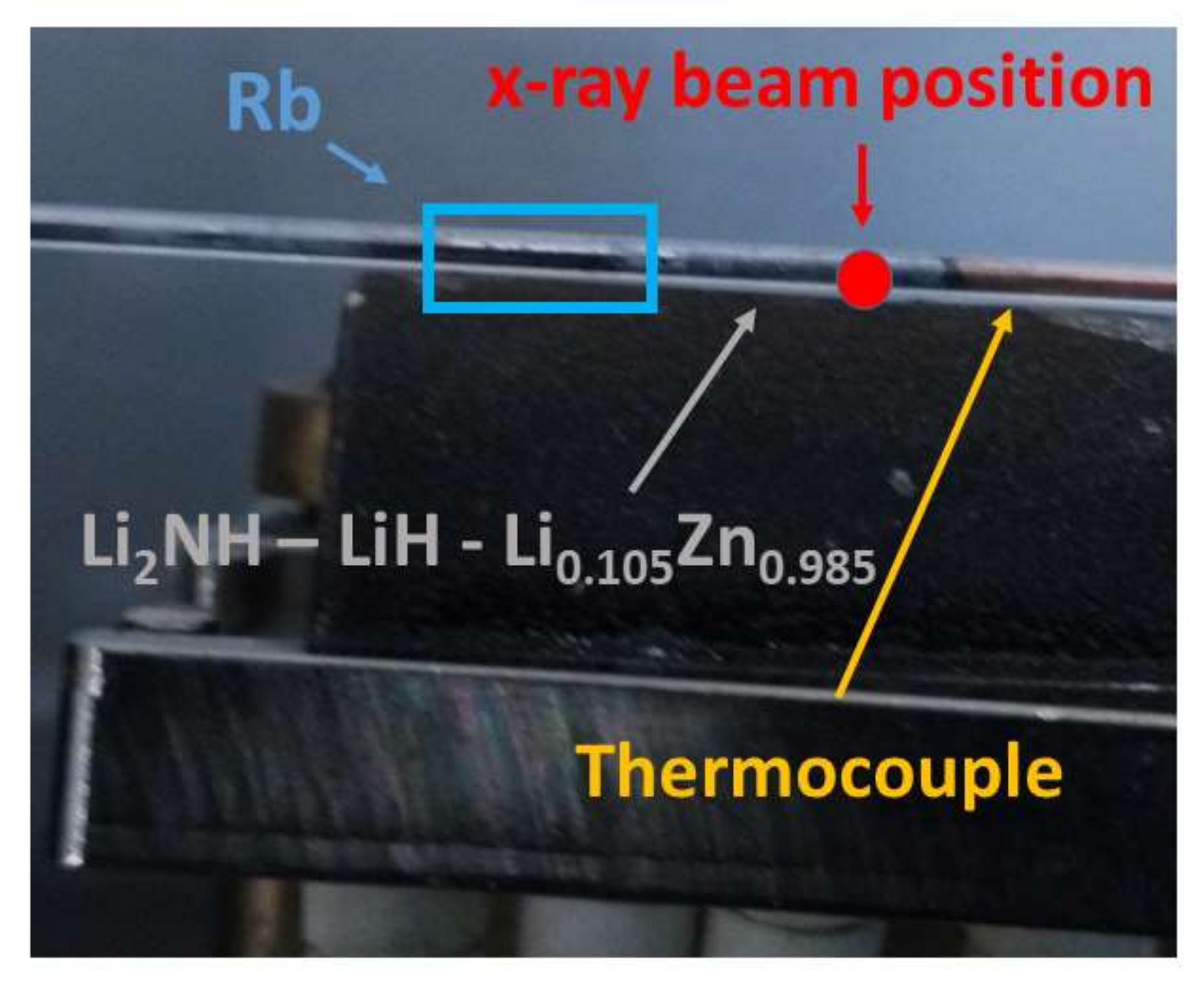
2. Experimental Details
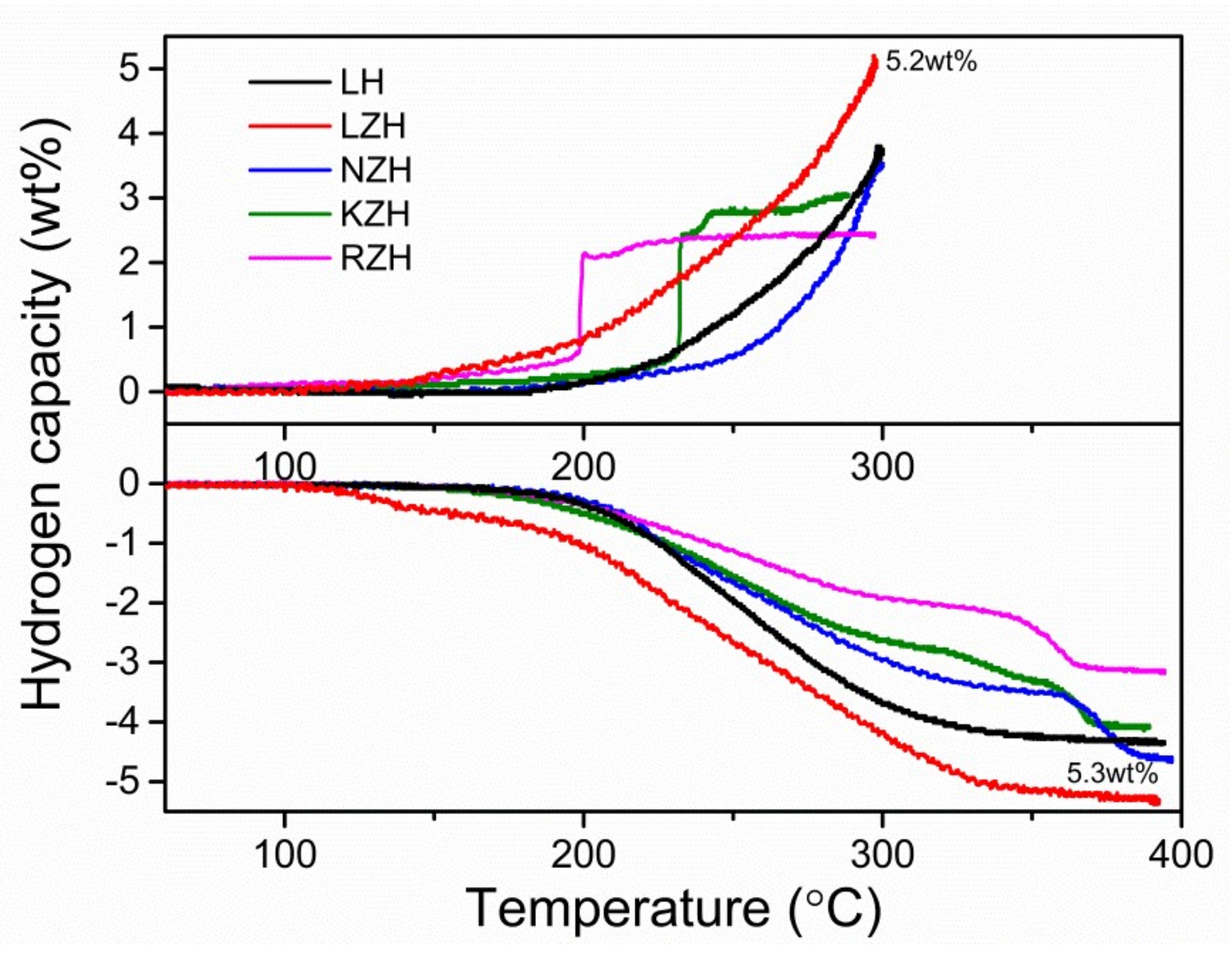
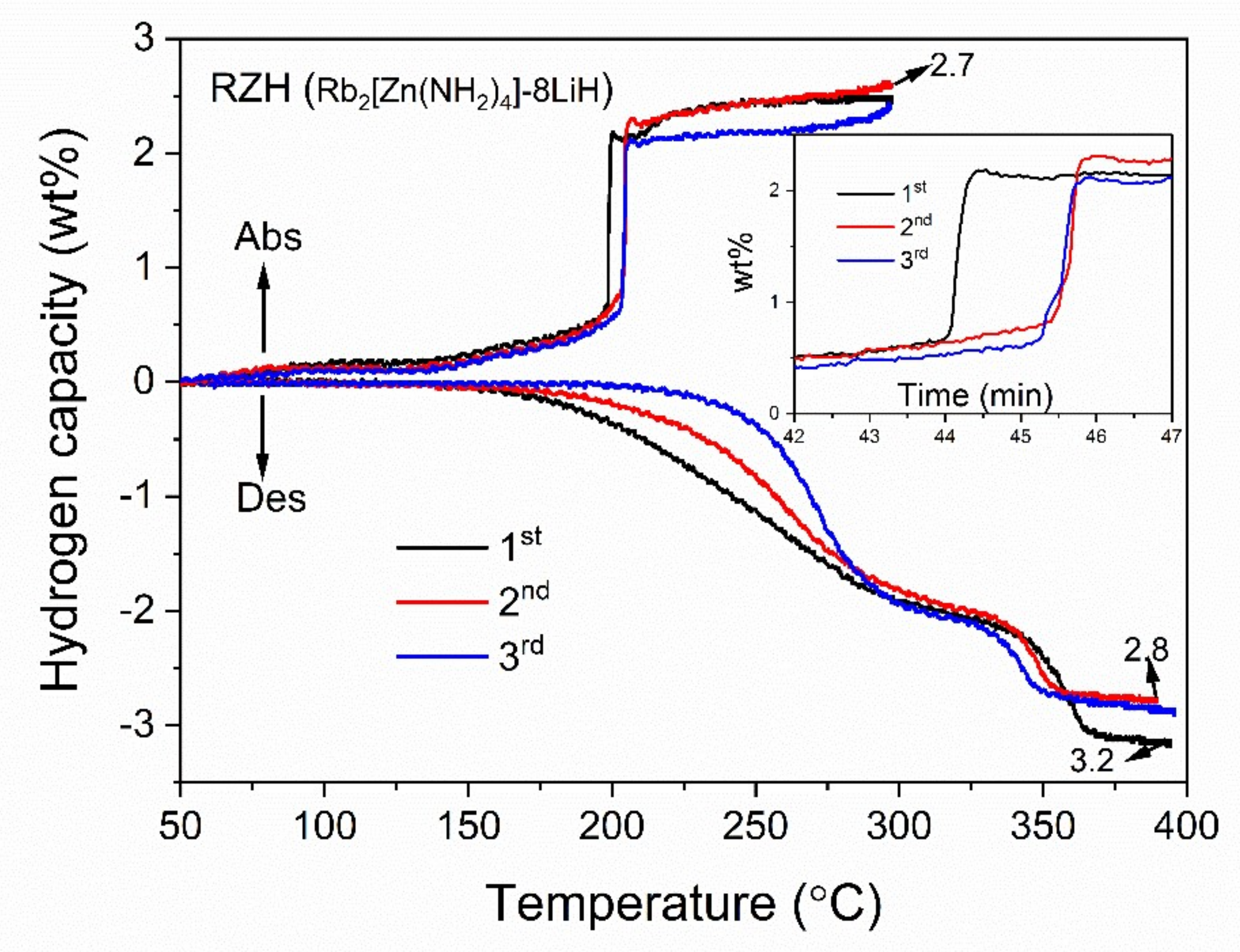
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Schlapbach, L.; Zuttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Pistidda, C. Solid-State Hydrogen Storage for a Decarbonized Society. Hydrogen 2021, 2, 428–443. [Google Scholar] [CrossRef]
- Gautam, Y.K.; Chawla, A.K.; Khan, S.A.; Agrawal, R.; Chandra, R. Hydrogen absorption and optical properties of Pd/Mg thin films prepared by DC magnetron sputtering. Int. J. Hydrogen Energy 2012, 37, 3772–3778. [Google Scholar] [CrossRef]
- Gautam, Y.K.; Chawla, A.K.; Walia, R.; Agrawal, R.; Chandra, R. Hydrogenation of Pd-capped Mg thin films prepared by DC magnetron sputtering. Appl. Surf. Sci. 2011, 257, 6291–6295. [Google Scholar] [CrossRef]
- Gautam, Y.K.; Kumar, M.; Chandra, R. Hydrogen absorption and desorption properties of Pd/Mg/Pd tri-layers prepared by magnetron sputtering. Surf. Coatings Technol. 2013, 237, 450–455. [Google Scholar] [CrossRef]
- Shang, Y.; Pistidda, C.; Gizer, G.; Klassen, T.; Dornheim, M. Mg-based materials for hydrogen storage. J. Magnes. Alloy. 2021. [Google Scholar] [CrossRef]
- He, T.; Cao, H.; Chen, P. Complex Hydrides for Energy Storage, Conversion, and Utilization. Adv. Mater. 2019, 31, e1902757. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Pachfule, P.; Wu, H.; Xu, Q.; Chen, P. Hydrogen carriers. Nat. Rev. Materials. 2016, 1, 1–17. [Google Scholar]
- Milanese, C.; Jensen, T.R.; Hauback, B.; Pistidda, C.; Dornheim, M.; Yang, H.; Lombardo, L.; Zuettel, A.; Filinchuk, Y.; Ngene, P.; et al. Complex hydrides for energy storage. Int. J. Hydrogen Energy 2019, 44, 7860–7874. [Google Scholar] [CrossRef] [Green Version]
- Orimo, S.I.; Nakamori, Y.; Eliseo, J.R.; Zuttel, A.; Jensen, C.M. Complex hydrides for hydrogen storage. Chem. Reviews. 2007, 107, 4111–4132. [Google Scholar] [CrossRef]
- Eghbali, P.; Nişancı, B.; Metin, Ö. Graphene hydrogel supported palladium nanoparticles as an efficient and reusable heterogeneous catalysts in the transfer hydrogenation of nitroarenes using ammonia borane as a hydrogen source. Pure Appl. Chem. 2018, 90, 327–335. [Google Scholar] [CrossRef]
- Eghbali, P.; Gürbüz, M.U.; Ertürk, A.S.; Metin, Ö. In situ synthesis of dendrimer-encapsulated palladium(0) nanoparticles as catalysts for hydrogen production from the methanolysis of ammonia borane. Int. J. Hydrogen Energy 2020, 45, 26274–26285. [Google Scholar] [CrossRef]
- Chen, P.; Xiong, Z.; Luo, J.; Lin, J.; Tan, K.L. Interaction of hydrogen with metal nitrides and imides. Nature 2002, 420, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Baricco, M.; Bang, M.; Fichtner, M.; Hauback, B.; Linder, M.; Luetto, C.; Moretto, P.; Sgroi, M. SSH2S: Hydrogen storage in complex hydrides for an auxiliary power unit based on high temperature proton exchange membrane fuel cells. J. Power Sources 2017, 342, 853–860. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.R.; Zhang, W.J.; Cao, H.J.; Chen, P. Mild-condition synthesis of A(2)ZnH(4) (A = K, Rb, Cs) and their effects on the hydrogen storage properties of 2LiH-Mg(NH2)(2). J. Energy Chemistry. 2020, 50, 358–364. [Google Scholar] [CrossRef]
- Garroni, S.; Santoru, A.; Cao, H.; Dornheim, M.; Klassen, T.; Milanese, C.; Gennari, F.; Pistidda, C. Recent Progress and New Perspectives on Metal Amide and Imide Systems for Solid-State Hydrogen Storage. Energies 2018, 11, 1027. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Fichtner, M.; Baricco, M. Preparation of Li-Mg-N-H hydrogen storage materials for an auxiliary power unit. Int. J. Hydrogen Energy 2017, 42, 17144–17148. [Google Scholar] [CrossRef]
- Hu, J.; Wu, G.; Liu, Y.; Xiong, A.Z.; Chen, P.; Murata, K.; Sakata, K.; Wolf, G. Hydrogen Release from Mg(NH2)2−MgH2 through Mechanochemical Reaction. J. Phys. Chem. B 2006, 110, 14688–14692. [Google Scholar] [CrossRef]
- Li, C.; Li, C.; Fan, M.; Chen, H.; Shu, K.; Zhang, Y.; Gao, M.; Liu, Y.; Pan, H. Synthesis of a ternary amide LixKy(NH2)(x+y) and a novel Li3K(NH2)(4)-xMgH(2) combination system for hydrogen storage. J. Energy Chemistry. 2019, 35, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.-J.; Li, H.-W.; Murakami, H.; Akiba, E. Remarkably improved hydrogen storage properties of LiNH2-LiH composite via the addition of CeF4. J. Alloy. Compd. 2018, 735, 1017–1022. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, H.; Zhang, W.; Cui, J.; Xiong, Z.; Chen, P. Thermochemical transformation and reversible performance of Mg(NH2)2–NaMgH3 system. Int. J. Hydrogen Energy 2020, 45, 23069–23075. [Google Scholar] [CrossRef]
- Albanesi, L.F.; Garroni, S.; Larochette, P.A.; Nolis, P.; Mulas, G.; Enzo, S.; Baró, M.D.; Gennari, F.C. Role of aluminum chloride on the reversible hydrogen storage properties of the Li–N–H system. Int. J. Hydrog. Energy. 2015, 40, 13506–13517. [Google Scholar] [CrossRef]
- Wu, G.; Xiong, Z.; Liu, T.; Liu, Y.; Hu, J.; Chen, P.; Feng, Y.; Wee, A.T. Synthesis and Characterization of a New Ternary ImideLi2Ca(NH)2. Inorg. Chem. 2007, 46, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Leng, H.Y.; Ichikawa, T.; Isobe, S.; Hino, S.; Hanada, N.; Fujii, H. Desorption behaviours from metal-N-H systems synthesized by ball milling. J. Alloy. Compounds. 2005, 404, 443–447. [Google Scholar] [CrossRef]
- Luo, W. (LiNH2–MgH2): A viable hydrogen storage system. J. Alloy. Compd. 2004, 381, 284–287. [Google Scholar] [CrossRef]
- Nakamori, Y.; Kitahara, G.; Miwa, K.; Ohba, N.; Noritake, T.; Towata, S.; Orimo, S. Hydrogen storage properties of Li-Mg-N-H systems. J. Alloy. Compounds. 2005, 404, 396–398. [Google Scholar] [CrossRef]
- Xiong, Z.; Wu, G.; Hu, J.; Chen, P. Ternary Imides for Hydrogen Storage. Adv. Mater. 2004, 16, 1522–1525. [Google Scholar] [CrossRef]
- Xiong, Z.; Hu, J.; Wu, G.; Chen, P.; Luo, W.; Gross, K.; Wang, J. Thermodynamic and kinetic investigations of the hydrogen storage in the Li-Mg-N-H system. J. Alloy. Compounds. 2005, 398, 235–239. [Google Scholar] [CrossRef]
- Cao, H.; Pistidda, C.; Riglos, M.V.C.; Chaudhary, A.-L.; Capurso, G.; Tseng, J.-C.; Puszkiel, J.; Wharmby, M.T.; Gemming, T.; Chen, P.; et al. Conversion of magnesium waste into a complex magnesium hydride system: Mg(NH2)2–LiH. Sustain. Energy Fuels 2020, 4, 1915–1923. [Google Scholar] [CrossRef]
- Durojaiye, T.; Hayes, J.; Goudy, A. Potassium, rubidium and cesium hydrides as dehydrogenation catalysts for the lithium amide/magnesium hydride system. Int. J. Hydrogen Energy 2015, 40, 2266–2273. [Google Scholar] [CrossRef] [Green Version]
- Gizer, G.; Puszkiel, J.; Cao, H.; Pistidda, C.; Le, T.T.; Dornheim, M.; Klassen, T. Tuning the reaction mechanism and hydrogenation/dehydrogenation properties of 6Mg(NH2)29LiH system by adding LiBH4. Int. J. Hydrogen Energy 2019, 44, 11920–11929. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Li, B.; Gao, M.; Pan, H. Metathesis Reaction-Induced Significant Improvement in Hydrogen Storage Properties of the KF-Added Mg(NH2)2–2LiH System. J. Phys. Chem. C 2013, 117, 866–875. [Google Scholar] [CrossRef]
- Miao, N.; Zhou, X.L.; Lin, X.; Shi, Y.; Leng, H.Y.; Li, Q. Effect of LiCe(BH4)(3)Cl with a high Li ion conductivity on the hydrogen storage properties of Li-Mg-N-H system. Int. J. Hydrog. Energy. 2019, 44, 29150–29158. [Google Scholar] [CrossRef]
- Wang, H.; Wu, G.; Cao, H.; Pistidda, C.; Chaudhary, A.-L.; Garroni, S.; Dornheim, M.; Chen, P. Near Ambient Condition Hydrogen Storage in a Synergized Tricomponent Hydride System. Adv. Energy Mater. 2017, 7. [Google Scholar] [CrossRef]
- Wang, J.; Liu, T.; Wu, G.; Li, W.; Liu, Y.; Araújo, C.M.; Scheicher, R.H.; Blomqvist, A.; Ahuja, R.; Xiong, Z.; et al. Potassium-Modified Mg(NH2)(2)/2 LiH System for Hydrogen Storage. Angew. Chem. -Int. Edition. 2009, 48, 5828–5832. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.C.; Stavila, V.; Poonyayant, N.; Heo, T.W.; Ray, K.G.; Klebanoff, L.E.; Udovic, T.J.; Lee, J.R.I.; Angboonpong, N.; Sugar, J.D.; et al. Hydrogen Storage: Nanointerface-Driven Reversible Hydrogen Storage in the Nanoconfined Li-N-H System (Adv. Mater. Interfaces 3/2017). Adv. Mater. Interfaces 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Xia, G.; Li, D.; Chen, X.; Tan, Y.; Tang, Z.; Guo, Z.; Liu, H.; Liu, Z.; Yu, X. Carbon-Coated Li3N Nanofibers for Advanced Hydrogen Storage. Adv. Mater. 2013, 25, 6238–6244. [Google Scholar] [CrossRef]
- Yan, M.-Y.; Sun, F.; Liu, X.-P.; Ye, J.-H.; Wang, S.-M.; Jiang, L.-J. Effects of graphite content and compaction pressure on hydrogen desorption properties of Mg(NH2)2–2LiH based tank. J. Alloy. Compd. 2015, 628, 63–67. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Zhang, X.; Yang, Y.; Zhang, Q.; Jin, T.; Wang, Y.; Gao, M.; Sun, L.; Pan, H. Synthesis of CsH and its effect on the hydrogen storage properties of the Mg(NH2)2-2LiH system. Int. J. Hydrogen Energy 2016, 41, 11264–11274. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Yang, Y.; Li, Y.; Hu, J.; Gao, M.; Pan, H. Superior catalytic activity of in situ reduced metallic Co for hydrogen storage in a Co(OH)(2)-containing LiBH4/2LiNH(2) composite. Mater. Res. Bulletin. 2018, 97, 544–552. [Google Scholar] [CrossRef]
- Gizer, G.; Cao, H.; Puszkiel, J.; Pistidda, C.; Santoru, A.; Zhang, W.; He, T.; Chen, P.; Klassen, T.; Dornheim, M. Enhancement Effect of Bimetallic Amide K2Mn(NH2)4 and In-Situ Formed KH and Mn4N on the Dehydrogenation/Hydrogenation Properties of Li–Mg–N–H System. Energies 2019, 12, 2779. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, T.; Hanada, N.; Isobe, S.; Leng, H.Y.; Fujii, H. Hydrogen storage properties in Ti catalyzed Li-N-H system. J. Alloy. Compounds. 2005, 404, 435–438. [Google Scholar] [CrossRef]
- Isobe, S.; Ichikawa, T.; Hanada, N.; Leng, H.; Fichtner, M.; Fuhr, O.; Fujii, H. Effect of Ti catalyst with different chemical form on Li–N–H hydrogen storage properties. J. Alloy. Compd. 2005, 404–406, 439–442. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Reed, D.; Book, D.; Anderson, P. Hydrogen release and uptake in the Li-Zn-N system. J. Alloy. Compounds. 2015, 645, S295–S298. [Google Scholar] [CrossRef]
- Zhang, T.; Isobe, S.; Matsuo, M.; Orimo, S.-I.; Wang, Y.; Hashimoto, N.; Ohnuki, S. Effect of Lithium Ion Conduction on Hydrogen Desorption of LiNH2–LiH Solid Composite. ACS Catal. 2015, 5, 1552–1555. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Cao, H.; He, T.; Guo, J.; Wu, G.; Chen, P. Effects of doping FeCl 3 on hydrogen storage properties of Li-N-H system. Prog. Nat. Sci. 2017, 27, 139–143. [Google Scholar] [CrossRef]
- Cao, H.; Pistidda, C.; Richter, T.M.M.; Santoru, A.; Milanese, C.; Garroni, S.; Bednarcik, J.; Chaudhary, A.-L.; Gizer, G.; Liermann, H.-P.; et al. In Situ X-ray Diffraction Studies on the De/rehydrogenation Processes of the K2[Zn(NH2)4]-8LiH System. J. Phys. Chem. C 2017, 121, 1546–1551. [Google Scholar] [CrossRef]
- Cao, H.; Richter, T.M.M.; Pistidda, C.; Chaudhary, A.-L.; Santoru, A.; Gizer, G.; Niewa, R.; Chen, P.; Klassen, T.; Dornheim, M. Ternary Amides Containing Transition Metals for Hydrogen Storage: A Case Study with Alkali Metal Amidozincates. ChemSusChem 2015, 8, 3777–3782. [Google Scholar] [CrossRef]
- Cao, H.; Santoru, A.; Pistidda, C.; Richter, T.M.M.; Chaudhary, A.-L.; Gizer, G.; Niewa, R.; Chen, P.; Klassen, T.; Dornheim, M. New synthesis route for ternary transition metal amides as well as ultrafast amide–hydride hydrogen storage materials. Chem. Commun. 2016, 52, 5100–5103. [Google Scholar] [CrossRef] [Green Version]
- Richter, T.M.M.; Zhang, S.; Niewa, R. Ammonothermal synthesis of dimorphic K2[Zn(NH2)4]. Z. Für Krist. - Cryst. Materials. 2013, 228, 351–358. [Google Scholar] [CrossRef]
- Richter, T.M.M.; Alt, N.S.A.; Schlücker, E.; Niewa, R. Ammonothermal Synthesis and Characterization of Li4[Zn(NH2)4](NH2)2. Chemistry 2015, 641, 1016–1023. [Google Scholar] [CrossRef]
- Uribe, F.A.; Gottesfeld, S.; Zawodzinski, T.A., Jr. Effect of ammonia as potential fuel impurity on proton exchange membrane fuel cell performance. J. Electrochem. Society. 2002, 149, A293–A296. [Google Scholar] [CrossRef]
- Soto, H.J.; Lee, W.-K.; Van Zee, J.W.; Murthy, M. Effect of Transient Ammonia Concentrations on PEMFC Performance. Electrochem. Solid-State Lett. 2003, 6, A133–A135. [Google Scholar] [CrossRef]
- Lonardelli, I.; Wenk, H.-R.; Lutterotti, L.; Goodwin, M. Texture analysis from synchrotron diffraction images with the Rietveld method: Dinosaur tendon and salmon scale. J. Synchrotron Radiat. 2005, 12, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Lutterotti, L.; Bortolotti, M.; Ischia, G.; Lonardelli, I.; Wenk, H.R. Rietveld texture analysis from diffraction images. Z. Fur Kristallographie 2007, 26, 125–130. [Google Scholar] [CrossRef]
- Ghanbari, F.; Hassani, A.; Wacławek, S.; Wang, Z.; Matyszczak, G.; Lin, K.-Y.A.; Dolatabadi, M. Insights into paracetamol degradation in aqueous solutions by ultrasound-assisted heterogeneous electro-Fenton process: Key operating parameters, mineralization and toxicity assessment. Sep. Purif. Technol. 2021, 266, 118533. [Google Scholar] [CrossRef]
- Wang, H.; Cao, H.J.; Wu, G.T.; He, T.; Chen, P. The improved Hydrogen Storage Performances of the Multi-Component Composite: 2Mg(NH2)(2)-3LiH-LiBH4. Energies 2015, 8, 6898–6909. [Google Scholar] [CrossRef] [Green Version]
- Dematteis, E.M.; Berti, N.; Cuevas, F.; Latroche, M.; Baricco, M. Substitutional effects in TiFe for hydrogen storage: A comprehensive review. Mater. Adv. 2021, 2, 2524–2560. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Dreistadt, D.M.; Capurso, G.; Jepsen, J.; Cuevas, F.; Latroche, M. Fundamental hydrogen storage properties of TiFe-alloy with partial substitution of Fe by Ti and Mn. J. Alloy. Compounds. 2021, 874, 159925. [Google Scholar] [CrossRef]
- Kölbig, M.; Bürger, I.; Linder, M. Thermal applications in vehicles using Hydralloy C5 in single and coupled metal hydride systems. Appl. Energy 2021, 287, 116534. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, H.; Pistidda, C.; Richter, T.M.M.; Capurso, G.; Milanese, C.; Tseng, J.-C.; Shang, Y.; Niewa, R.; Chen, P.; Klassen, T.; et al. De-hydrogenation/Rehydrogenation Properties and Reaction Mechanism of AmZn(NH2)n-2nLiH Systems (A = Li, K, Na, and Rb). Sustainability 2022, 14, 1672. https://doi.org/10.3390/su14031672
Cao H, Pistidda C, Richter TMM, Capurso G, Milanese C, Tseng J-C, Shang Y, Niewa R, Chen P, Klassen T, et al. De-hydrogenation/Rehydrogenation Properties and Reaction Mechanism of AmZn(NH2)n-2nLiH Systems (A = Li, K, Na, and Rb). Sustainability. 2022; 14(3):1672. https://doi.org/10.3390/su14031672
Chicago/Turabian StyleCao, Hujun, Claudio Pistidda, Theresia M. M. Richter, Giovanni Capurso, Chiara Milanese, Jo-Chi Tseng, Yuanyuan Shang, Rainer Niewa, Ping Chen, Thomas Klassen, and et al. 2022. "De-hydrogenation/Rehydrogenation Properties and Reaction Mechanism of AmZn(NH2)n-2nLiH Systems (A = Li, K, Na, and Rb)" Sustainability 14, no. 3: 1672. https://doi.org/10.3390/su14031672
APA StyleCao, H., Pistidda, C., Richter, T. M. M., Capurso, G., Milanese, C., Tseng, J.-C., Shang, Y., Niewa, R., Chen, P., Klassen, T., & Dornheim, M. (2022). De-hydrogenation/Rehydrogenation Properties and Reaction Mechanism of AmZn(NH2)n-2nLiH Systems (A = Li, K, Na, and Rb). Sustainability, 14(3), 1672. https://doi.org/10.3390/su14031672











