Effect of Using Aluminum Sulfate (Alum) as a Surface Amendment in Beef Cattle Feedlots on Ammonia and Sulfide Emissions
Abstract
1. Introduction
2. Materials and Methods
2.1. Lab-Scale Studies
2.2. Field-Scale Studies
2.3. Statistical Analysis
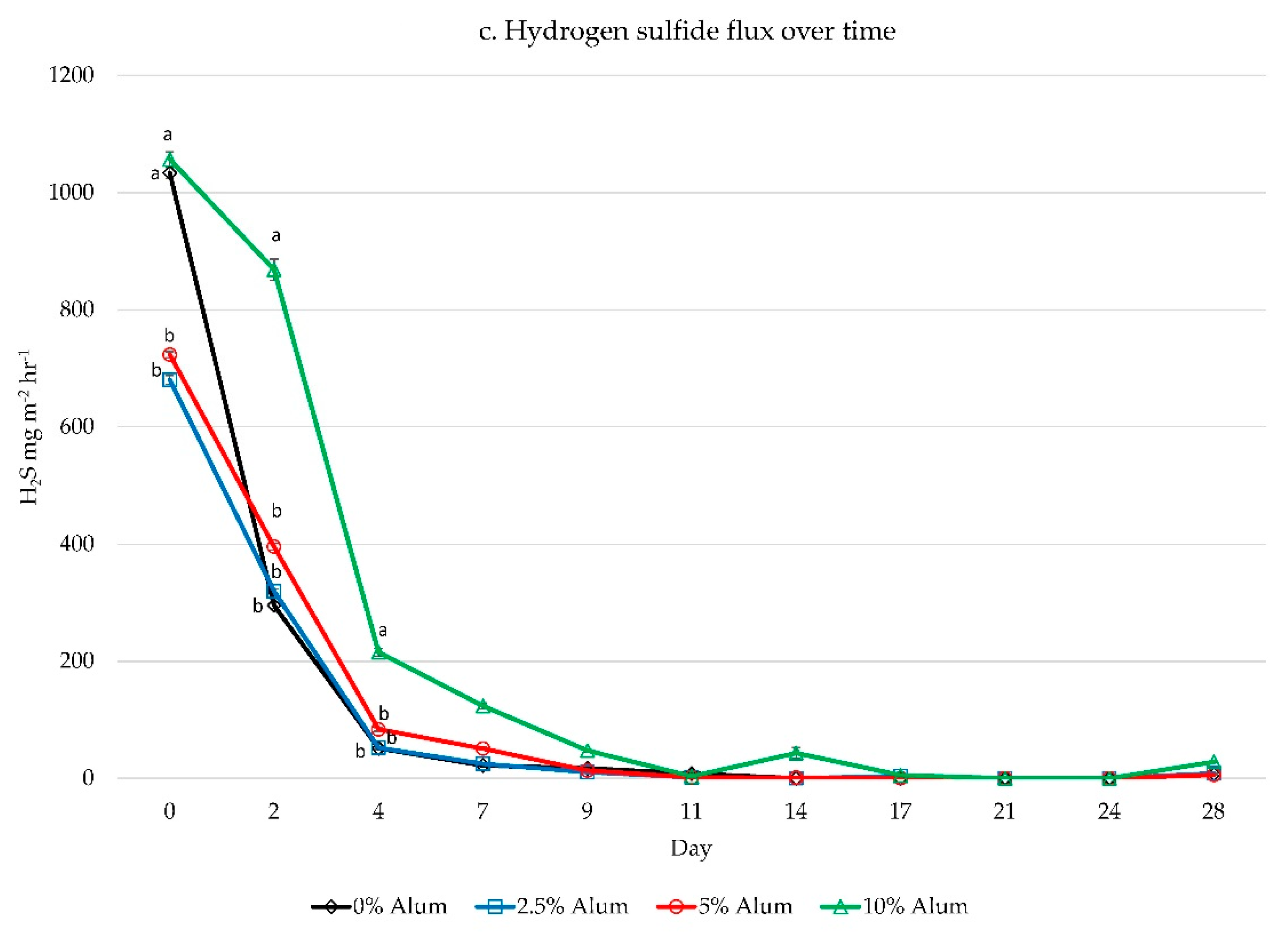
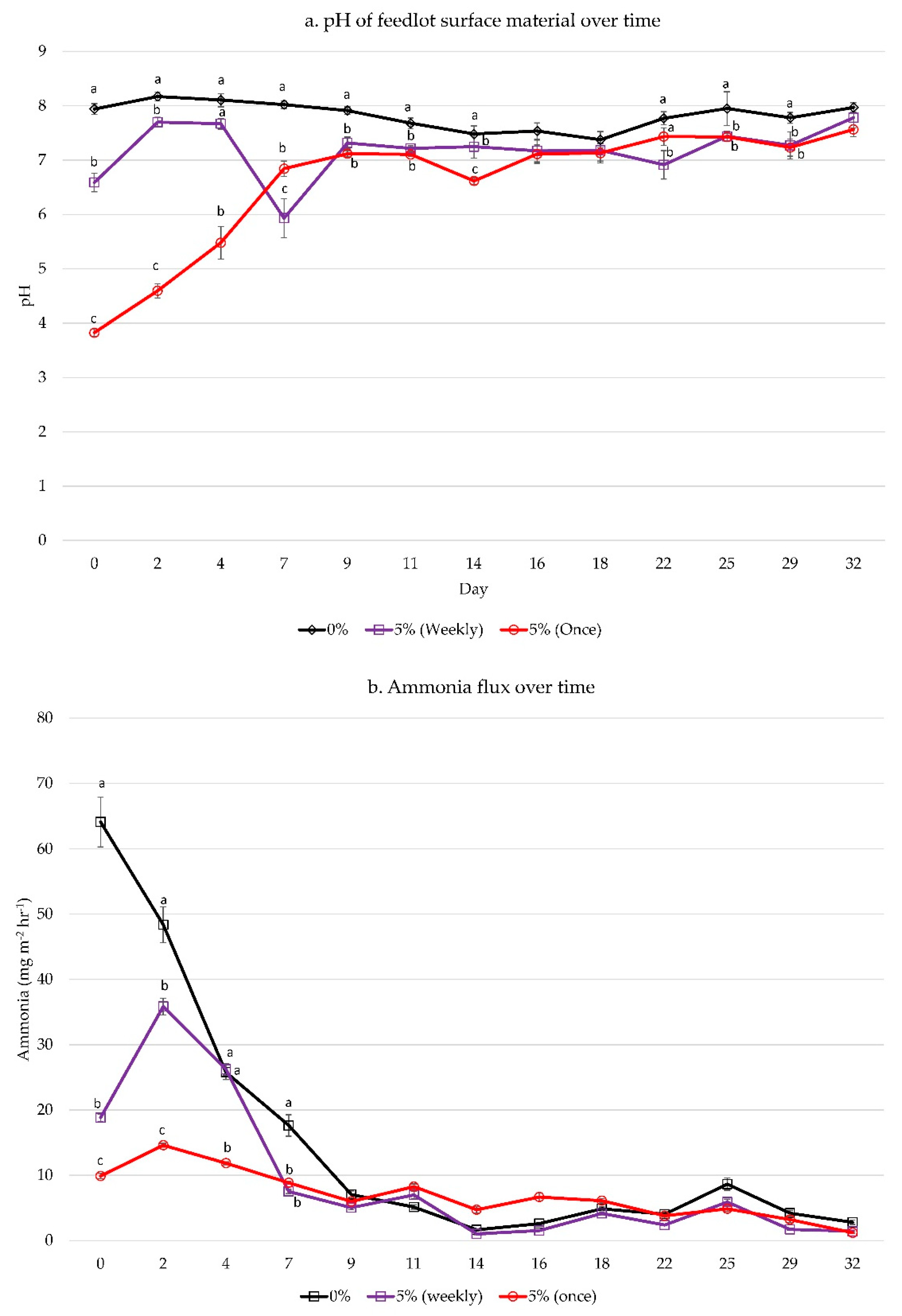
3. Results
3.1. Titration Study
3.2. Dose Frequency Study
3.3. Feedlot Surface Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mackie, R.I.; Stroot, P.G.; Varel, V.H. Biochemical identification and biological origin of key odor components in livestock waste. J. Anim. Sci. 1998, 76, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.N.; Varel, V.H. In vitro study of the biochemical origin and production limits of odorous compounds in cattle feedlots. J. Anim. Sci. 2001, 79, 2949–2956. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.N.; Berry, E.D. Cattle feedlot soil moisture and manure content: I. Impacts on greenhouse gases, odor compounds, nitrogen losses, and dust. J. Environ. Qual. 2005, 34, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Rappert, S.; Muller, R. Odor compounds in waste gas emission form agricultural operation and food industries. Waste Manag. 2005, 25, 887–907. [Google Scholar] [CrossRef]
- Trabue, S.; Kerr, B.; Bearson, B.; Ziemer, C. Swine odor analyzed by odor panels and chemical techniques. J. Environ. Qual. 2011, 40, 1510–1520. [Google Scholar] [CrossRef]
- Trabue, S.; Scoggin, K.; McConnell, L.; Maghirang, R.; Razote, E.; Hatfield, J. Identifying and tracking key odorants from cattle feedlots. Atmos. Environ. 2011, 45, 4243–4251. [Google Scholar] [CrossRef]
- Preece, S.L.M.; Casey, K.D.; Auvermann, B.W. Hydrogen Sulfide Emissions from Open/Dry-Lot Cattle-Feeding Operations, Texas Agri-Life Extension, E-620. 2012. Available online: https://agrilifeextension.tamu.edu/library/ranching/hydrogen-sulfide-emissions-from-open-dry-lot-cattle-feeding-operations/ (accessed on 20 December 2021).
- National Research Council (US) Committee on Acute Exposure Guideline Levels. Acute Exposure Guideline Levels for Selected Airborne Chemicals; National Academies Press: Washington, DC, USA, 2008; Volume 6. Available online: https://www.ncbi.nlm.nih.gov/books/NBK207883/ (accessed on 19 January 2022).
- Fazzalari, F.A. (Ed.) Compilation of Odor and Taste Threshold Data; ASTM Data Series DS 48A; ASTM Data: Philadelphia, PA, USA, 1978. [Google Scholar]
- Buttery, B.G.; Turnbaugh, J.G.; Ling, L.C. Contribution of volatiles to rice aroma. J. Agric. Food Chem. 1988, 36, 1006–1009. [Google Scholar] [CrossRef]
- O’Neill, D.H.; Phillips, V.R. A review of the control of odour nuisance from livestock buildings: Part 3, properties of the odorous substances which have been identified in livestock waste or in the air around them. J. Agric. Eng. Res. 1992, 53, 23–50. [Google Scholar] [CrossRef]
- Moulders, E.J. The odour of white bread IV. Quantitative determination of constituents in the vapour and their odour values. Z. Lesensm. Unters. Forsch. 1973, 151, 310–317. [Google Scholar]
- Guadagni, G.; Buttery, R.G.; Okano, S. Odour thresholds of some organic compounds associated with food flavours. J. Sci. Food Agric. 1963, 14, 761–765. [Google Scholar] [CrossRef]
- Buttery, R.G.; Teranishi, R.; Flath, R.A.; Ling, L.C. Identification of additional tomato paste volatiles. J. Agric. Food Chem. 1990, 38, 792–795. [Google Scholar] [CrossRef]
- Buttery, B.G.; Guadagni, D.; Ling, L.; Seifert, R.; Lipton, W. Additional volatile compounds of cabbage, broccoli, and cauliflower. J. Agric. Food Chem. 1976, 24, 829–832. [Google Scholar] [CrossRef]
- Stowell, R. Ammonia Loss and Emission Reporting: Considerations for Cattle Operations. UNL BeefWatch, 1 February 2018. Available online: http://newsroom.unl.edu/announce/beef/7498/42942 (accessed on 20 December 2021).
- Bierman, S.; Klopfenstein, T.J.; Stock, R.; Shain, D. Evaluation of Nitrogen, Phosphorus, and Organic Matter Balance in the Feedlot as Affected by Nutrition; Beef Cattle Report MP66-A; University of Nebraska-Lincoln: Lincoln, NE, USA, 1996; pp. 74–76. [Google Scholar]
- Van Horn, H.H.; Newton, G.L.; Kunkle, W.E. Ruminant nutrition from an environmental perspective: Factors affecting whole-farm nutrient balance. J. Anim. Sci. 1996, 74, 3082–3102. [Google Scholar] [CrossRef]
- Hausinger, R.P. Metabolic versatility of prokaryotes for urea deposition. J. Bacteriol. 2004, 186, 2520–2522. [Google Scholar] [CrossRef] [PubMed]
- Bierman, S.; Erickson, G.E.; Klopfenstein, T.J.; Stock, R.A.; Shain, D.H. Evaluation of nitrogen and organic matter balance in the feedlot as affected by level and source of dietary fiber. J. Anim. Sci. 1999, 77, 1645–1653. [Google Scholar] [CrossRef]
- Erickson, G.; Klopfenstein, T. Managing N inputs and the effect on N volatilization following excretion in open-dirt feedlots in Nebraska. Nitrogen in the environment. Sci. World J. 2001, 52, 830. [Google Scholar] [CrossRef]
- Todd, R.; Cole, N.; Clark, R.; Flesch, T.; Harper, L.; Baek, B. Ammonia emissions form beef cattle feedyard on the southern High Plains. Atmos. Environ. 2008, 42, 6769–6805. [Google Scholar] [CrossRef]
- Homolka, M.N.; Erickson, G.E.; Koelsch, R.K. Predicting nitrogen and phosphorus balance in beef open lots. Appl. Ani. Sci. 2021, 37, 641–653. [Google Scholar] [CrossRef]
- Hartung, J.; Phillips, V.R. Control of gaseous emissions from livestock buildings and manure stores. J. Agric. Eng. Res. 1994, 57, 173–189. [Google Scholar] [CrossRef]
- Rhoades, M.; Parker, D.; Cole, N.; Todd, R.; Caraway, E.; Auvermann, B.; Topliff, D.; Schuster, G. Continuous ammonia emission measurements from a commercial beef feedyard in Texas. Trans. ASABE 2010, 53, 1823–1831. [Google Scholar] [CrossRef]
- Shi, Y.; Parker, D.B.; Cole, N.A.; Auvermann, B.W.; Mehlhorn, J.E. Surface amendments to minimize ammonia emissions from beef cattle feedlots. Trans. ASABE 2001, 44, 677–682. [Google Scholar] [CrossRef]
- Varel, V.H.; Wells, J.E.; Miller, D.N. Combination of urease inhibitor and plant essential oil to control coliform bacteria, odour production and ammonia loss from cattle waste. J. Appl. Microbiol. 2007, 102, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Cole, N.A.; Todd, R.W.; Parker, D.B. Use of fat and zeolite to reduce ammonia emissions from beef cattle feedyards. In Proceedings of the International Symposium on Air Quality and Waste Management for Agriculture, Broomfield, CO, USA, 16–19 September 2007. [Google Scholar]
- Moore, P.A., Jr.; Daniel, T.C.; Edwards, D.R.; Miller, D.N. Effect of chemical amendments on ammonia volatilization from poultry litter. J. Environ. Qual. 1995, 24, 293–300. [Google Scholar] [CrossRef]
- Moore, P.A., Jr.; Daniel, T.C.; Edwards, D.R. Reducing phosphorus runoff and inhibiting ammonia loss from poultry manure with aluminum sulfate. J. Environ. Qual. 2000, 29, 37–49. [Google Scholar] [CrossRef]
- Moore, P.A.; Edwards, D.R. Long-term effects of poultry litter, alum-treated litter, and ammonium nitrate on phosphorus availability in soils. J. Environ. Qual. 2007, 36, 163–174. [Google Scholar] [CrossRef]
- Warren, J.G.; Phillips, S.B.; Mullins, G.L.; Keahey, D.; Penn, C.J. Environmental and production consequences of using alum-amended poultry litter as a nutrient source for corn. J. Environ. Qual. 2006, 35, 172–183. [Google Scholar] [CrossRef][Green Version]
- Spiehs, M.J.; Woodbury, B.L.; Parker, D.B. Ammonia, hydrogen sulfide, and greenhouse gas emissions from lab-scaled manure bedpacks with and without aluminum sulfate additions. Environments 2019, 6, 108. [Google Scholar] [CrossRef]
- Woodbury, B.L.; Gilley, J.E.; Parker, D.B.; Stromer, B.S. Greenhouse gas emissions from beef feedlot surface materials as affected by diet, moisture, temperature, and time. Trans. ASABE 2018, 61, 571–582. [Google Scholar] [CrossRef]
- Woodbury, B.L.; Gilley, J.E.; Parker, D.B.; Marx, D.B.; Eigenberg, R.A. VOC emissions from beef feedlot pen surfaces as affected by within-pen location, moisture, and temperature. Biosystem. Engng. 2015, 134, 31–41. [Google Scholar] [CrossRef]
- Parker, D.B.; Caraway, E.A.; Rhoades, M.B.; Cole, N.A.; Todd, R.W.; Casey, K.D. Effect of wind tunnel air velocity on VOC flux from standard solutions and CAFO manure/wastewater. Trans ASABE 2010, 53, 831–845. [Google Scholar] [CrossRef]
- Watson, M.A.; Wolf, A.; Wolf, N. Total Nitrogen. In Recommended Methods of Manure Analysis; Publication No. A3769; University of Wisconsin Cooperative Extension: Madison, WI, USA, 2003; pp. 18–24. [Google Scholar]
- Wolf, A.; Watson, M.; Wolf, N. Digestion and dissolution methods for P, K, Ca, Mg, and trace elements. In Recommended Methods of Manure Analysis; Publication No. A3769; University of Wisconsin Cooperative Extension: Madison, WI, USA, 2003; pp. 30–38. [Google Scholar]
- Mielke, L.N.; Swanson, N.P.; McCalla, T.M. Soil profile conditions of cattle feedlots. J. Environ. Qual. 1974, 3, 14–17. [Google Scholar] [CrossRef]
- Miller, J.J.; Curtis, T.; Larney, F.J.; McAllister, T.A.; Olson, B.M. Physical and chemical properties of feedlot pen surface located on moderately coarse- and moderately fine-textured soils in Southern Alberta. J. Environ. Qual. 2008, 37, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Parkin, T.B.; Venterea, R.T. USDA-ARS GRACEnet Project Protocols, Chapter 3. Chamber-Based Trace Gas Flux Measurements4; Follett, R.F., Ed.; Sampling Protocols: Beltsville, MD, USA, 2010; pp. 1–39. Available online: http://www.ars.usda.gov/SP2UserFiles/Program/212/Chapter%203.%20GRACEnet%20Trace%20Gas%20Sampling%20Protocols.pdf (accessed on 26 January 2022).
- Miller, D.N.; Woodbury, B.L. A solid-phase microextraction chamber method for analysis of feces and manures volatiles. J. Environ. Qual. 2006, 35, 2383–2394. [Google Scholar] [CrossRef]
- Woodbury, B.L.; Miller, D.N.; Eigenberg, R.A.; Nienaber, J.A. An inexpensive laboratory and field chamber for manure volatile gas flux analysis. Trans. ASABE 2006, 49, 767–772. [Google Scholar] [CrossRef]
- Spiehs, M.J. Lab-scale model to evaluate odor and gas concentrations emitted by deep bedded pack manure. J. Visualized Expt. 2018, 137, e57332. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.B.; Ham, J.; Woodbury, B.; Cai, L.; Spiehs, M.; Rhoades, M.; Trabue, S.; Casey, K.D.; Todd, R.; Cole, N.A. Standardization of flux chamber and wind tunnel flux measurements for quantifying volatile organic compound and ammonia emissions from area sources at animal feeding operations. Atmos. Environ. 2013, 66, 72–83. [Google Scholar] [CrossRef]
- Parker, D.B.; Gilley, J.; Woodbury, B.; Kim, K.H.; Bartelt-Hunt, S.; Galvin, G. Odorous VOC emission following land application of swine manure slurry. Atmos. Environ. 2013, 66, 91–100. [Google Scholar] [CrossRef]
- SAS. SAS 9.4; SAS Institute Inc.: Cary, NC, USA, 2016. [Google Scholar]
- Smith, D.R.; Moore, P.A., Jr.; Griffis, C.L.; Daniel, T.C.; Edwards, D.R.; Boothe, D.L. Effects of alum and aluminum chloride on phosphorus runoff from swine manure. J. Environ. Qual. 2001, 30, 992–998. [Google Scholar] [CrossRef]
- Ueki, A. Sulfate-reduction in anaerobic digestion of animal waste. J. Gen. Appl. Microbiol. 1986, 32, 111–123. [Google Scholar] [CrossRef]
- Choi, I.H.; Moore, P.A., Jr. Effects of liquid aluminum chloride additions to poultry litter on broiler performance, ammonia emissions, soluble phosphorus, total volatile fatty acids, and nitrogen content of litter. Poultry Sci. 2008, 87, 1955–1963. [Google Scholar] [CrossRef]
- Euken, R.; Doran, B.; Clark, C.; Shouse, S.; Ellis, S.; Loy, D.; Schultz, L. Beef Feedlot Systems Manual; PM 1867; Iowa State University Extension and Outreach: Ames, IA, USA, 2015. [Google Scholar]
- Hooser, S.B.; Van Alstine, W.; Kiupel, M.; Sojka, J. Acute pit gas (hydrogen sulfide) poisoning in confinement cattle. J. Vet. Diag. Invest. 2000, 12, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Zang, B.; Shuyan, L.; Frederick, M.C.; Guoxue, L.; Zhang, D.; Yangyang, L. Control of dimethyl sulfide and dimethyl disulfide odors during pig manure composting using nitrogen amendment. Bioresour. Technol. 2017, 224, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Linderholm, A.L.; Findleton, C.L.; Kumar, G.; Hong, Y.; Bisson, L.F. Identification of genes affecting hydrogen sulfide formation in Saccharomyces cerevisiae. Appl. Environ. Microb. 2008, 74, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Andriamanohiarisoamanana, F.J.; Sakamoto, Y.; Yamashiro, T.; Yasui, S.; Iwasaki, M.; Ihara, I.; Umetsu, K. Effects of handling parameters on hydrogen sulfide emission from stored dairy manure. J. Environ. Manag. 2015, 154, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Yongsiri, C.; Vollertsen, J.; Hvitved-Jacobsen, T. Effect of temperature on air-water transfer of hydrogen sulfide. J. Environ. Eng. 2004, 130, 104–109. [Google Scholar] [CrossRef]







| Nutrient | Original FSM | 0% Alum | 2.5% Alum | 5% Alum | 10% Alum | p-Value |
|---|---|---|---|---|---|---|
| Dry matter, % | 88.4 ± 0.9 a | 41.3 ± 0.4 c | 40.8 ± 0.5 c | 42.3 ± 1.0 bc | 44.0 ± 0.7 b | <0.01 |
| Total N, g kg−1 | 23 ± 0.3 | 20.9 ± 0.7 | 20.4 ± 0.6 | 20.5 ± 0.9 | 22.6 ± 0.7 | 0.09 |
| Organic-N, g kg−1 | 21.1 ± 0.1 | 20.5 ± 0.6 | 20.0 ± 0.5 | 19.7 ± 0.9 | 19.9 ± 0.6 | 0.77 |
| Ammonium-N, g kg−1 | 1.9 ± 0.3 b | 0.2 ± 0.1 d | 0.3 ± 0.1 d | 0.8 ± 0.2 c | 2.6 ± 0.2 a | <0.01 |
| Nitrate-N, g kg−1 | 0.02 ± 0.0 b | 0.17 ± 0.1 a | 0.08 ± 0.0 ab | 0.05 ± 0.0 b | 0.00 ± 0.0 b | <0.01 |
| Total P, g kg−1 | 13.2 ± 0.1 a | 13.5 ± 0.1 a | 12.4 ± 0.1 b | 12.7 ± 0.2 b | 12.5 ± 0.1 b | <0.01 |
| Total S, g kg−1 | 6.1 ± 0.2 d | 6.9 ± 0.1 d | 17.3 ± 0.7 c | 22.4 ± 0.3 b | 37.1 ± 0.6 a | <0.01 |
| pH upper 2 cm | ----- | 8.3 ± 0.0 a | 6.4 ± 0.1 bB | 4.1 ± 0.0 cB | 3.9 ± 0.0 cB | <0.01 |
| pH bottom 2 cm | ----- | 8.1 ± 0.1 a | 7.3 ± 0.1 bA | 7.0 ± 0.1 cA | 6.6 ± 0.2 dA | <0.01 |
| Nutrient | Original FSM | 0% Alum | 5% Alum (Once) | 5% Alum (Weekly) | p-Value |
|---|---|---|---|---|---|
| Dry matter, % | 85.4 ± 1.0 a | 36.5 ± 0.2 b | 36.7 ± 0.2 b | 36.6 ± 0.2 b | <0.01 |
| Total N, g kg−1 | 24.1 ± 0.4 | 20.5 ± 0.3 | 21.6 ± 0.9 | 21.6 ± 0.6 | 0.37 |
| Organic-N, g kg−1 | 21.3 ± 0.1 | 19.3 ± 0.3 | 20.5 ± 0.6 | 20.2 ± 0.5 | 0.23 |
| Ammonium-N, g kg−1 | 1.3 ± 0.3 | 1.1 ± 0.2 | 1.1 ± 0.3 | 1.4 ± 0.5 | 0.76 |
| Nitrate-N, g kg−1 | 0.01 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.00 ± 0.0 | --- |
| Total Phosphorus, g kg−1 | 34.2 ± 0.2 | 34.8 ± 0.7 | 34.1 ± 0.6 | 33.4 ± 0.4 | 0.21 |
| Total Sulfur, g kg−1 | 17.1 ± 0.1 b | 17.8 ± 0.5 b | 40.3 ± 1.1 a | 41.8 ± 1.2 a | <0.01 |
| Ambient Temp Range, °C | Average Soil Temp, °C | Average Soil Moisture, % | |
|---|---|---|---|
| Experiment 3, Day | |||
| 0 | 20.6–25.6 | 23.1 ± 0.2 b | 49.9 ± 2.3 xy |
| 3 | 20.0–25.0 | 22.8 ± 0.1 c | 52.3 ± 2.4 xy |
| 7 | 21.7–30.6 | 24.7 ± 0.1 a | 52.0 ± 1.5 xy |
| 10 | 23.3–30.6 | 22.5 ± 0.2 c | 55.0 ± 1.4 x |
| 14 | 18.9–25.0 | 22.5 ± 0.1 c | 48.1 ± 1.8 y |
| 17 | 23.3–28.3 | 21.9 ± 0.1 d | 49.4 ± 1.3 y |
| 21 | 20.0–25.6 | 21.6 ± 0.1 e | 36.2 ± 2.2 z |
| 24 | 18.9–22.8 | 21.8 ± 0.1 d | 51.0 ± 1.1 y |
| Experiment 4, Day | |||
| 0 | 28.9–32.2 | 27.7 ± 0.3 xy | 65.7 ± 2.4 b |
| 3 | 21.7–27.8 | 29.4 ± 0.3 xy | 59.3 ± 3.5 b |
| 7 | 21.1–26.7 | 29.1 ± 0.3 xy | 63.1 ± 3.8 b |
| 10 | 23.9–28.3 | 28.5 ± 0.2 xy | 68.3 ± 2.3 b |
| 14 | 23.3–27.8 | 33.0 ± 6.3 x | 66.3 ± 0.1 b |
| 17 | 20.6–25.0 | 25.1 ± 0.2 y | 80.6 ± 1.3 a |
| 21 | 20.0–26.7 | 20.8 ± 0.1 z | 80.0 ± 0.1 a |
| 24 | 25.6–31.7 | 24.6 ± 0.1 y | 66.4 ± 2.7 b |
| Statistical Analysis | |||
| Treatment | ----- | 0.23 | 0.79 |
| Day | ----- | <0.01 | <0.01 |
| Treatment x Day | ----- | 0.98 | 0.89 |
| Nutrient | Initial FSM | 0% Alum | 10% Alum | p-Value |
|---|---|---|---|---|
| Dry matter, % | 44.9 ± 1.0 | 51.4 ± 2.0 | 57.5 ± 1.5 | 0.01 |
| Total N, g kg−1 | 19.8 ± 1.3 | 18.1 ± 0.5 | 19.7 ± 0.4 | 0.01 |
| Organic-N, g kg−1 | 17.4 ± 1.3 | 17.6 ± 0.4 | 17.8 ± 0.4 | 0.68 |
| Ammonium-N, g kg−1 | 2.4 ± 0.3 | 0.9 ± 0.3 | 3.1 ± 1.1 | 0.08 |
| Nitrate-N, g kg−1 | 0.01 ± 0.0 | 0.01 ± 0.0 | 0.01 ± 0.0 | ----- |
| Total Sulfur, g kg−1 | 6.7 ± 0.9 | 6.5 ± 0.2 b | 16.8 ± 0.6 a | >0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spiehs, M.J.; Woodbury, B. Effect of Using Aluminum Sulfate (Alum) as a Surface Amendment in Beef Cattle Feedlots on Ammonia and Sulfide Emissions. Sustainability 2022, 14, 1984. https://doi.org/10.3390/su14041984
Spiehs MJ, Woodbury B. Effect of Using Aluminum Sulfate (Alum) as a Surface Amendment in Beef Cattle Feedlots on Ammonia and Sulfide Emissions. Sustainability. 2022; 14(4):1984. https://doi.org/10.3390/su14041984
Chicago/Turabian StyleSpiehs, Mindy J., and Bryan Woodbury. 2022. "Effect of Using Aluminum Sulfate (Alum) as a Surface Amendment in Beef Cattle Feedlots on Ammonia and Sulfide Emissions" Sustainability 14, no. 4: 1984. https://doi.org/10.3390/su14041984
APA StyleSpiehs, M. J., & Woodbury, B. (2022). Effect of Using Aluminum Sulfate (Alum) as a Surface Amendment in Beef Cattle Feedlots on Ammonia and Sulfide Emissions. Sustainability, 14(4), 1984. https://doi.org/10.3390/su14041984





