Modified Activated Carbon Synthesized from Oil Palm Leaves Waste as a Novel Green Adsorbent for Chemical Oxygen Demand in Produced Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
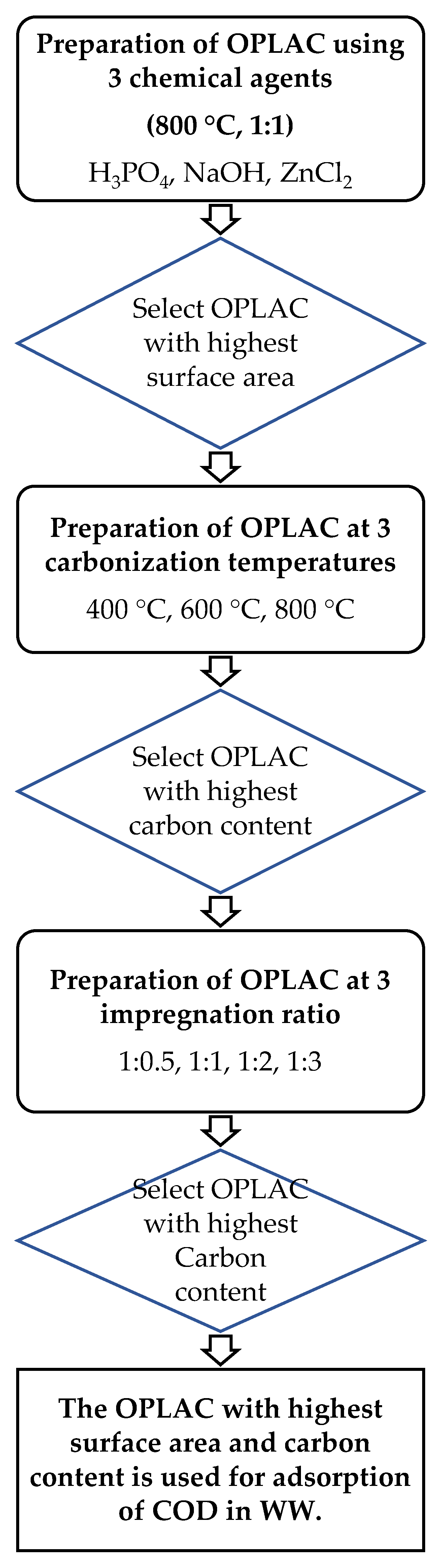
2.2. Synthesis of OPLAC
2.3. Characterization of AC and PW
2.4. Adsorption Batch Experiments
2.5. Adsorption Isotherm Modelling
2.6. Adsorption Kinetic Modelling
3. Results and Discussion
3.1. Surface Areas of OPLACS
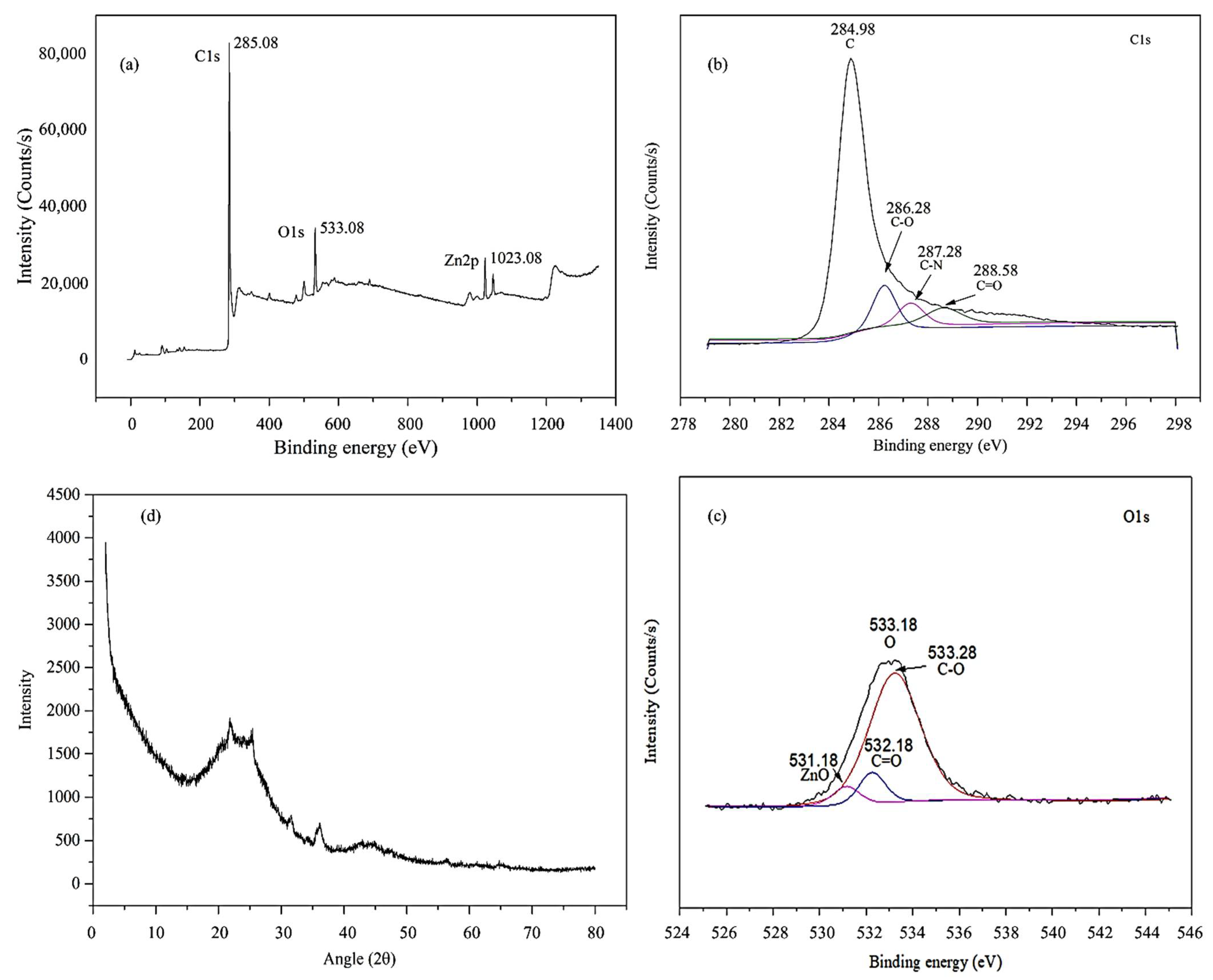
3.2. Surface Functional Groups of OPLAC-ZC
3.3. Elemental Composition of OPLAC-ZC
3.4. Chemical Composition and Crystallinity of OPLAC-ZC
3.5. Effect of Initial Solution pH on Removal of COD
3.6. Effect of OPLAC-ZC Dosage on Removal of COD
3.7. Effect of Contact Time on Removal of COD
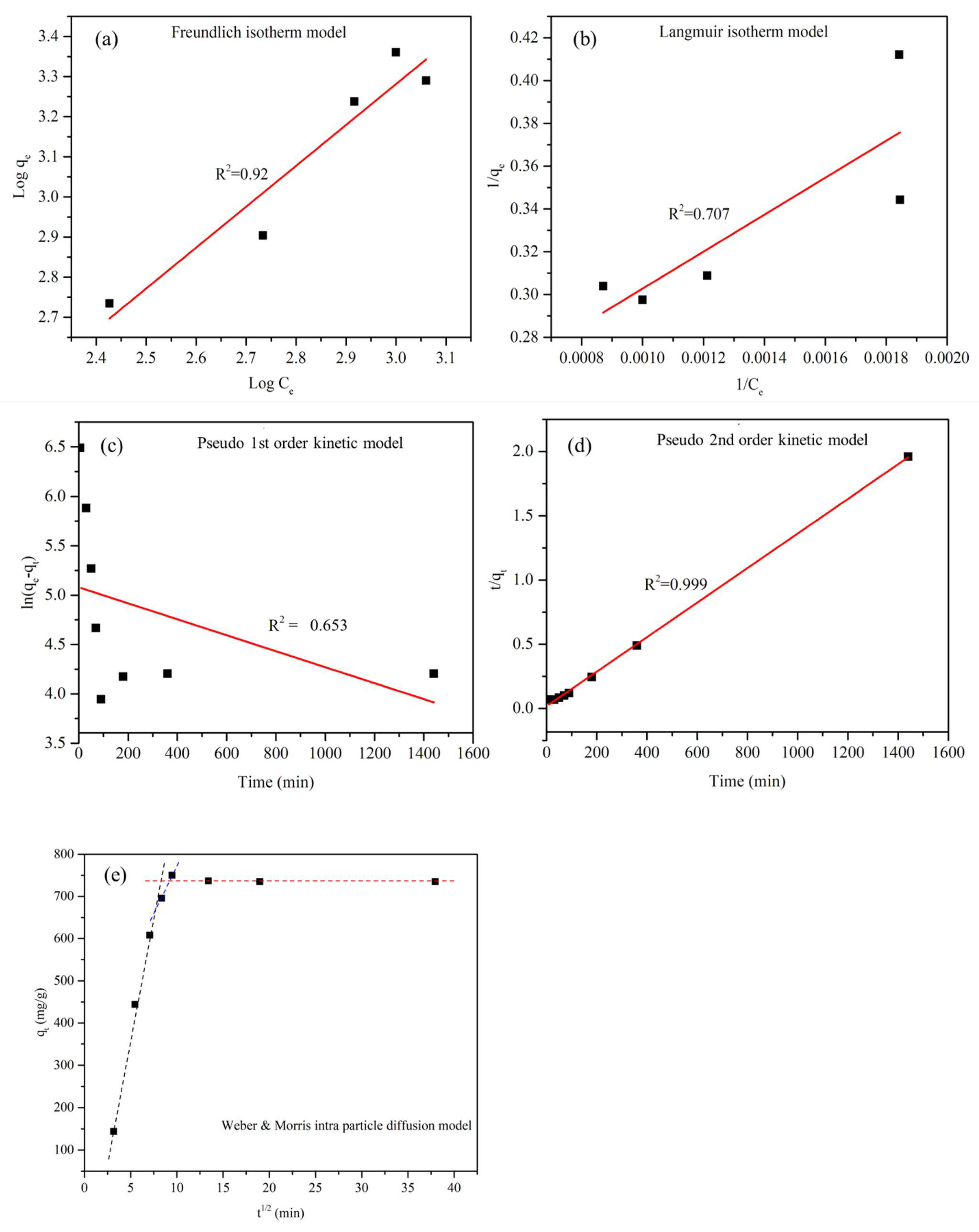
3.8. Adsorption Isotherm Modeling
3.9. Adsorption Kinetic Modeling
4. COD Adsorption Efficiency of Various Materials
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borhan, A.; Abdullah, N.A.; Rashidi, N.A.; Taha, M.F. Removal of Cu2+ and Zn2+ from Single Metal Aqueous Solution Using Rubber-Seed Shell Based Activated Carbon. Procedia Eng. 2016, 148, 694–701. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhang, L.; Cheng, Z. Removal of organic pollutants from aqueous solution using agricultural wastes: A review. J. Mol. Liq. 2015, 212, 739–762. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P.; Singh, V.K. Wastewater treatment using low cost activated carbons derived from agricultural byproducts—A case study. J. Hazard. Mater. 2008, 152, 1045–1053. [Google Scholar] [CrossRef]
- Affam, A.C. Conventional steam activation for conversion of oil palm kernel shell biomass into activated carbon via biochar product. Glob. J. Environ. Sci. Manag. 2020, 6, 15–30. [Google Scholar] [CrossRef]
- Aguayo-Villarreal, I.A.; Bonilla-Petriciolet, A.; Muñiz-Valencia, R. Preparation of activated carbons from pecan nutshell and their application in the antagonistic adsorption of heavy metal ions. J. Mol. Liq. 2017, 230, 686–695. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Karri, R.R.; Yeganeh, Z.T.; Mahvi, A.H.; Nourmoradi, H.; Salari, M.; Zarei, A.; Sillanpää, M. Statistical modelling of endocrine disrupting compounds adsorption onto activated carbon prepared from wood using CCD-RSM and DE hybrid evolutionary optimization framework: Comparison of linear vs non-linear isotherm and kinetic parameters. J. Mol. Liq. 2020, 302, 112526. [Google Scholar] [CrossRef]
- Salman, J.M.; Njoku, V.O.; Hameed, B.H. Batch and fixed-bed adsorption of 2,4-dichlorophenoxyacetic acid onto oil palm frond activated carbon. Chem. Eng. J. 2011, 174, 33–40. [Google Scholar] [CrossRef]
- Khan, T.; Sabariah, T.; Abd, B.; Isa, M.H.; Ghanim, A.A.J.; Beddu, S.; Jusoh, H.; Iqbal, M.S.; Ayele, G.T.; Jami, M.S. Modeling of Cu(II) Adsorption from an Aqueous Solution Using an Artificial Neural Network (ANN). Molecules 2020, 25, 3263. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, C.; Padilla-Ortega, E.; Robledo-Cabrera, A.; López-Valdivieso, A. Cr(VI) adsorption on activated carbon: Mechanisms, modeling and limitations in water treatment. J. Environ. Chem. Eng. 2020, 8, 104031. [Google Scholar] [CrossRef]
- Nasrullah, A.; Bhat, A.H.; Naeem, A.; Isa, M.H.; Danish, M. High surface area mesoporous activated carbon-alginate beads for efficient removal of methylene blue. Int. J. Biol. Macromol. 2018, 107, 1792–1799. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Preparation of waste tea activated carbon using potassium acetate as an activating agent for adsorption of Acid Blue 25 dye. Chem. Eng. J. 2011, 171, 502–509. [Google Scholar] [CrossRef]
- Kumar, J.A.; Amarnath, D.J.; Sathish, S.; Jabasingh, S.A.; Saravanan, A.; Hemavathy, R.V.; Anand, K.V.; Yaashikaa, P.R. Enhanced PAHs removal using pyrolysis-assisted potassium hydroxide induced palm shell activated carbon: Batch and column investigation. J. Mol. Liq. 2019, 279, 77–87. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Optimized waste tea activated carbon for adsorption of Methylene Blue and Acid Blue 29 dyes using response surface methodology. Chem. Eng. J. 2011, 175, 233–243. [Google Scholar] [CrossRef]
- Akar, E.; Altinişik, A.; Seki, Y. Using of activated carbon produced from spent tea leaves for the removal of malachite green from aqueous solution. Ecol. Eng. 2013, 52, 19–27. [Google Scholar] [CrossRef]
- Khan, T.; Mustafa, M.R.U.; Isa, M.H.; Manan, T.S.B.A.; Ho, Y.C.; Lim, J.W.; Yusof, N.Z. Artificial Neural Network (ANN) for Modelling Adsorption of Lead (Pb (II)) from Aqueous Solution. Water. Air. Soil Pollut. 2017, 228, 1–15. [Google Scholar] [CrossRef]
- Tsibranska, I.; Hristova, E. Comparison of different kinetic models for adsorption of heavy metals onto activated carbon from apricot stones. Bulg. Chem. Commun. 2011, 43, 370–377. [Google Scholar]
- Devi, R.; Singh, V.; Kumar, A. COD and BOD reduction from coffee processing wastewater using Avacado peel carbon. Bioresour. Technol. 2008, 99, 1853–1860. [Google Scholar] [CrossRef]
- Van Thuan, T.; Quynh, B.T.P.; Nguyen, T.D.; Ho, V.T.T.; Bach, L.G. Response surface methodology approach for optimization of Cu2+, Ni2+ and Pb2+ adsorption using KOH-activated carbon from banana peel. Surf. Interfaces 2017, 6, 209–217. [Google Scholar] [CrossRef]
- Lim, W.C.; Srinivasakannan, C.; Al Shoaibi, A. Cleaner production of porous carbon from palm shells through recovery and reuse of phosphoric acid. J. Clean. Prod. 2015, 102, 501–511. [Google Scholar] [CrossRef]
- Ahmed, M.J. Preparation of activated carbons from date (Phoenix dactylifera L.) palm stones and application for wastewater treatments: Review. Process Saf. Environ. Prot. 2016, 102, 168–182. [Google Scholar] [CrossRef]
- Ayinla, R.T.; Dennis, J.O.; Zaid, H.M.; Sanusi, Y.K.; Usman, F.; Adebayo, L.L. A review of technical advances of recent palm bio-waste conversion to activated carbon for energy storage. J. Clean. Prod. 2019, 229, 1427–1442. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Utilization of palm solid residue as a source of renewable and sustainable energy in Malaysia. Renew. Sustain. Energy Rev. 2014, 40, 621–632. [Google Scholar] [CrossRef]
- Sidik, S.M.; Jalil, A.A.; Triwahyono, S.; Adam, S.H.; Satar, M.A.H.; Hameed, B.H. Modified oil palm leaves adsorbent with enhanced hydrophobicity for crude oil removal. Chem. Eng. J. 2012, 203, 9–18. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Zhang, Z.; Doherty, W.O.S.; O’Hara, I.M. The outlook of the production of advanced fuels and chemicals from integrated oil palm biomass biorefinery. Renew. Sustain. Energy Rev. 2019, 109, 386–411. [Google Scholar] [CrossRef]
- Elias, N.; Chandren, S.; Attan, N.; Mahat, N.A.; Razak, F.I.A.; Jamalis, J.; Wahab, R.A. Structure and properties of oil palm-based nanocellulose reinforced chitosan nanocomposite for efficient synthesis of butyl butyrate. Carbohydr. Polym. 2017, 176, 281–292. [Google Scholar] [CrossRef]
- Awalludin, M.F.; Sulaiman, O.; Hashim, R.; Nadhari, W.N.A.W. An overview of the oil palm industry in Malaysia and its waste utilization through thermochemical conversion, specifically via liquefaction. Renew. Sustain. Energy Rev. 2015, 50, 1469–1484. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of basic dye using activated carbon prepared from oil palm shell: Batch and fixed bed studies. Desalination 2008, 225, 13–28. [Google Scholar] [CrossRef]
- Sahu, J.N.; Karri, R.R.; Jayakumar, N.S. Improvement in phenol adsorption capacity on eco-friendly biosorbent derived from waste Palm-oil shells using optimized parametric modelling of isotherms and kinetics by differential evolution. Ind. Crops Prod. 2021, 164, 113333. [Google Scholar] [CrossRef]
- Chew, T.L.; Husni, H. Oil palm frond for the adsorption of Janus Green dye. Mater. Today Proc. 2019, 16, 1766–1771. [Google Scholar] [CrossRef]
- Wafti, N.S.A.; Lau, H.L.N.; Loh, S.K.; Aziz, A.A.; Rahman, Z.A.; Yuen, C. Activated Carbon from oil Palm Biomass as Potential Adsorbent for Palm Oil Mill Effluent Treatment. J. Oil Palm Res. 2017, 29, 278–290. [Google Scholar] [CrossRef] [Green Version]
- Isa, M.H.; Ibrahim, N.; Aziz, H.A.; Adlan, M.N.; Sabiani, N.H.M.; Zinatizadeh, A.A.L.; Kutty, S.R.M. Removal of chromium (VI) from aqueous solution using treated oil palm fibre. J. Hazard. Mater. 2008, 152, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Farma, R.; Lestari, O.; Taer, E.; Apriwandi; Minarni; Awitdrus, A. Removal of Cu, Fe, and Zn from Peat Water by Using Activated Carbon Derived from Oil Palm Leaves. Adv. Mater. Res. 2021, 1162, 65–73. [Google Scholar] [CrossRef]
- Haghbin, M.R.; Niknam Shahrak, M. Process conditions optimization for the fabrication of highly porous activated carbon from date palm bark wastes for removing pollutants from water. Powder Technol. 2021, 377, 890–899. [Google Scholar] [CrossRef]
- Hendges, L.T.; Costa, T.C.; Temochko, B.; Gómez González, S.Y.; Mazur, L.P.; Marinho, B.A.; da Silva, A.; Weschenfelder, S.E.; de Souza, A.A.U.; de Souza, S.M.A.G.U. Adsorption and desorption of water-soluble naphthenic acid in simulated offshore oilfield produced water. Process Saf. Environ. Prot. 2021, 145, 262–272. [Google Scholar] [CrossRef]
- Promraksa, A.; Rakmak, N. Biochar production from palm oil mill residues and application of the biochar to adsorb carbon dioxide. Heliyon 2020, 6, 1–9. [Google Scholar] [CrossRef]
- Shokrollahzadeh, S.; Golmohammad, F.; Naseri, N.; Shokouhi, H.; Arman-Mehr, M. Chemical oxidation for removal of hydrocarbons from gas-field produced water. Procedia Eng. 2012, 42, 942–947. [Google Scholar] [CrossRef] [Green Version]
- Desta, M.B. Mulu Berhe Desta Batch sorption experiments: Langmuir and Freundlich isotherm studies for the adsorption of textile metal ions onto teff straw (Eragrostis tef) agricultural waste. J. Thermodyn. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yurdakal, S.; Garlisi, C.; Özcan, L.; Bellardita, M.; Palmisano, G. Chapter 4—(Photo)catalyst Characterization Techniques: Adsorption Isotherms and BET, SEM, FTIR, UV-Vis, Photoluminescence, and Electrochemical Characterizations. In Heterogeneous Photocatalysis; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Khurshid, H.; Mustafa, M.R.U.; Rashid, U.; Isa, M.H.; Chia, H.Y.; Shah, M.M. Adsorptive removal of COD from produced water using tea waste biochar. Environ. Technol. Innov. 2021, 23, 101563. [Google Scholar] [CrossRef]
- Kharrazi, S.M.; Mirghaffari, N.; Dastgerdi, M.M.; Soleimani, M. A novel post-modification of powdered activated carbon prepared from lignocellulosic waste through thermal tension treatment to enhance the porosity and heavy metals adsorption. Powder Technol. 2020, 366, 358–368. [Google Scholar] [CrossRef]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O.J.I.J.C. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+ Unto Phosphoric Acid Modified Rice Husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar] [CrossRef]
- Halim, A.A.; Aziz, H.A.; Johari, M.A.M.; Ariffin, K.S. Comparison study of ammonia and COD adsorption on zeolite, activated carbon and composite materials in landfill leachate treatment. Desalination 2010, 262, 31–35. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Gayatri, S.L. Activated tea waste as a potential low-cost adsorbent for the removal of p -nitrophenol from wastewater. J. Chem. Eng. Data 2010, 55, 4614–4623. [Google Scholar] [CrossRef]
- Wan, S.; Hua, Z.; Sun, L.; Bai, X.; Liang, L. Biosorption of nitroimidazole antibiotics onto chemically modified porous biochar prepared by experimental design: Kinetics, thermodynamics, and equilibrium analysis. Process Saf. Environ. Prot. 2016, 104, 422–435. [Google Scholar] [CrossRef]
- Edet, U.A.; Ifelebuegu, A.O. Kinetics, isotherms, and thermodynamic modeling of the adsorption of phosphates from model wastewater using recycled brick waste. Processes 2020, 8, 665. [Google Scholar] [CrossRef]
- Kohler, T.; Hinze, M.; Müller, K.; Schwieger, W. Temperature independent description of water adsorption on zeotypes showing a type V adsorption isotherm. Energy 2017, 135, 227–236. [Google Scholar] [CrossRef]
- Valderrama, C.; Gamisans, X.; de las Heras, X.; Farrán, A.; Cortina, J.L. Sorption kinetics of polycyclic aromatic hydrocarbons removal using granular activated carbon: Intraparticle diffusion coefficients. J. Hazard. Mater. 2008, 157, 386–396. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Van Tran, T.; Bui, Q.T.P.; Nguyen, T.D.; Thanh Ho, V.T.; Bach, L.G. Application of response surface methodology to optimize the fabrication of ZnCl2-activated carbon from sugarcane bagasse for the removal of Cu2+. Water Sci. Technol. 2017, 75, 2047–2055. [Google Scholar] [CrossRef]
- Soysa, R.; Choi, Y.S.; Kim, S.J.; Choi, S.K. Fast pyrolysis characteristics and kinetic study of Ceylon tea waste. Int. J. Hydrog. Energy 2016, 41, 16436–16443. [Google Scholar] [CrossRef]
- Nasri, N.S.; Hamza, U.D.; Ismail, S.N.; Ahmed, M.M.; Mohsin, R. Assessment of porous carbons derived from sustainable palm solid waste for carbon dioxide capture. J. Clean. Prod. 2014, 71, 148–157. [Google Scholar] [CrossRef]
- Deng, S.; Yu, G.; Chen, Z.; Wu, D.; Xia, F.; Jiang, N. Characterization of suspended solids in produced water in Daqing oilfield. Colloids Surf. A Physicochem. Eng. Asp. 2009, 332, 63–69. [Google Scholar] [CrossRef]
- Zbair, M.; Ait Ahsaine, H.; Anfar, Z. Porous carbon by microwave assisted pyrolysis: An effective and low-cost adsorbent for sulfamethoxazole adsorption and optimization using response surface methodology. J. Clean. Prod. 2018, 202, 571–581. [Google Scholar] [CrossRef]
- Ghorbani, F.; Kamari, S.; Zamani, S.; Akbari, S.; Salehi, M. Optimization and modeling of aqueous Cr(VI) adsorption onto activated carbon prepared from sugar beet bagasse agricultural waste by application of response surface methodology. Surf. Interfaces 2020, 18, 100444. [Google Scholar] [CrossRef]
- Eid, M.E.-S. Synthesis of Polyethylenimine-magnetic amorphous carbon nano composite as a novel adsorbent for Hg(II) from aqueous solutions. Aust. J. Basic Appl. Sci. 2016, 10, 323–335. [Google Scholar]
- Soliman, A.M.; Elwy, H.M.; Thiemann, T.; Majedi, Y.; Labata, F.T.; Al-Rawashdeh, N.A.F. Removal of Pb(II) ions from aqueous solutions by sulphuric acid-treated palm tree leaves. J. Taiwan Inst. Chem. Eng. 2016, 58, 264–273. [Google Scholar] [CrossRef]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Lübken, M. Biochar for wastewater treatment-conversion technologies and applications. Appl. Sci. 2020, 10, 3493. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Farag, R.S.; Elshfai, M.M. Reduction of organic matter from municipal wastewater at low cost using green synthesis nano iron extracted from black tea: Artificial intelligence with regression analysis. Egypt. J. Pet. 2020, 29, 9–20. [Google Scholar] [CrossRef]
- Santoso, E.; Ediati, R.; Kusumawati, Y.; Bahruji, H.; Sulistiono, D.O.; Prasetyoko, D. Review on recent advances of carbon based adsorbent for methylene blue removal from waste water. Mater. Today Chem. 2020, 16, 100233. [Google Scholar] [CrossRef]
- Chaouki, Z.; Hadri, M.; Nawdali, M.; Benzina, M.; Zaitan, H. Treatment of a landfill leachate from Casablanca city by a coagulation-flocculation and adsorption process using a palm bark powder (PBP). Sci. Afr. 2021, 12, e00721. [Google Scholar] [CrossRef]
- Pinto Brito, M.J.; Flores Santos, M.P.; de Souza Júnior, E.C.; Santos, L.S.; Ferreira Bonomo, R.C.; Da Costa Ilhéu Fontan, R.; Veloso, C.M. Development of activated carbon from pupunha palm heart sheaths: Effect of synthesis conditions and its application in lipase immobilization. J. Environ. Chem. Eng. 2020, 8, 1–11. [Google Scholar] [CrossRef]
- Cheraghipour, E.; Pakshir, M. Process optimization and modeling of Pb(II) ions adsorption on chitosan-conjugated magnetite nano-biocomposite using response surface methodology. Chemosphere 2020, 260, 127560. [Google Scholar] [CrossRef]
- Martini, S.; Afroze, S.; Ahmad Roni, K. Modified eucalyptus bark as a sorbent for simultaneous removal of COD, oil, and Cr(III) from industrial wastewater. Alex. Eng. J. 2020, 59, 1637–1648. [Google Scholar] [CrossRef]
- Halim, A.A.; Aziz, H.A.; Johari, M.A.M.; Ariffin, K.S.; Adlan, M.N. Ammoniacal nitrogen and COD removal from semi-aerobic landfill leachate using a composite adsorbent: Fixed bed column adsorption performance. J. Hazard. Mater. 2010, 175, 960–964. [Google Scholar] [CrossRef]
- Azoulay, K.; Bencheikh, I.; Moufti, A.; Dahchour, A.; Mabrouki, J.; El Hajjaji, S. Comparative study between static and dynamic adsorption efficiency of dyes by the mixture of palm waste using the central composite design. Chem. Data Collect. 2020, 27, 1–19. [Google Scholar] [CrossRef]
- El-Sayed, M.; Nada, A.A. Polyethylenimine—Functionalized amorphous carbon fabricated from oil palm leaves as a novel adsorbent for Cr(VI) and Pb(II) from aqueous solution. J. Water Process Eng. 2017, 16, 296–308. [Google Scholar] [CrossRef]
- Isam, M.; Baloo, L.; Sapari, N.; Nordin, I.; Yavari, S.; Al-Madhoun, W. Removal of Lead using Activated Carbon Derived from Red Algae (Gracilaria Changii). MATEC Web Conf. 2018, 203, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hao, Z.; Wang, C.; Yan, Z.; Jiang, H.; Xu, H. Magnetic particles modification of coconut shell-derived activated carbon and biochar for effective removal of phenol from water. Chemosphere 2018, 211, 962–969. [Google Scholar] [CrossRef]
- Bagheri, R.; Ghaedi, M.; Asfaram, A.; Alipanahpour Dil, E.; Javadian, H. RSM-CCD design of malachite green adsorption onto activated carbon with multimodal pore size distribution prepared from Amygdalus scoparia: Kinetic and isotherm studies. Polyhedron 2019, 171, 464–472. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, Z.; Lu, B.; Xian, J.; Tsang, E.P.; Cheng, W.; Fang, J.; Fang, Z. Magnetic biochar for environmental remediation: A review. Bioresour. Technol. 2019, 298, 122468. [Google Scholar] [CrossRef]
- Pan, Y.; Zhu, Y.; Xu, Z.; Lu, R.; Zhang, Z.; Liang, M.; Liu, H. RETRACTED ARTICLE: Adsorption removal of COD from wastewater by the activated carbons prepared from sugarcane bagasse. In Proceedings of the 5th International Conference on Bioinformatics and Biomedical Engineering, iCBBE, Wuhan, China, 10–12 May 2011. [Google Scholar] [CrossRef]
- Gallo-Cordova, A.; Silva-Gordillo, M.D.M.; Muñoz, G.A.; Arboleda-Faini, X.; Almeida Streitwieser, D. Comparison of the adsorption capacity of organic compounds present in produced water with commercially obtained walnut shell and residual biomass. J. Environ. Chem. Eng. 2017, 5, 4041–4050. [Google Scholar] [CrossRef]



| Activated Carbon | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Size (nm) | Yield (%) |
|---|---|---|---|---|
| OPLAC-HP | 255.840 | 0.166 | 2.5 | 42 |
| OPLAC-NO | 267.719 | 0.0521 | 0.8 | 46 |
| OPLAC-ZC | 331.153 | 0.206 | 2.5 | 42 |
| Elements | C | O | Zn | Si | Ca |
|---|---|---|---|---|---|
| Temperature °C | |||||
| 400 | 68.3 | 20.2 | 2.8 | 1.2 | 0.4 |
| 600 | 74.2 | 14.29 | 3.2 | 2.2 | 0.9 |
| 800 | 81.2 | 9.6 | 4 | 4.2 | 1.1 |
| Impregnation ratio | |||||
| 1:0.5 | 76.4 | 11.3 | 0.8 | 6.6 | 3 |
| 1:1 | 81.2 | 9.6 | 4 | 4.2 | 1.1 |
| 1:2 | 70.9 | 15.9 | 1.7 | 6.1 | 1.9 |
| 1:3 | 68.3 | 11.6 | 7.4 | 5.4 | 4.3 |
| Model | Parameters | Units | Values |
|---|---|---|---|
| LIM | qmax | mg/g | 4.62 |
| KL | L/mg | 0.0025 | |
| R2 | 0.707 | ||
| FIM | KF | mg/g | 1.67505 |
| 1/n | 0.98 | ||
| R2 | 0.92 |
| Model | Parameters | Units | Values |
|---|---|---|---|
| PFO model | qe, exp | mg/g | 802 |
| qe | mg/g | 160.737 | |
| K1 | min−1 | 0.0008 | |
| R2 | 0.653 | ||
| PSO model | qe | mg/g | 746.26 |
| K2 | g/mg/min | 0.000107 | |
| R2 | 0.999 | ||
| W&M model | Ki | mg/g/min1/2 | 119.41 |
| R2 | 0.98 |
| Adsorbent | Surface Area (m2/g) | Adsorption Capacity | References |
|---|---|---|---|
| Eucalyptus bark | 6.1178 | [63] | |
| Palm bark powder biochar | 1.80 | 59% | [60] |
| Zeolite Carbon | 60.94 | 3.23 mg/g | [64] |
| Palm waste | 272 | [65] | |
| Oil palm leaves carbon | 122 | [66] | |
| Activated carbon from red algae | 0.8950 | [67] | |
| Coal AC Magnetic Coal AC | 29.7 53.4 | [68] | |
| Amygdalus scoparia AC | 209.3 | [69] | |
| OPLAC | 331.153 | 4.62 mg/g, 59.6 ± 5% | This study |
| Sludge magnetic biochar | 47% | [70] | |
| Sugarcane Bagasse AC | 58.73% | [71] | |
| Commercial walnut shell | 3.93 mg/g | [72] | |
| Commercial activated carbon | 99.02% | [17] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khurshid, H.; Mustafa, M.R.U.; Isa, M.H. Modified Activated Carbon Synthesized from Oil Palm Leaves Waste as a Novel Green Adsorbent for Chemical Oxygen Demand in Produced Water. Sustainability 2022, 14, 1986. https://doi.org/10.3390/su14041986
Khurshid H, Mustafa MRU, Isa MH. Modified Activated Carbon Synthesized from Oil Palm Leaves Waste as a Novel Green Adsorbent for Chemical Oxygen Demand in Produced Water. Sustainability. 2022; 14(4):1986. https://doi.org/10.3390/su14041986
Chicago/Turabian StyleKhurshid, Hifsa, Muhammad Raza Ul Mustafa, and Mohamed Hasnain Isa. 2022. "Modified Activated Carbon Synthesized from Oil Palm Leaves Waste as a Novel Green Adsorbent for Chemical Oxygen Demand in Produced Water" Sustainability 14, no. 4: 1986. https://doi.org/10.3390/su14041986








