Catalytic Ozonation of the Secondary Effluents from the Largest Chinese Petrochemical Wastewater Treatment Plant—A Stability Assessment
Abstract
:1. Introduction
2. Materials and Methods
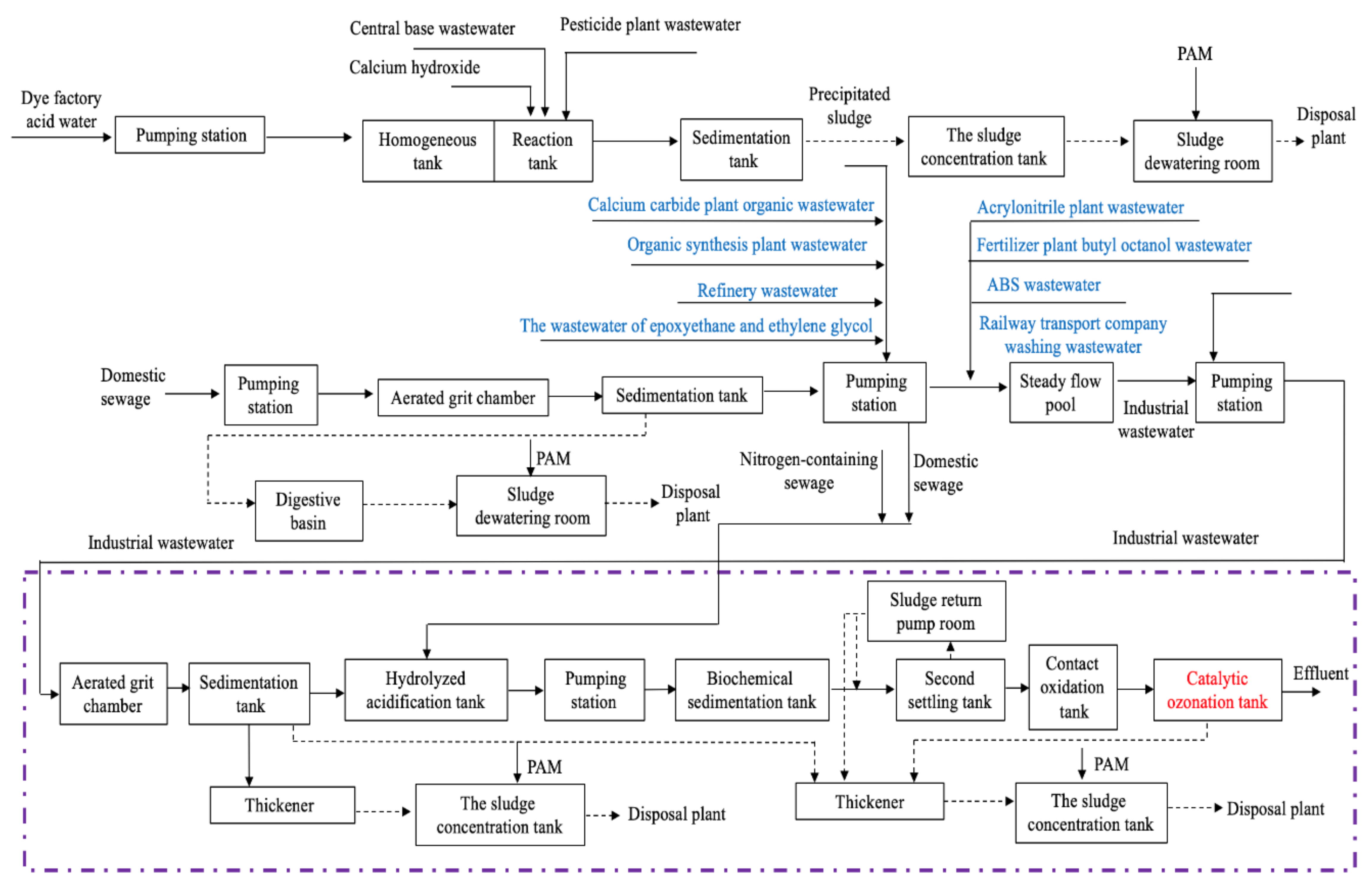
2.1. The Petrochemical WWTP
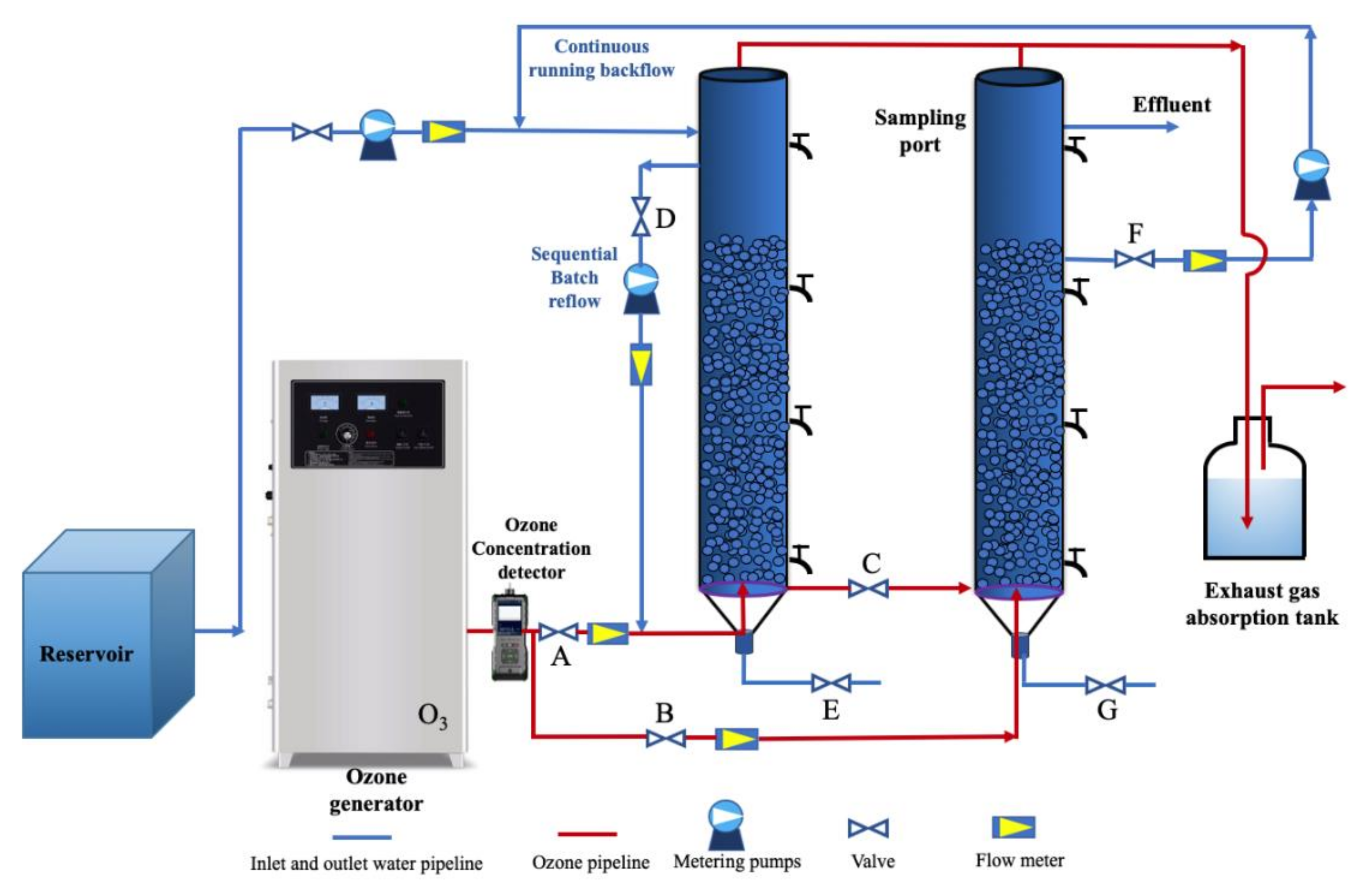
2.2. Experimental Set-Up
2.3. Wastewater Treatment Process
2.4. Analytic Items and Methods
2.4.1. Water Sample Test Methods
2.4.2. Stability Assessment Indicators and Evaluation Criteria
3. Results and Discussion
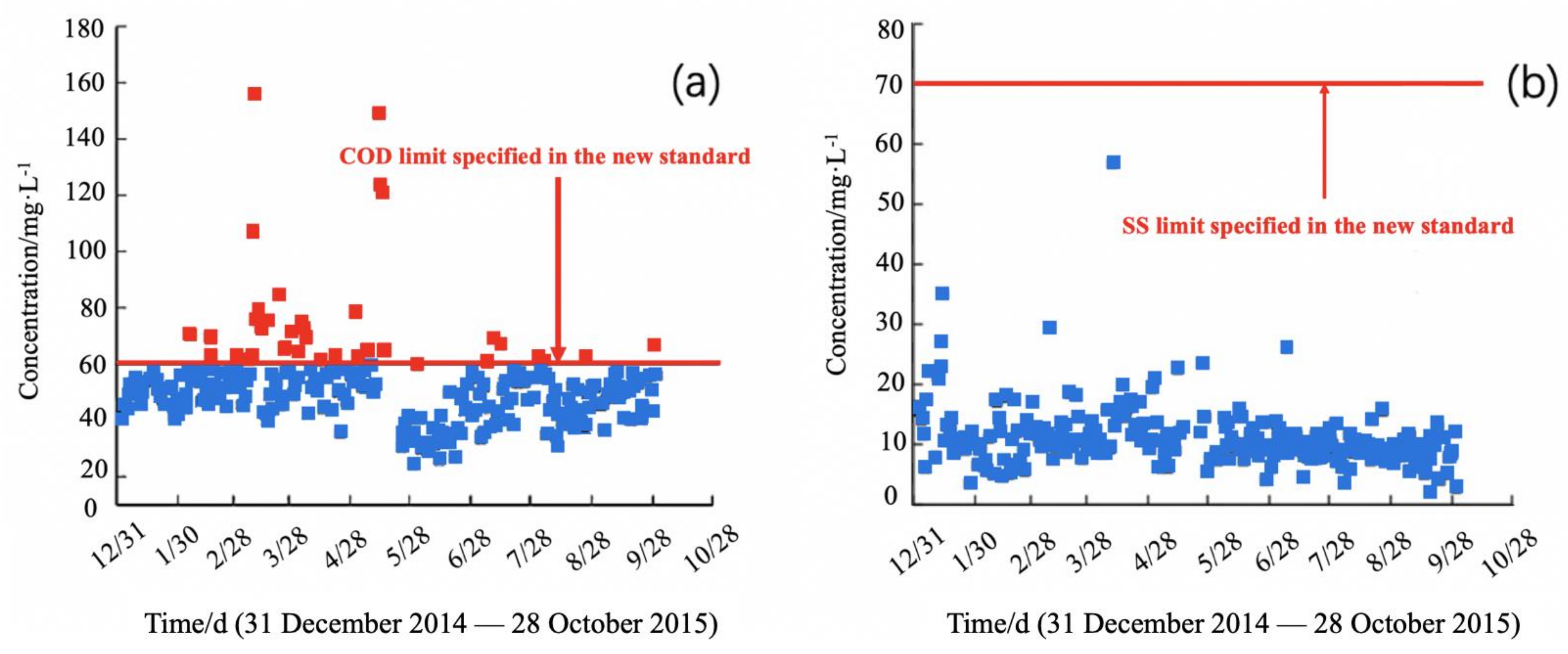
3.1. Stability Assessment
3.2. Influencing Parameters
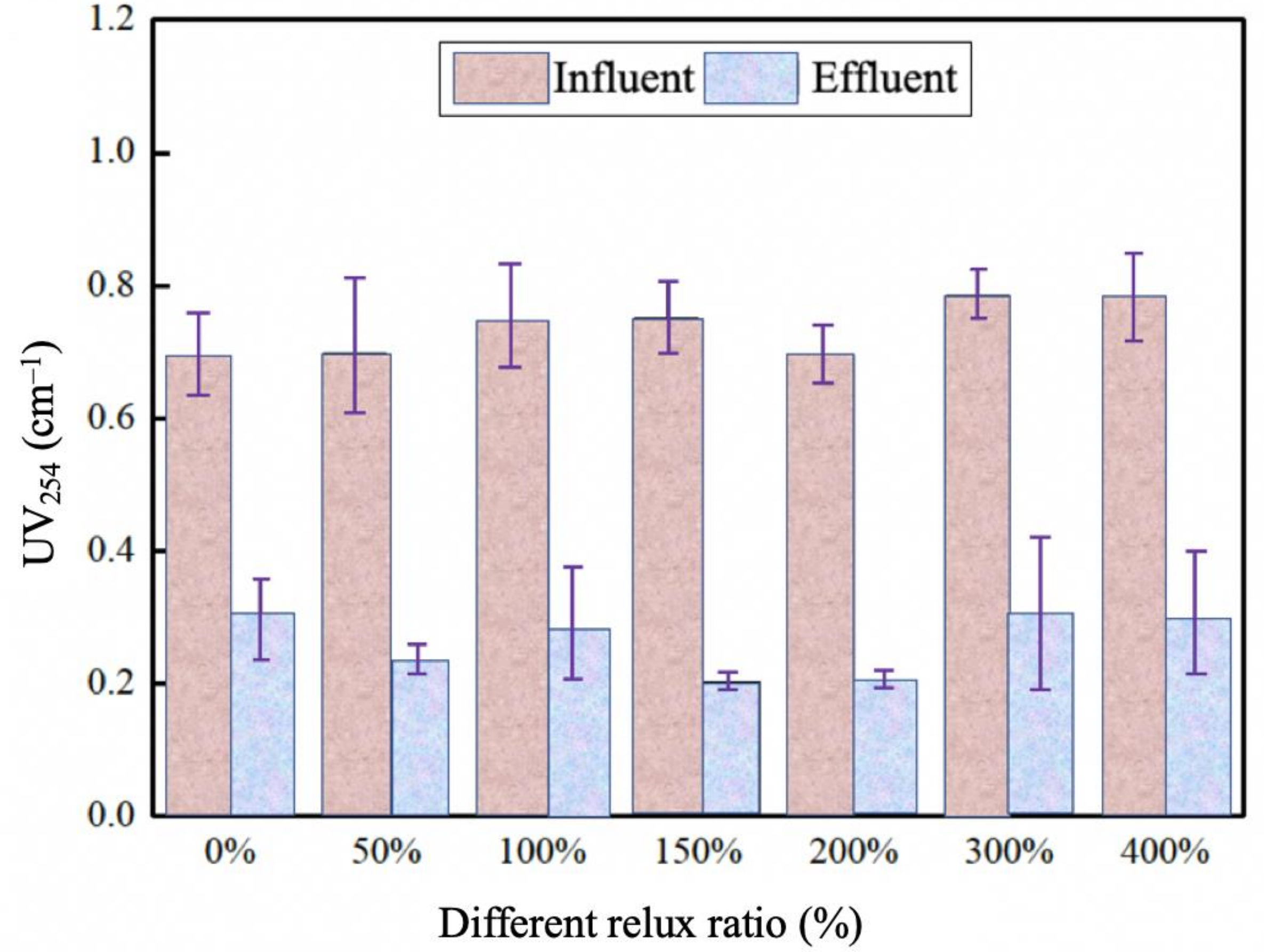
3.2.1. Reflux Ratios
Effect of Different Reflux Ratio on COD Removal by Catalytic Ozonation
Effect of Different Reflux Ratio on UV254 by Catalytic Ozonation
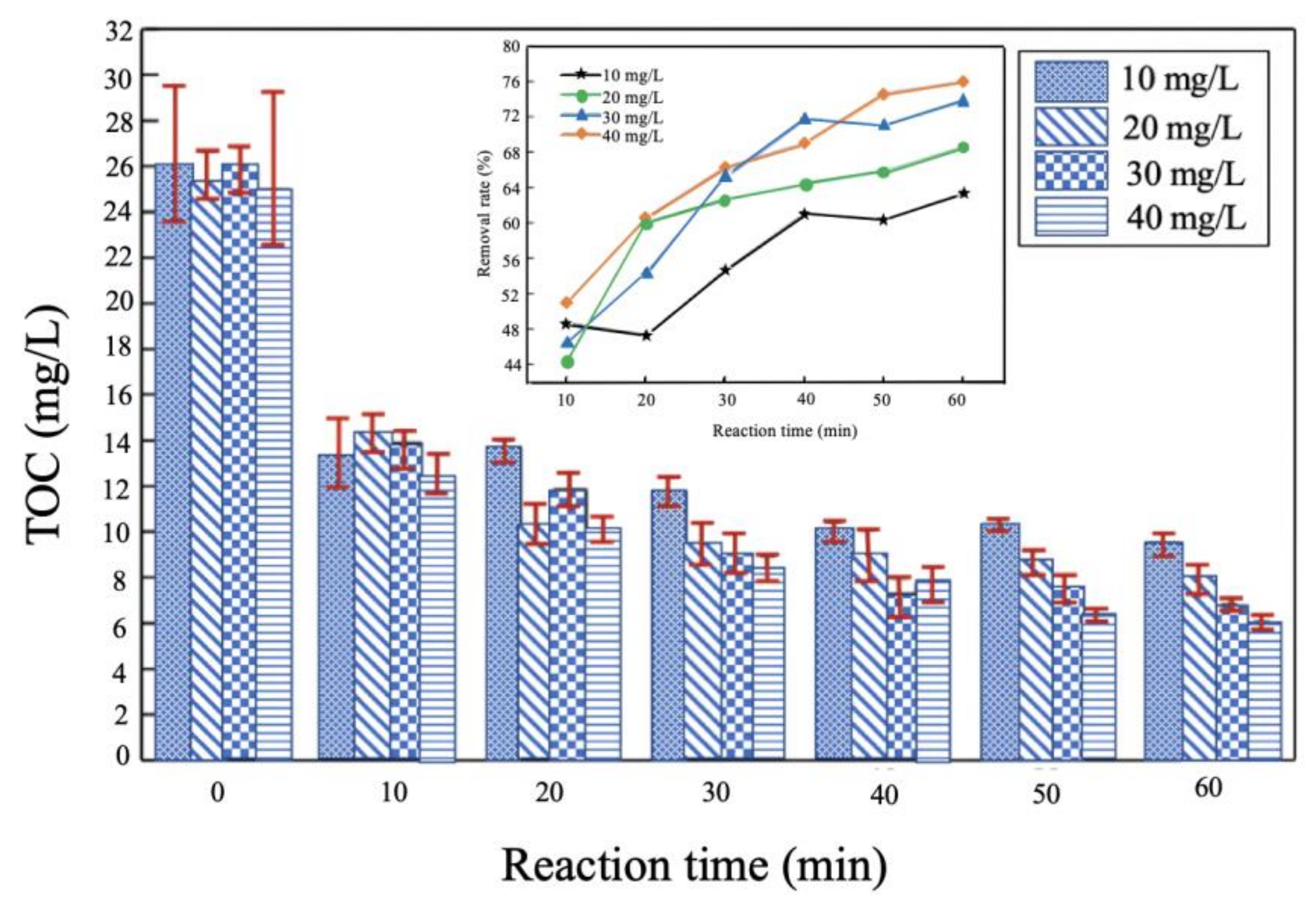
3.2.2. Ozone Concentration
3.2.3. Effect of Different SS Concentrations
3.2.4. Effect of Different Aeration Ratios
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of nanomaterials in the treatment of wastewater: A review. Water 2020, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Environmental Protection of the People’s Republic of China (MEPPRC). Emission of Pollutants in Petrochemical Industry; Ministry of Environmental Protection of the People’s Republic of China (MEPPRC): Beijing, China, 2015.
- Yaqoob, A.A.; Mohd Noor, N.H.B.; Serrà, A.; Ibrahim, M.N.M. Advances and Challenges in Developing Efficient Graphene Oxide-Based ZnO Photocatalysts for Dye Photo-Oxidation. Nanomaterials 2020, 10, 932. [Google Scholar] [CrossRef] [PubMed]
- Sui, M.; Sheng, L.; Lu, K.; Tian, F. FeOOH catalytic ozonation of oxalic acid and the effect of phosphate binding on its catalytic activity. Appl. Catal. B Environ. 2010, 96, 94–100. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Ahmad, A.; Reddy, A.V.B. Toxicology and Environmental Application of Carbon Nanocomposite. In Environmental Remediation through Carbon Based Nano Composites; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–18. [Google Scholar]
- Umar, K.; Yaqoob, A.A.; Ibrahim, M.N.M.; Parveen, T.; Safian, M.T. Environmental applications of smart polymer composites. In Smart Polymer Nanocomposites: Biomedical and Environmental Applications; Bhawani, S.A., Khan, A., Jawaid, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 295–312. ISBN 978-0-12-819961-9. [Google Scholar]
- Zhou, X.; Li, Y.; Zhao, Y.; Yue, X. Pilot-scale anaerobic/anoxic/oxic/oxic biofilm process treating coking wastewater. J. Chem. Technol. Biotechnol. 2012, 88, 305–310. [Google Scholar] [CrossRef]
- Fu, L.; Wu, C.; Zhou, Y.; Zuo, J.; Song, G.; Tan, Y. Ozonation reactivity characteristics of dissolved organic matter in secondary petrochemical wastewater by single ozone, ozone/H2O2, and ozone/catalyst. Chemosphere 2019, 233, 34–43. [Google Scholar] [CrossRef]
- Geng, C.J.; Pu, W.J.; Gao, W.; Wang, H.W.; Zhong, D.H.; Rao, H.K. Pilot study on treatment of secondary effluent of sewage treatment plant by ozone catalytic oxidation. Sci. Technol. Chem. Ind. 2016, 24, 44–46. [Google Scholar]
- Cui, D.; Pang, C.L.; Kong, X.Z.; Chen, X.; Li, A.; Ma, F.; Yang, J.X. Application of ozone/photocatalytic oxidation to advanced treatment of printing and dyeing wastewater. China Water Wastewater 2013, 29, 114–116. [Google Scholar]
- Chu, J.Y.; Chen, J.N.; Wang, C.; Fu, P. Wastewater reuse potential analysis: Implications for China’s water resources management. Water Res. 2004, 38, 2746–2756. [Google Scholar] [CrossRef]
- Wang, M. Study on the Nitrogen Removal of Anaerobic Ammonia Oxidation in Oil Refinery Wastewater. Master’s Thesis, Beijing University of Technology, Beijing, China, 2016. [Google Scholar]
- Andrés-Doménech, I.; Montanari, A.; Marco, J.B. Efficiency of Storm Detention Tanks for Urban Drainage Systems under Climate Variability. J. Water Resour. Plan. Manag. 2012, 138, 36–46. [Google Scholar] [CrossRef]
- Kamali, M.; Costa, M.E.; Aminabhavi, T.M.; Capela, I. Sustainability of treatment technologies for industrial biowastes effluents. Chem. Eng. J. 2019, 368, 474–494. [Google Scholar] [CrossRef]
- Guerrero, J.; Guisasola, A.; Comas, J.; Rodríguez-Roda, I.; Baeza, J.A. Multi-criteria selection of optimum WWTP control set-points based on microbiology-related failures, effluent quality and operating costs. Chem. Eng. J. 2012, 188, 23–29. [Google Scholar] [CrossRef]
- Martínez, S.A.; Rodríguez, M.G.; Morales, M.A. Stability Analysis of an Activated Sludge Bioreactor at a Petrochemical Plant at Different Temperatures. Int. J. Chem. React. Eng. 2006, 3, 1–7. [Google Scholar] [CrossRef]
- Ødegaard, H.; Skrøvseth, A.F. An evaluation of performance and process stability of different processes for small wastewater treatment plants. Water Sci. Technol. 1997, 35, 119–127. [Google Scholar] [CrossRef]
- Rakness, K.; Gordon, G.; Langlais, B.; Masschelein, W.; Matsumoto, N.; Richard, Y.; Robson, C.M.; Somiya, I. Guideline for Measurement of Ozone Concentration in the Process Gas from an Ozone Generator. Ozone Sci. Eng. 1996, 18, 209–229. [Google Scholar] [CrossRef]
- Bader, H.; Hoigné, J. Determination of ozone in water by the indigo method. Water Res. 1981, 15, 449–456. [Google Scholar] [CrossRef]
- Zhou, Q.; Cabaniss, S.E.; Maurice, P.A. Considerations in the use of high-pressure size exclusion chromatography (HPSEC) for determining molecular weights of aquatic humic substances. Water Res. 2000, 34, 3505–3514. [Google Scholar] [CrossRef]
- State Environmental Protection Administration of China (SEPA). Monitoring and Analysis Methods of Water and Wastewater, 4th ed.; Environmental Science Press: Beijing, China, 2002.
- Namguk, H.; Gray, A.; David, F.; Jaeweon, C. Varations of molecular weight estimation by HP-size exclusion chromatography with UVA versus online DOC detection. Environ. Sci. Technol. 2002, 36, 3393–3399. [Google Scholar]
- Su, W.; Du, P.F.; Chen, J.N. Preliminary Study on Evaluation Method of Operational Stability of Urban Sewage Treatment Plant. Environ. Pollut. Control Technol. Equip. 2005, 6, 85–87. [Google Scholar]
- Shi, X.L.; Hu, X.B.; Wang, Z.; Ding, L.L.; Ren, H.Q. Effect of reflux ratio on COD and nitrogen removals from coke plant wastewaters. Water Sci. Technol. 2010, 61, 3017–3025. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Sun, X.; Fu, L. The recent development of advanced wastewater treatment by ozone and biological aerated filter. Environ. Sci. Pollut. Res. 2018, 25, 8315–8329. [Google Scholar] [CrossRef]
- Altmann, J.; Massa, L.; Sperlich, A.; Gnirss, R.; Jekel, M. UV254 absorbance as real-time monitoring and control parameter for micropollutant removal in advanced wastewater treatment with powdered activated carbon. Water Res. 2016, 94, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Wert, E.C.; Rosario-Ortiz, F.L.; Snyder, S.A. Using ultraviolet absorbance and color to assess pharmaceutical oxidation during ozonation of wastewater. Environ. Sci. Technol. 2009, 43, 4858–4863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Sheng, F.; Chen, W.T. Indication of UV254 in the treatment of coal gasification wastewater. J. Environ. Eng. 2015, 9, 1809–1814. [Google Scholar]
- Zheng, X.Y.; Wang, X.L.; Li, X.X. Study on advanced treatment of effluent from advanced treatment by ozone oxidation. China Environ. Sci. 2014, 5, 1159–1165. [Google Scholar]
- Zhang, S.; Wu, C.; Zhou, Y.; Wang, Y.; He, X. Effect of wastewater particles on catalytic ozonation in the advanced treatment of petrochemical secondary effluent. Chem. Eng. J. 2018, 345, 280–289. [Google Scholar] [CrossRef]
- Tian, X.M.; Song, Y.D.; Shen, Z.Q.; Zhou, Y.X.; Wang, K.J.; Jin, X.G.; Zhen, Z.F.; Liu, T. A comprehensive review on toxic pet-rochemical wastewater pretreatment and advanced treatment. J. Clean Prod. 2019, 245, 118692. [Google Scholar] [CrossRef]



| No. | Effluent Source | Water Volume (m3/h) | COD (mg/L) | COD (g/h) |
|---|---|---|---|---|
| 1 | The wastewater of the dye factory | 227.14 | 662.46 | 150,471.16 |
| 2 | The wastewater of fertilizer plant | 90.35 | 2179.39 | 196,907.89 |
| 3 | The wastewater of acrylonitrile plant | 370.79 | 394.03 | 146,102.38 |
| 4 | The wastewater of calcium carbide plant | 221.89 | 1485.1 | 329,528.84 |
| 5 | Organic wastewater of calcium carbide plant | 201.27 | 1223.79 | 246,312.21 |
| 6 | Glycol wastewater of glycol plant | 70.59 | 612.95 | 43,268.14 |
| 7 | Ethylene oxide wastewater of glycol plant | 31.09 | 376.52 | 11,706.01 |
| 8 | The wastewater of the organic synthesis plant | 545.66 | 374.56 | 204,382.41 |
| 9 | The wastewater of refinery plant | 1089.54 | 412.41 | 449,337.19 |
| 10 | The wastewater of synthetic resin plant | 176.77 | 409.11 | 72,318.37 |
| 11 | The wastewater of fine chemicals factory | 11.76 | 566.56 | 6662.75 |
| 12 | Lusheng pesticide factory | 11.06 | 1281.79 | 14,176.60 |
| 13 | CITIC Chemical Co., Ltd. wastewater | 5.44 | 2049.64 | 11,150.04 |
| 14 | Yingtai Chemical Co., Ltd. | 0.73 | 2049.64 | 1496.24 |
| 15 | Fu Landa Chemical Co., Ltd. | 0.35 | 26.91 | 9.42 |
| 16 | Zongheng Plastic Packaging Co., Ltd. | 5.27 | 4110 | 21,659.70 |
| 17 | Northern cleaning | 12.09 | 179.6 | 2171.36 |
| 18 | Entering industry and trade | 0.05 | 120.77 | 6.04 |
| 19 | Bei Dagou municipal drainage | 350.00 | 240 | 84,000.00 |
| 20 | Industrial park drainage | 84.44 | 225 | 18,999.00 |
| 21 | Buried plant permeate | 10.00 | 1300 | 13,000.00 |
| 22 | 805, 806 tank drainage | 100.00 | 50 | 5000.00 |
| 23 | Domestic waste heaping plant permeate | 0.00 | 0 | 0.00 |
| 24 | Domestic sewage | 1412.28 | 224.57 | 317,155.72 |
| 25 | Ash dam drainage | 100.00 | 120 | 12,000.00 |
| 26 | Fertilizer plant containing nitrogen water | 100.00 | 150 | 15,000.00 |
| Subtotal | 5228.56 | 453.84 | 2,372,921.47 | |
| New wastewater volume (Standardization) | 767.76 | 318.8 | 244,763.40 | |
| Direct drainage water consumption | 473 | 30 | 14,190 | |
| Total displacement | 6469.32 | 406.83 | 2,631,874.87 | |
| Items | COD (mg/L) | TOD (mg/L) | BOD5 (mg/L) | NH4+-N (mg/L) | TN (mg/L) | TP (mg/L) | SS (mg/L) |
|---|---|---|---|---|---|---|---|
| Secondary effluent before catalytic ozonation | 84.7 ± 20.9 | 26.0 ± 8.90 | 5.22 ± 0.62 | 1.22 ± 2.85 | 13.6 ± 2.11 | 0.90 ± 0.25 | 26.6 ± 12.4 |
| GB 8978-1996 * | 100 | - | 30 | 15 | - | 0.5 | 70 |
| GB 31571-2015 ** | 60 (50) | 20 (15) | 20 (10) | 5 | 30 | 0.5 | 50 |
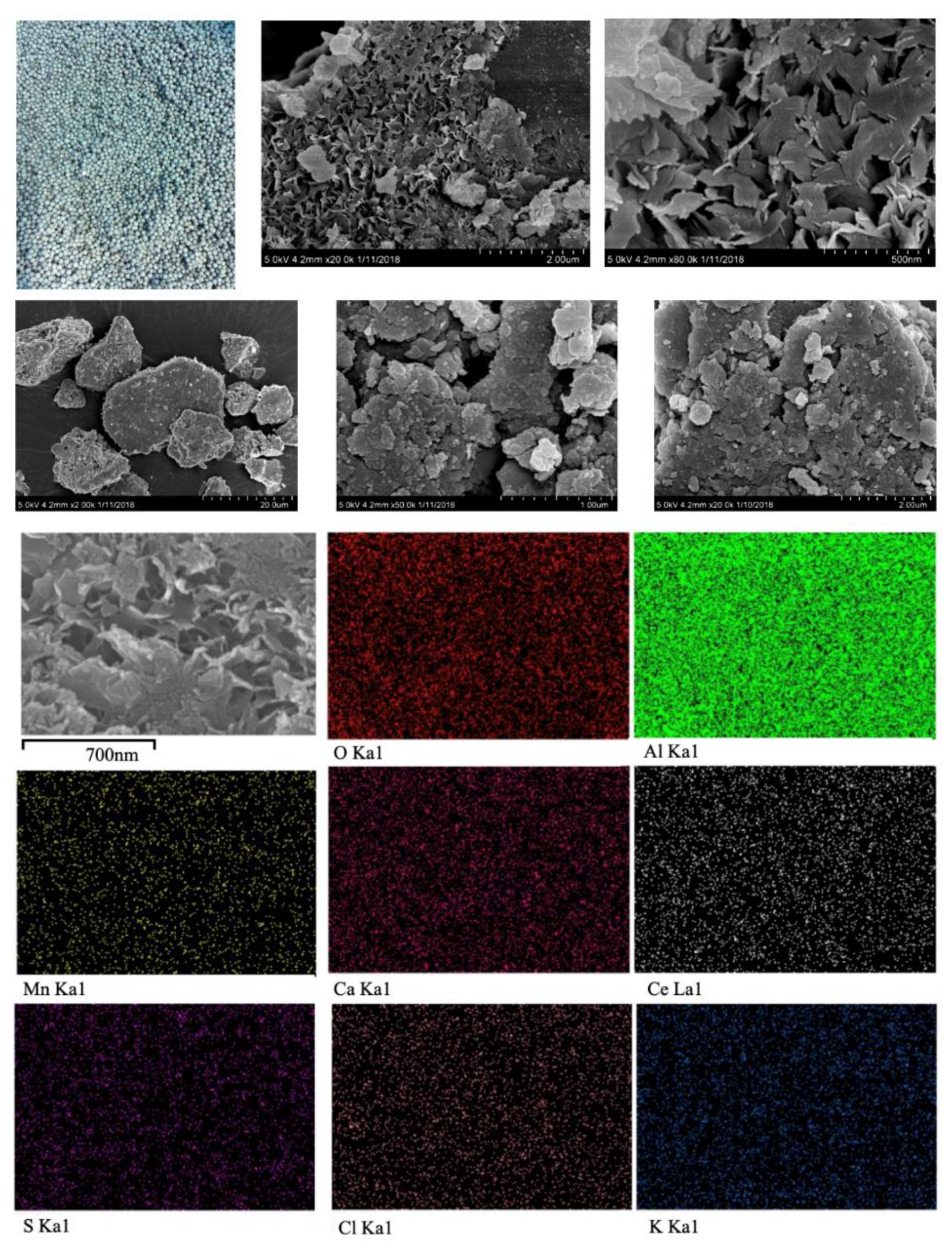
| Element | App | Intensity | Weight % | Weight % | Atomic % |
|---|---|---|---|---|---|
| Conc. | Corrn. | Sigma | |||
| O | 29.06 | 0.8443 | 43.46 | 0.48 | 59.19 |
| Mg | 0.15 | 0.7629 | 0.25 | 0.06 | 0.23 |
| Al | 29.55 | 0.8466 | 44.06 | 0.43 | 35.59 |
| S | 0.76 | 0.7236 | 1.32 | 0.13 | 0.90 |
| Cl | 0.91 | 0.6791 | 1.70 | 0.15 | 1.04 |
| K | 1.02 | 0.9749 | 1.32 | 0.15 | 0.73 |
| Ca | 2.11 | 0.9466 | 2.82 | 0.16 | 1.53 |
| Ce | 3.25 | 0.8101 | 5.07 | 0.48 | 0.79 |
| Item | Unit | Value |
|---|---|---|
| Particle size | mm | 2~4 |
| Density | g/mL | 0.66 |
| Specific surface area | m2/g | 206.9 |
| Void volume | cm3/g | 0.47 |
| Compressive strength | N/particle | 137 |
| Wear rate | % | 0.31 |
| Water absorption | % | 47 |
| Qualified size | % | 94 |
| Major catalytic component | - | Activated alumina as a carrier; the ingredients are a trade secret |
| Years of use | year | ≥6 |
| Degree | η | |||
|---|---|---|---|---|
| A | η = 100% | ≤ 0.5 | ≤ 0.5 | ≤ 1 |
| B | η 100% | 0.5 ≤ 1 | 0.5 ≤ 3 | 1 ≤ 1.5 |
| C | - | 1 | 3 | 1.5 |
| No. | η | Vσ | Cso | VR | Evaluation Result |
|---|---|---|---|---|---|
| 1 | A | A | A | A | 1 |
| 2 | A | A | A | B | 1 |
| 3 | A | A | A | C | 2 |
| 4 | A | A | B | A | 1 |
| 5 | A | A | B | B | 2 |
| 6 | A | A | B | C | 2 |
| 7 | A | A | C | A | 2 |
| 8 | A | A | C | B | 3 |
| 9 | A | A | C | C | 3 |
| 10 | A | B | B | B | 3 |
| 11 | A | B | B | C | 4 |
| 12 | A | B | C | B | 3 |
| 13 | A | B | C | C | 4 |
| 14 | A | C | C | C | 4 |
| 15 | B | A | A | A | 3 |
| 16 | B | A | A | B | 3 |
| 17 | B | A | A | C | 3 |
| 18 | B | A | B | A | 3 |
| 19 | B | A | B | B | 3 |
| 20 | B | A | B | C | 3 |
| 21 | B | A | C | A | 3 |
| 22 | B | A | C | B | 4 |
| 23 | B | A | C | C | 4 |
| 24 | B | B | B | B | 4 |
| 25 | B | B | B | C | 4 |
| 26 | B | B | C | B | 4 |
| 27 | B | B | C | C | 4 |
| 28 | B | C | C | C | 4 |
| Remarks | (1) Evaluation result 1 means the water quality of long-term running effluent meets the standard and has good stability. The time series are concentrated near the average value, and there is no abnormal value. | ||||
| (2) Evaluation result 2 means the water quality of long-term operation effluent meets the standard and has good stability. Most of the values are relatively concentrated, some values are abnormal, or the values are evenly dispersed | |||||
| (3) Evaluation result 3 means, in the long-term operation process, the effluent quality may not meet the standard, the operation stability may be general, the time series is scattered, and there are many abnormal values. | |||||
| (4) Evaluation result 4 means, in the long-term operation process, there must be a situation that the effluent quality cannot meet the standard. The operation stability is poor and the time series dispersion is high. | |||||
| Contaminants | η | |||
|---|---|---|---|---|
| COD | 92.6% | 0.37 | 3.00 | 3.14 |
| SS | 100% | 0.46 | 3.75 | 4.82 |
| Contaminants | η | Evaluation Result | |||
|---|---|---|---|---|---|
| COD | B | A | B | C | 3 |
| SS | A | A | C | C | 3 |
| Aeration Ratio | Ozone in Water (mg/L) | Ozone in the Exhaust Gas (>mg/L) | Ozone Concentration (mg/L) | Transfer Rate (%) | Utilization Rate (%) |
|---|---|---|---|---|---|
| 1:1 | 0.21 | 0.82 | 36 | 96.59 | 98.50 |
| 2:1 | 0.12 | 0.60 | 36 | 97.49 | 99.37 |
| 3:1 | 0.19 | 0.55 | 36 | 97.69 | 99.12 |
| 4:1 | 0.19 | 0.50 | 36 | 97.90 | 98.72 |
| 5:1 | 0.34 | 0.60 | 36 | 97.41 | 98.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wang, H.; Zhou, Y.; Kamali, M.; He, X.; Khalaj, M.; Xia, Y. Catalytic Ozonation of the Secondary Effluents from the Largest Chinese Petrochemical Wastewater Treatment Plant—A Stability Assessment. Sustainability 2022, 14, 2200. https://doi.org/10.3390/su14042200
Zhang S, Wang H, Zhou Y, Kamali M, He X, Khalaj M, Xia Y. Catalytic Ozonation of the Secondary Effluents from the Largest Chinese Petrochemical Wastewater Treatment Plant—A Stability Assessment. Sustainability. 2022; 14(4):2200. https://doi.org/10.3390/su14042200
Chicago/Turabian StyleZhang, Siyu, Hao Wang, Yuexi Zhou, Mohammadreza Kamali, Xuwen He, Mohammadreza Khalaj, and Yu Xia. 2022. "Catalytic Ozonation of the Secondary Effluents from the Largest Chinese Petrochemical Wastewater Treatment Plant—A Stability Assessment" Sustainability 14, no. 4: 2200. https://doi.org/10.3390/su14042200






