Effects of Extracellular Polymeric Substances and Specific Compositions on Enhancement of Copper Bioleaching Efficiency from Waste Printed Circuit Boards
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture
2.2. Metal Concentration of Waste PCBs Samples
2.3. Synthetic EPS
2.4. Leaching Experiments
2.5. Analytical Methods
3. Results
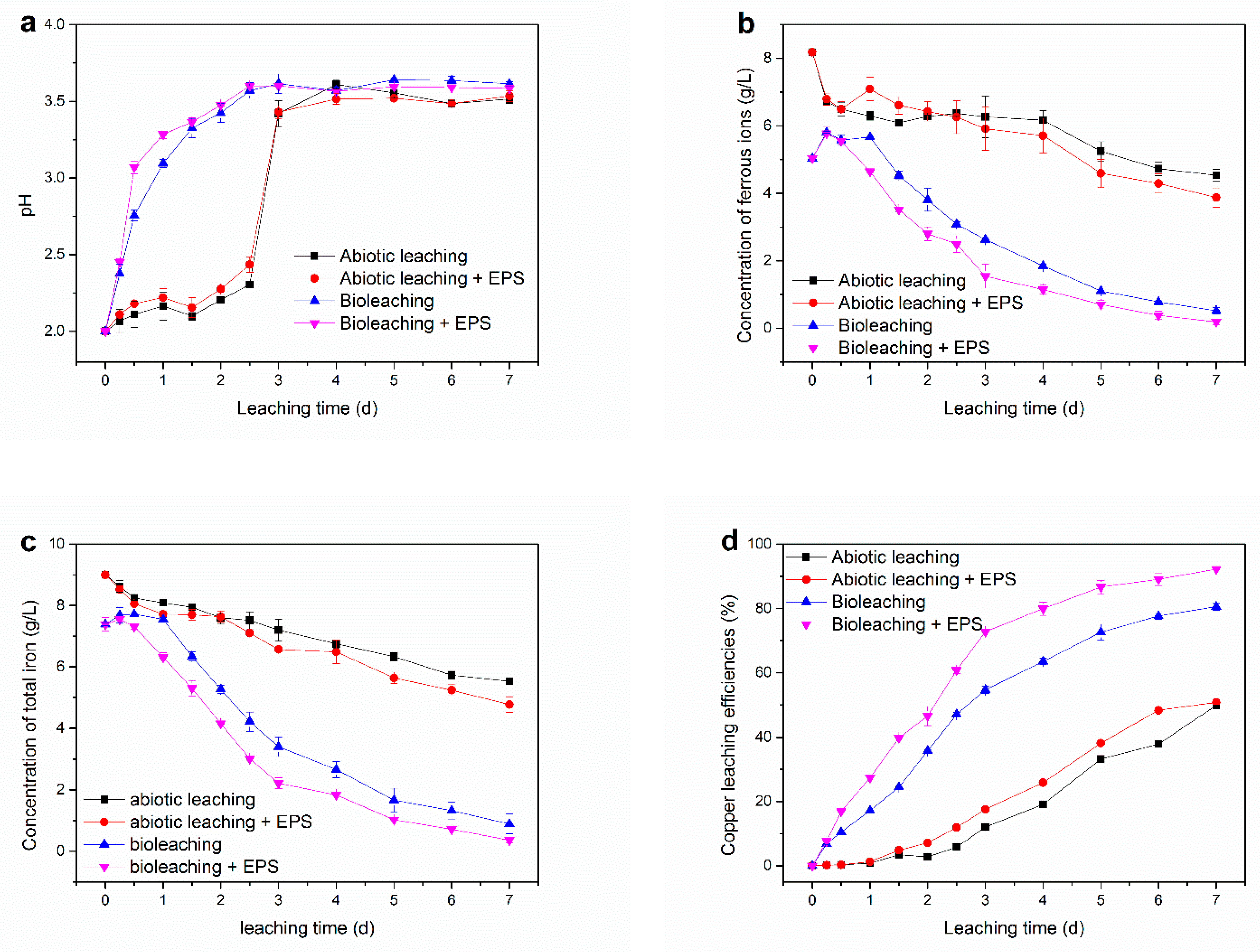
3.1. Synthetic EPS on Leaching Metal Concentrates of Waste PCBs
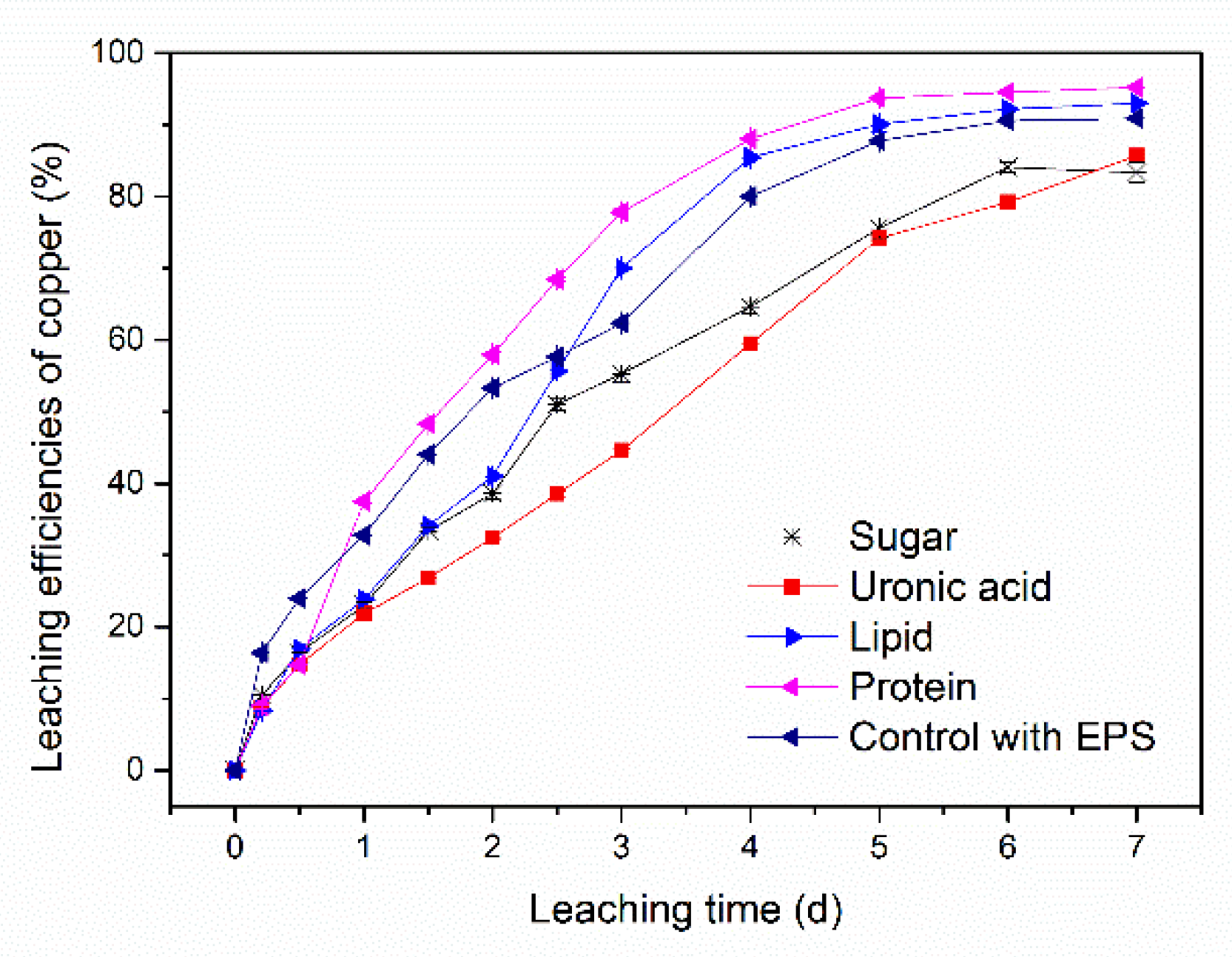
3.2. Function of Different Synthetic Compositions of EPS
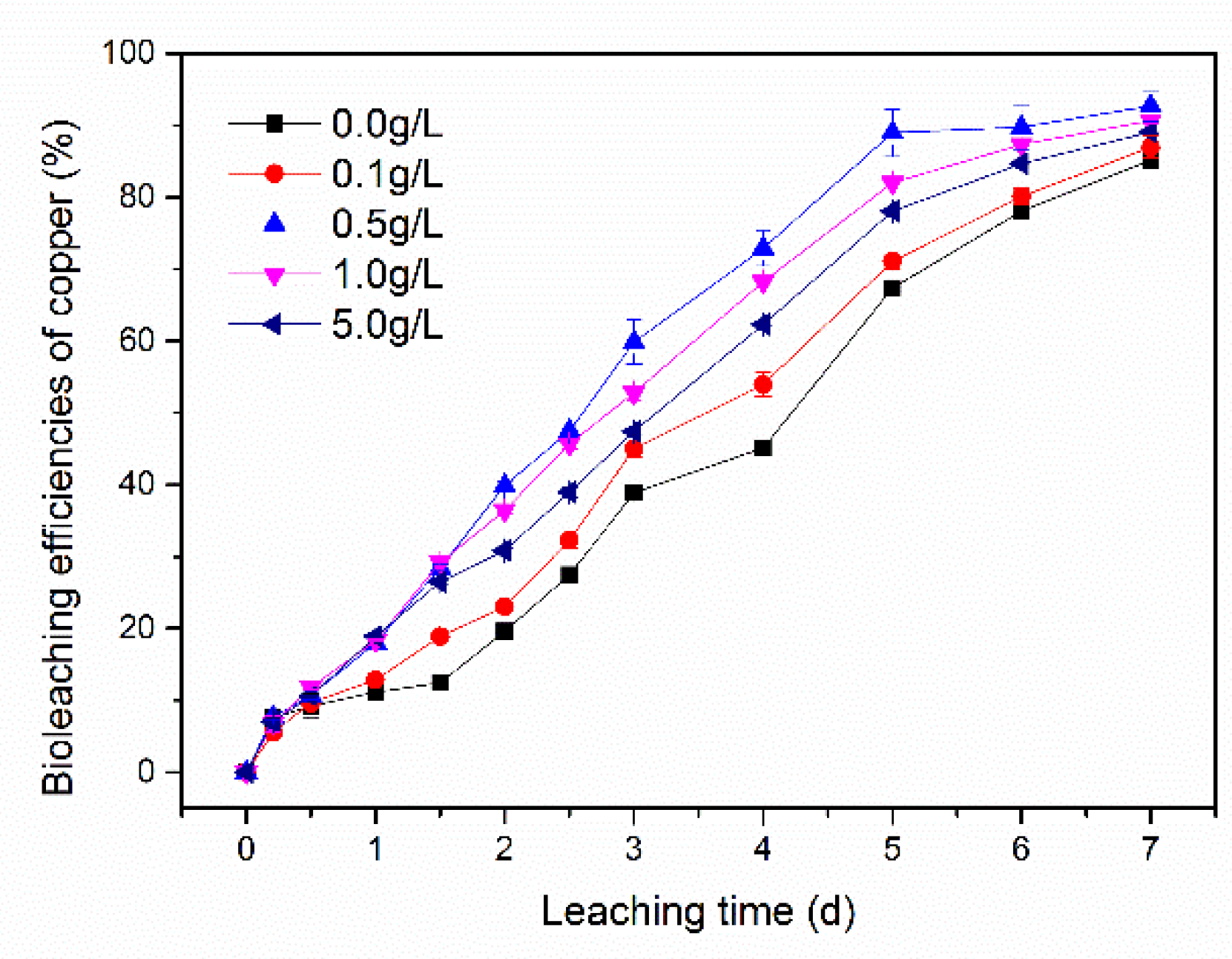
3.3. Effect of Different Concentrations of Synthetic EPS
- Dissolve the precipitate that covers the surface of waste PCBs or sulfide minerals, then bacteria and ferric ion can re-contact and react with copper;
- Re-dissolve the precipitate that entrap leached copper, thus the copper is released back into the bioleaching solution.
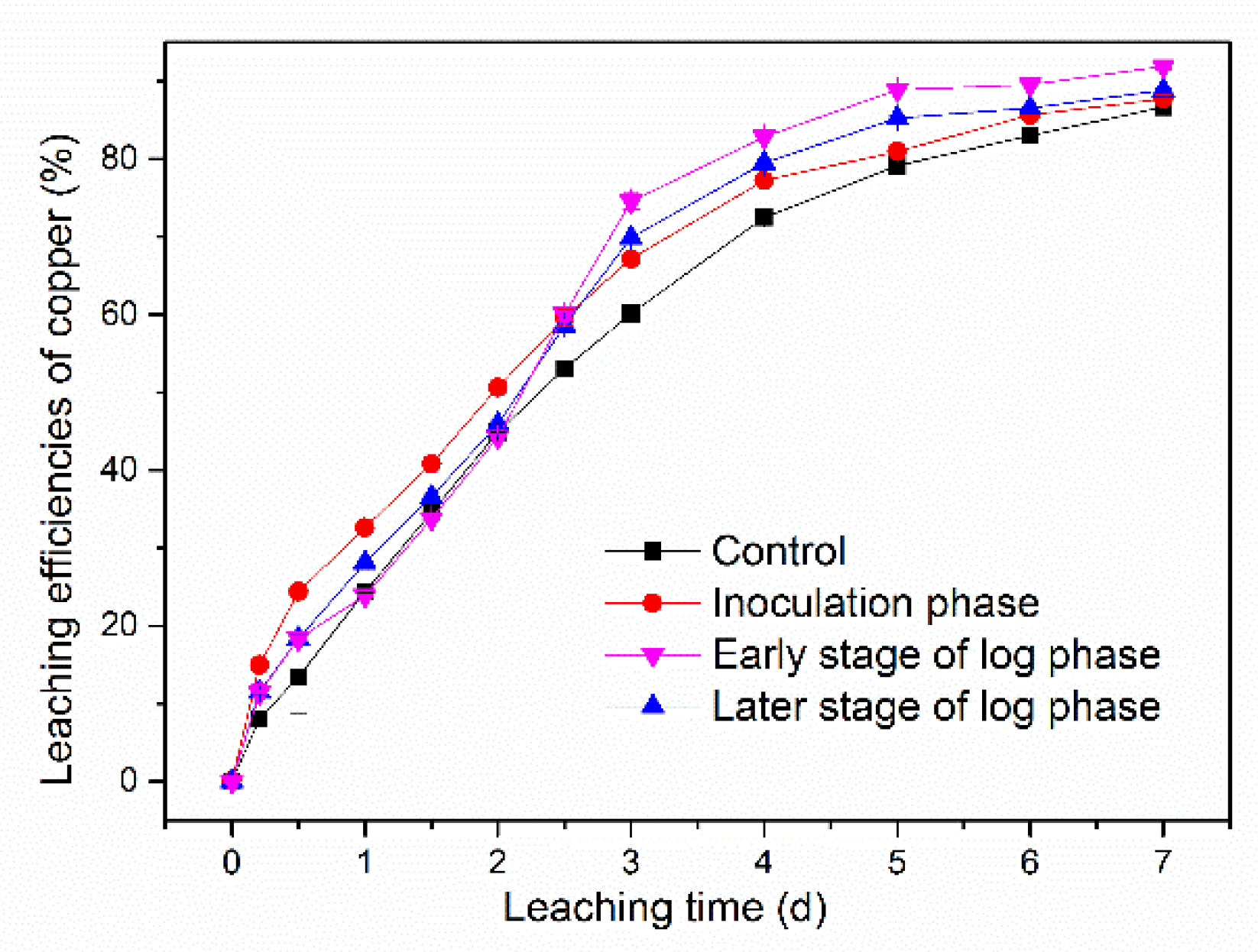
3.4. Effect of Addition of Synthetic EPS at Different Time Points
4. Discussion
4.1. EPS Promote the Bioleaching Process
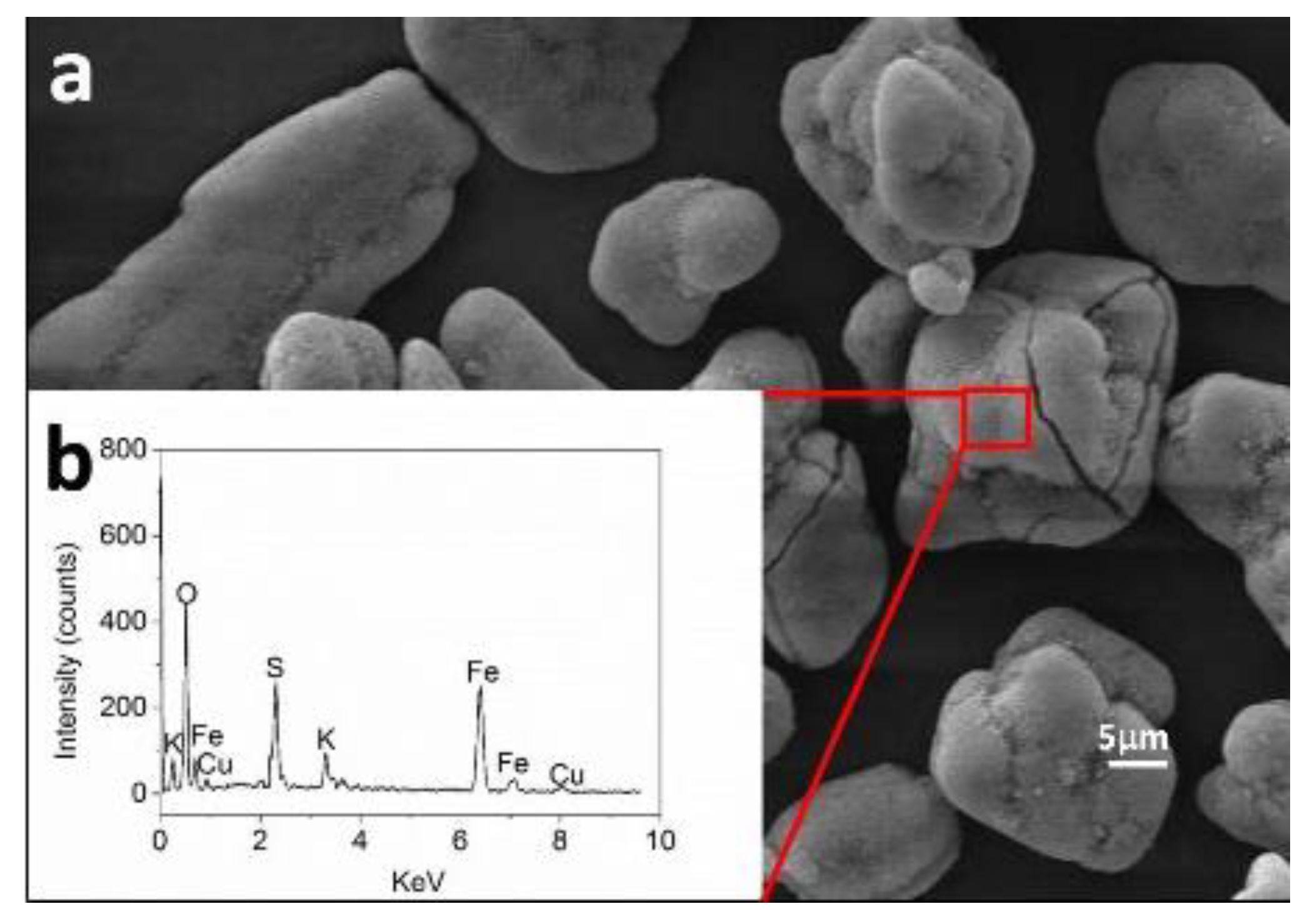
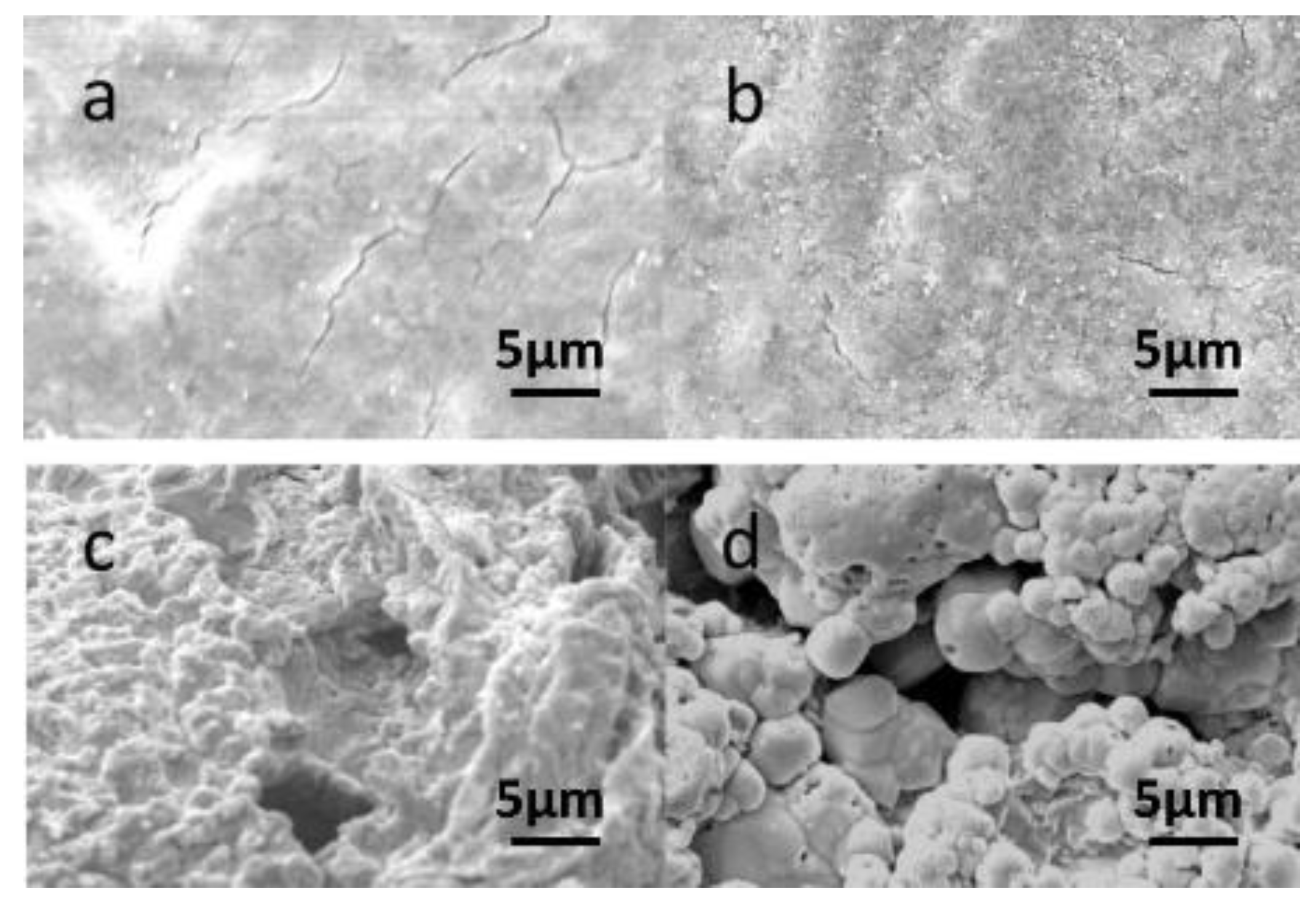
4.2. The Mechanism of Enhancing Bioleaching Process by EPS
4.3. Interaction between EPS and Environment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kitila, A.W.; Woldemikael, S.M. Waste electrical and electronic equipment management in the educational institutions and governmental sector offices of Addis Ababa, Ethiopia. Waste Manag. 2019, 85, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Balde, C.P.; Forti, V.; Gray, V.; Kuehr, R.; Stegmann, P. The Global E-Waste Monitor 2017; United Nations University (UNU): Bonn, Germany; International Telecommunication Union (ITU): Geneva, Switzerland; International Solid Waste Association (ISWA): Vienna, Austria, 2017. [Google Scholar]
- Abdelbasir, S.M.; Hassan, S.S.M.; Kamel, A.H.; El-Nasr, R.S. Status of electronic waste recycling techniques: A review. Environ. Sci. Pollut. Res. 2018, 25, 16533–16547. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, M.; Gurumurthy, K. Enhanced bioleaching of copper from circuit boards of computer waste by Acidithiobacillus ferrooxidans. Environ. Chem. Lett. 2019, 17, 1873–1879. [Google Scholar] [CrossRef]
- Tatariants, M.; Yousef, S.; Sidaraviciute, R.; Denafas, G.; Bendikiene, R. Characterization of waste printed circuit boards recycled using a dissolution approach and ultrasonic treatment at low temperatures. RSC Adv. 2017, 7, 37729–37738. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Y.; Wu, P.; Zhu, N.; Zhang, T.; Liu, W.; Wu, J.; Li, P. Bioleaching of copper from waste printed circuit boards by bacterial consortium enriched from acid mine drainage. J. Hazard. Mater. 2010, 184, 812–818. [Google Scholar] [CrossRef]
- Qiu, R.; Lin, M.; Ruan, J.; Fu, Y.; Hu, J.; Deng, M.; Tang, Y.; Qiu, R. Recovering full metallic resources from waste printed circuit boards: A refined review. J. Clean. Prod. 2020, 244, 118690. [Google Scholar] [CrossRef]
- de Carvalho, L.C.; da Silva, S.R.; Giardini, R.M.N.; de Souza, L.F.C.; Leão, V.A. Bio-oxidation of refractory gold ores containing stibnite and gudmundite. Environ. Technol. Innov. 2019, 15, 100390. [Google Scholar] [CrossRef]
- Wu, C.; Jiang, M.; Hsieh, L.; Cai, Y.; Shen, Y.; Wang, H.; Lin, Q.; Shen, C.; Hu, B.; Lou, L. Feasibility of bioleaching of heavy metals from sediment with indigenous bacteria using agricultural sulfur soil conditioners. Sci. Total Environ. 2020, 703, 134812. [Google Scholar] [CrossRef]
- Peng, T.J.; Zhou, D.; Liu, X.D.; Yu, R.L.; Jiang, T.; Gu, G.H.; Chen, M.; Qiu, G.Z.; Zeng, W.M. Enrichment of ferric iron on mineral surface during bioleaching of chalcopyrite. Trans. Nonferrous Met. Soc. China 2016, 26, 544–550. [Google Scholar] [CrossRef]
- Becci, A.; Amato, A.; Fonti, V.; Karaj, D.; Beolchini, F. An innovative biotechnology for metal recovery from printed circuit boards. Resour. Conserv. Recycl. 2020, 153, 104549. [Google Scholar] [CrossRef]
- Li, J.; Wen, J.; Guo, Y.; An, N.; Liang, C.; Ge, Z. Bioleaching of gold from waste printed circuit boards by alkali-tolerant Pseudomonas fluorescens. Hydrometallurgy 2020, 194, 105260. [Google Scholar] [CrossRef]
- Akbari, S.; Ahmadi, A. Recovery of copper from a mixture of printed circuit boards (PCBs) and sulphidic tailings using bioleaching and solvent extraction processes. Chem. Eng. Process. Process Intensif. 2019, 142, 107584. [Google Scholar] [CrossRef]
- Yang, T.; Xu, Z.; Wen, J.; Yang, L. Factors influencing bioleaching copper from waste printed circuit boards by Acidithiobacillus ferrooxidans. Hydrometallurgy 2009, 97, 29–32. [Google Scholar] [CrossRef]
- d’Abzac, P.; Bordas, F.; van Hullebusch, E.; Lens, P.N.L.; Guibaud, G. Effects of extraction procedures on metal binding properties of extracellular polymeric substances (EPS) from anaerobic granular sludges. Colloids Surf. B Biointerfaces 2010, 80, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, T.; Telegdi, J.; Thierry, D.; Sand, W. Importance of extracellular polymeric substances from Thiobacillus ferrooxidans for bioleaching. Appl. Environ. Microbiol. 1998, 64, 2743–2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, S.; Li, K.; Huang, Z.; Tong, Y.; Yang, H. Specific mechanism of Acidithiobacillus caldus extracellular polymeric substances in the bioleaching of copper-bearing sulfide ore. PLoS ONE 2019, 14, e0213945. [Google Scholar] [CrossRef]
- Afzal Ghauri, M.; Okibe, N.; Barrie Johnson, D. Attachment of acidophilic bacteria to solid surfaces: The significance of species and strain variations. Hydrometallurgy 2007, 85, 72–80. [Google Scholar] [CrossRef]
- Harneit, K.; Göksel, A.; Kock, D.; Klock, J.H.; Gehrke, T.; Sand, W. Adhesion to metal sulfide surfaces by cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans and Leptospirillum ferrooxidans. Hydrometallurgy 2006, 83, 245–254. [Google Scholar] [CrossRef]
- Frølund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- Kinzler, K.; Gehrke, T.; Telegdi, J.; Sand, W. Bioleaching—A result of interfacial processes caused by extracellular polymeric substances (EPS). Hydrometallurgy 2003, 71, 83–88. [Google Scholar] [CrossRef]
- Zeng, W.; Qiu, G.; Zhou, H.; Liu, X.; Chen, M.; Chao, W.; Zhang, C.; Peng, J. Characterization of extracellular polymeric substances extracted during the bioleaching of chalcopyrite concentrate. Hydrometallurgy 2010, 100, 177–180. [Google Scholar] [CrossRef]
- Sand, W.; Gehrke, T. Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron(III) ions and acidophilic bacteria. Res. Microbiol. 2006, 157, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Wolfaardt, G.M.; Lawrence, J.R.; Korber, D.R. Function of EPS. In Microbial Extracellular Polymeric Substances; Wingender, J., Neu, T.R., Flemming, H.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar] [CrossRef]
- Devasia, P.; Natarajan, K.A.; Sathyanarayana, D.N.; Rao, G.R. Surface chemistry of Thiobacillus ferrooxidans relevant to adhesion on mineral surfaces. Appl. Environ. Microbiol. 1993, 59, 4051–4055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nouha, K.; Kumar, R.S.; Balasubramanian, S.; Tyagi, R.D. Critical review of EPS production, synthesis and composition for sludge flocculation. J. Environ. Sci. 2018, 66, 225–245. [Google Scholar] [CrossRef] [Green Version]
- Nie, H.; Yang, C.; Zhu, N.; Wu, P.; Zhang, T.; Zhang, Y.; Xing, Y. Isolation of Acidithiobacillus ferrooxidans strain Z1 and its mechanism of bioleaching copper from waste printed circuit boards. J. Chem. Technol. Biotechnol. 2015, 90, 714–721. [Google Scholar] [CrossRef]
- Choi, M.-S.; Cho, K.-S.; Kim, D.-S.; Kim, D.-J. Microbial Recovery of Copper from Printed Circuit Boards of Waste Computer by Acidithiobacillus ferrooxidans. J. Environ. Sci. Health Part A 2004, 39, 2973–2982. [Google Scholar] [CrossRef]
- Franzmann, P.D.; Haddad, C.M.; Hawkes, R.B.; Robertson, W.J.; Plumb, J.J. Effects of temperature on the rates of iron and sulfur oxidation by selected bioleaching Bacteria and Archaea: Application of the Ratkowsky equation. Miner. Eng. 2005, 18, 1304–1314. [Google Scholar] [CrossRef]
- Yu, R.L.; Zhong, D.L.; Miao, L.; Wu, F.D.; Qiu, G.Z.; Gu, G.H. Relationship and effect of redox potential, jarosites and extracellular polymeric substances in bioleaching chalcopyrite by acidithiobacillus ferrooxidans. Trans. Nonferrous Met. Soc. China 2011, 21, 1634–1640. [Google Scholar] [CrossRef]
- Sand, W.; Gehrke, T.; Jozsa, P.G.; Schippers, A. (Bio)chemistry of bacterial leaching-direct vs. indirect bioleaching. Hydrometallurgy 2001, 59, 159–175. [Google Scholar] [CrossRef]
- Yu, R.L.; Liu, J.; Chen, A.; Zhong, D.L.; Li, Q.; Qin, W.Q.; Qiu, G.Z.; Gu, G.H. Interaction mechanism of Cu2+, Fe3+ ions and extracellular polymeric substances during bioleaching chalcopyrite by Acidithiobacillus ferrooxidans ATCC2370. Trans. Nonferrous Met. Soc. China 2013, 23, 231–236. [Google Scholar] [CrossRef]
- Zhu, N.; Xiang, Y.; Zhang, T.; Wu, P.; Dang, Z.; Li, P.; Wu, J. Bioleaching of metal concentrates of waste printed circuit boards by mixed culture of acidophilic bacteria. J. Hazard. Mater. 2011, 192, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Chang-Li, L.; Jin-Lan, X.; Zhen-Yuan, N.; Yi, Y.; Chen-Yan, M. Effect of sodium chloride on sulfur speciation of chalcopyrite bioleached by the extreme thermophile Acidianus manzaensis. Bioresour. Technol. 2012, 110, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Parhi, P.K.; Nayak, B.D.; Pradhan, N.; Mohapatra, U.B.; Sukla, L.B. Two step meso-acidophilic bioleaching of chalcopyrite containing ball mill spillage and removal of the surface passivation layer. Bioresour. Technol. 2013, 130, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Vera, M.; Schippers, A.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation-part A. Appl. Microbiol. Biotechnol. 2013, 97, 7529–7541. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.; Zhang, D.; Zhang, X.; Xia, Y.; Wu, F.; Chen, S.; Li, L. Bioleaching mechanism of Co and Li from spent lithium-ion battery by the mixed culture of acidophilic sulfur-oxidizing and iron-oxidizing bacteria. Bioresour. Technol. 2009, 100, 6163–6169. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Q.; Jiao, W.; Jiang, H.; Sand, W.; Xia, J.; Liu, X.; Qin, W.; Qiu, G.; Hu, Y.; et al. Adhesion forces between cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans or Leptospirillum ferrooxidans and chalcopyrite. Colloids Surf. B Biointerfaces 2012, 94, 95–100. [Google Scholar] [CrossRef]
- Tourney, J.; Ngwenya, B.T. The role of bacterial extracellular polymeric substances in geomicrobiology. Chem. Geol. 2014, 386, 115–132. [Google Scholar] [CrossRef]
- Vu, B.; Chen, M.; Crawford, R.J.; Ivanova, E.P. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 2009, 14, 2535–2554. [Google Scholar] [CrossRef]
- Bellenberg, S.; Leon-Morales, C.F.; Sand, W.; Vera, M. Visualization of capsular polysaccharide induction in Acidithiobacillus ferrooxidans. Hydrometallurgy 2012, 129–130, 82–89. [Google Scholar] [CrossRef]
- Jiao, Y.; Cody, G.D.; Harding, A.K.; Wilmes, P.; Schrenk, M.; Wheeler, K.E.; Banfield, J.F.; Thelen, M.P. Characterization of extracellular polymeric substances from acidophilic microbial biofilms. Appl. Environ. Microbiol. 2010, 76, 2916–2922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.; Song, Y.X.; Chai, L.Y.; Duan, C.S.; Tang, C.J.; Ali, M.; Peng, C. Comparative evaluation of short-term stress of Cd(II), Hg(II), Pb(II), As(III) and Cr(VI) on anammox granules by batch test. J. Biosci. Bioeng. 2016, 122, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Park, C.S.; Kim, Y.J.; Park, Y. Il Biosorption isotherms of Pb (II) and Zn (II) on Pestan, an extracellular polysaccharide, of Pestalotiopsis sp. KCTC 8637P. Process Biochem. 2006, 41, 312–316. [Google Scholar] [CrossRef]
- Oyetibo, G.O.; Miyauchi, K.; Suzuki, H.; Ishikawa, S.; Endo, G. Extracellular mercury sequestration by exopolymeric substances produced by Yarrowia spp.: Thermodynamics, equilibria, and kinetics studies. J. Biosci. Bioeng. 2016, 122, 701–707. [Google Scholar] [CrossRef]
| Metal | Cu | Sn | Pb | Zn | Fe | Ni | Mg | Rest |
|---|---|---|---|---|---|---|---|---|
| Contents (%) | 68.62 ± 1.01 | 7.48 ± 0.19 | 2.27 ± 0.08 | 0.91 ± 0.05 | 0.28 ± 0.10 | 0.17 ± 0.01 | 0.09 ± 0.06 | 20.18 ± 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zhu, N.; Yang, R.; Yang, C.; Wu, P. Effects of Extracellular Polymeric Substances and Specific Compositions on Enhancement of Copper Bioleaching Efficiency from Waste Printed Circuit Boards. Sustainability 2022, 14, 2503. https://doi.org/10.3390/su14052503
Xu J, Zhu N, Yang R, Yang C, Wu P. Effects of Extracellular Polymeric Substances and Specific Compositions on Enhancement of Copper Bioleaching Efficiency from Waste Printed Circuit Boards. Sustainability. 2022; 14(5):2503. https://doi.org/10.3390/su14052503
Chicago/Turabian StyleXu, Jin, Nengwu Zhu, Ruying Yang, Chong Yang, and Pingxiao Wu. 2022. "Effects of Extracellular Polymeric Substances and Specific Compositions on Enhancement of Copper Bioleaching Efficiency from Waste Printed Circuit Boards" Sustainability 14, no. 5: 2503. https://doi.org/10.3390/su14052503
APA StyleXu, J., Zhu, N., Yang, R., Yang, C., & Wu, P. (2022). Effects of Extracellular Polymeric Substances and Specific Compositions on Enhancement of Copper Bioleaching Efficiency from Waste Printed Circuit Boards. Sustainability, 14(5), 2503. https://doi.org/10.3390/su14052503





