Data Integration Analysis Indicates That Soil Texture and pH Greatly Influence the Acid Buffering Capacity of Global Surface Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search and Data Collection
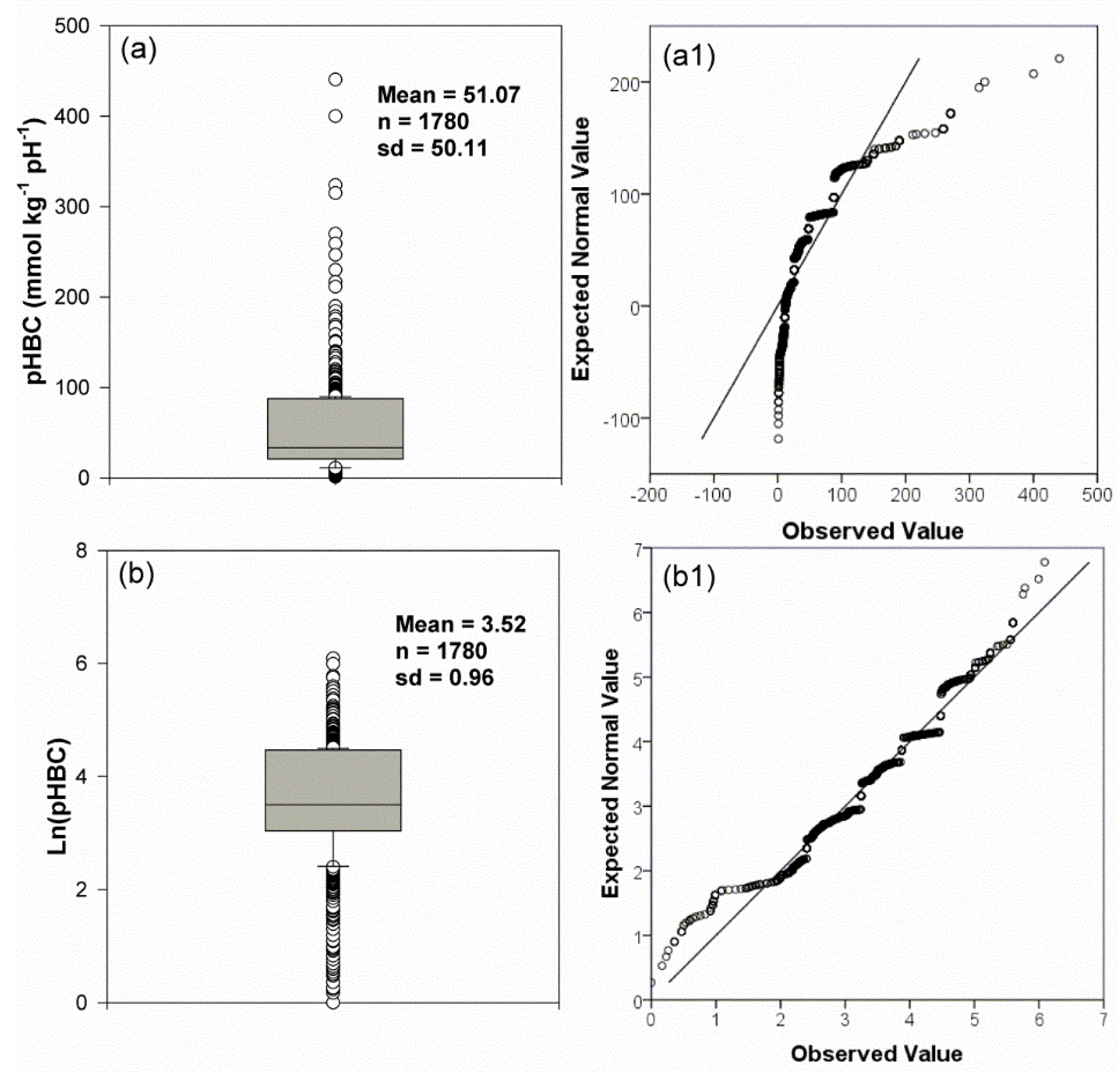
2.2. Data Processing and Analyses
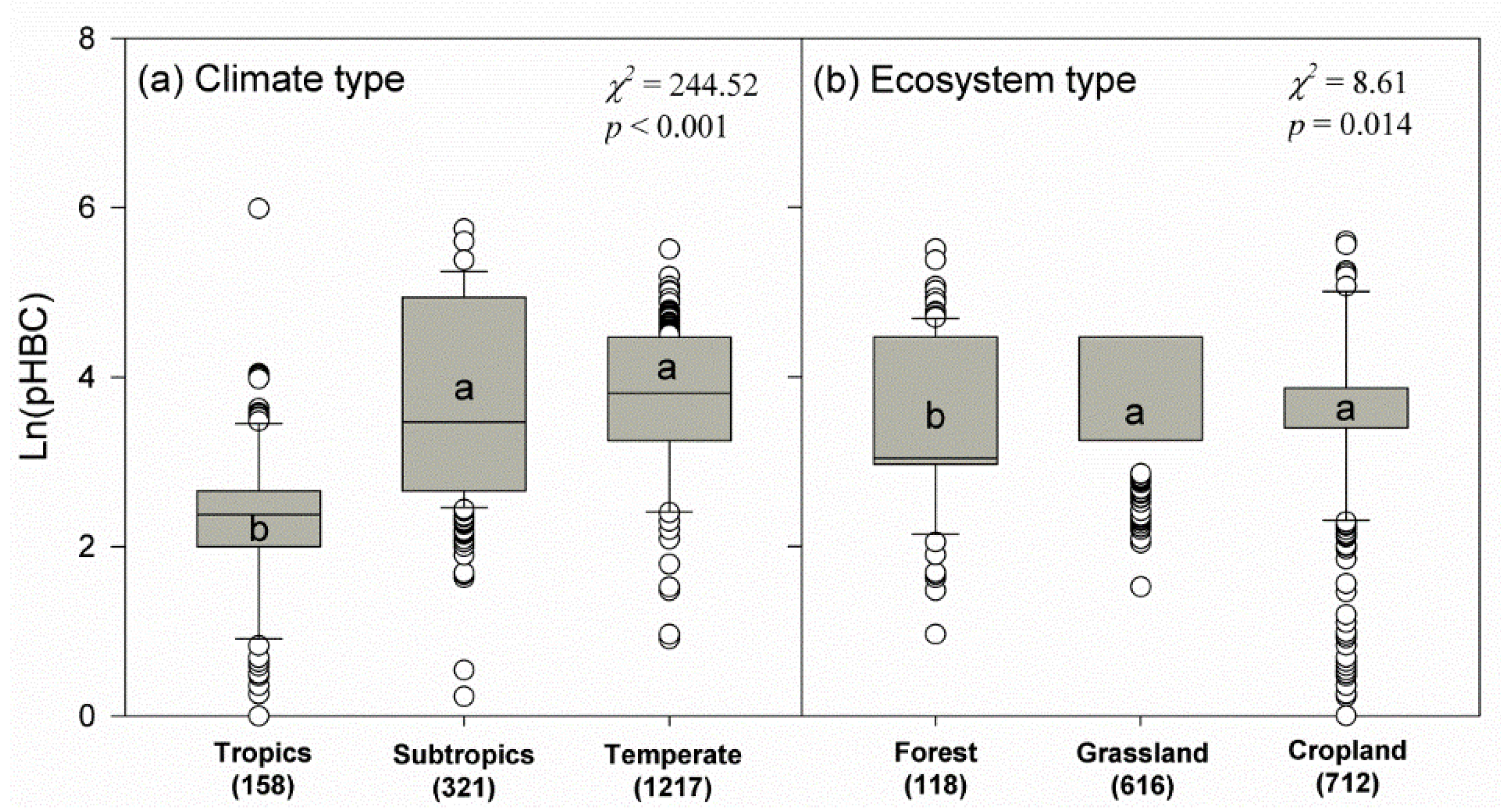
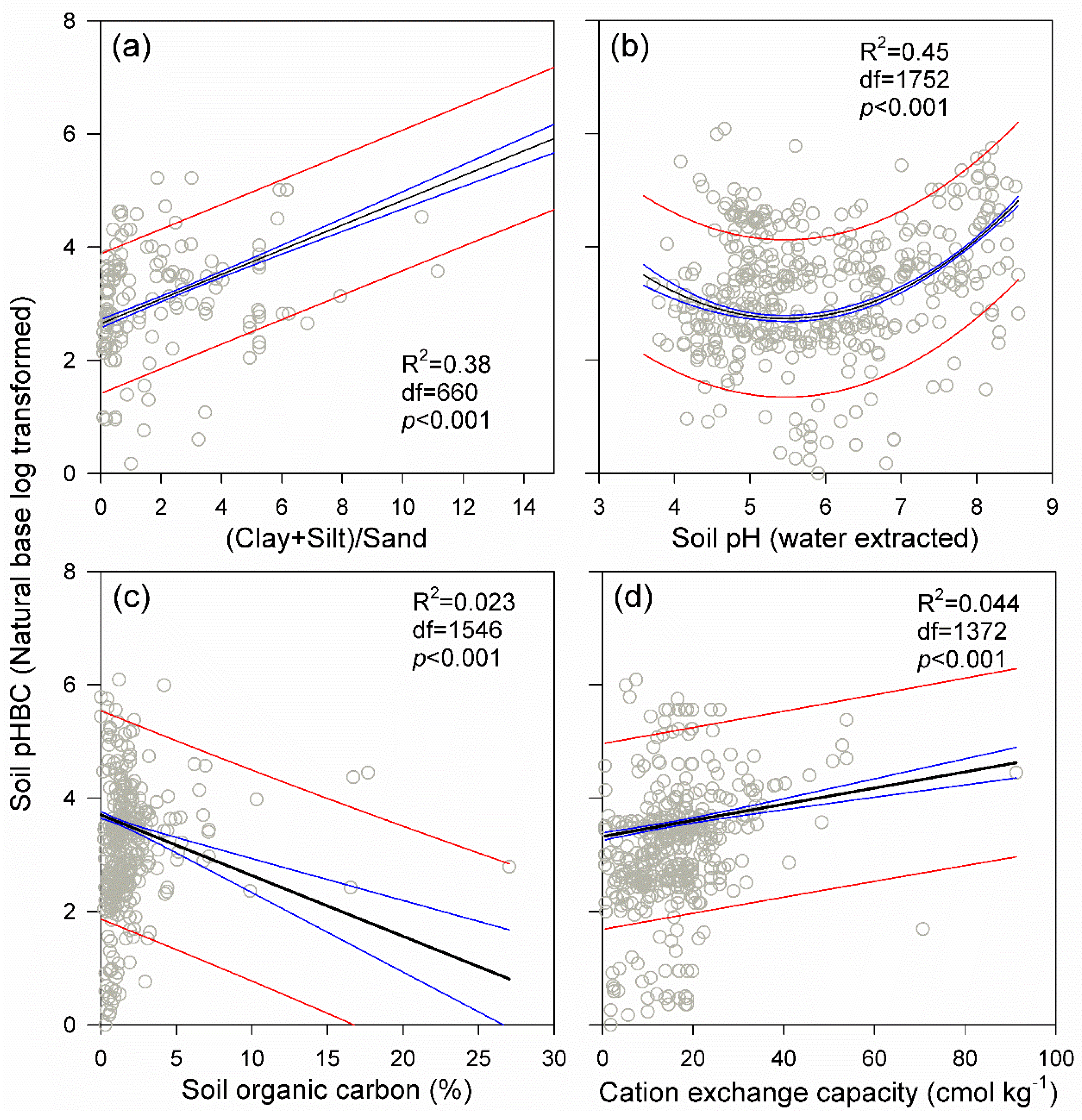
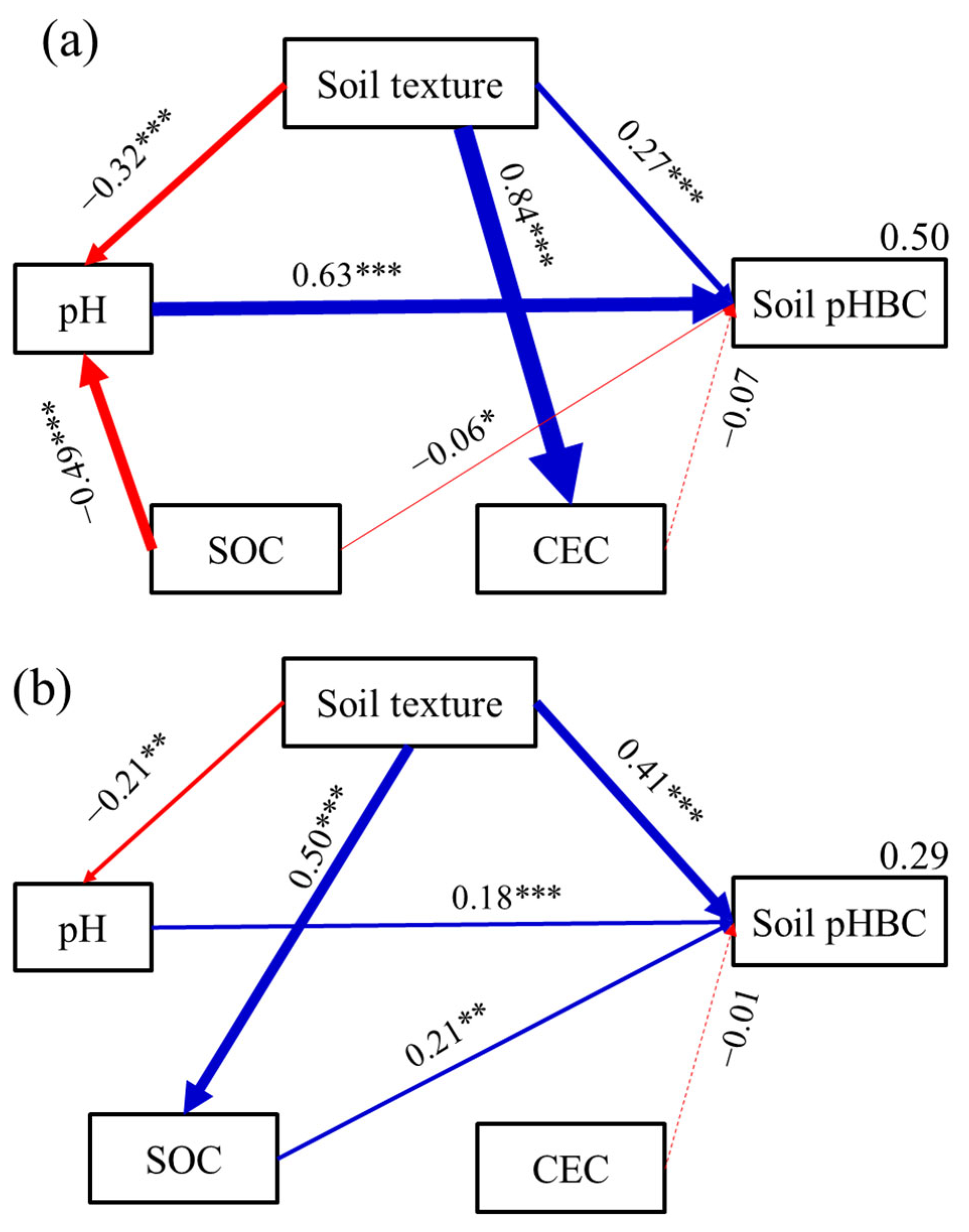
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Markewitz, D.; Richter, D.D.; Allen, H.L.; Urrego, J.B. Three Decades of Observed Soil Acidification in the Calhoun Experimental Forest: Has Acid Rain Made a Difference? Soil Sci. Soc. Am. J. 1998, 62, 1428–1439. [Google Scholar] [CrossRef]
- Raza, S.; Miao, N.; Wang, P.; Ju, X.; Chen, Z.; Zhou, J.; Kuzyakov, Y.; Na, M. Dramatic loss of inorganic carbon by nitrogen-induced soil acidification in Chinese croplands. Glob. Change Biol. 2020, 26, 3738–3751. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Ji, C.; Ma, W.; Wang, S.; Wang, S.; Han, W.; Mohammat, A.; Robinson, D.; Smith, P. Significant soil acidification across northern China’s grasslands during 1980s–2000s. Glob. Change Biol. 2012, 18, 2292–2300. [Google Scholar] [CrossRef]
- Deng, Q.; Hui, D.; Dennis, S.; Reddy, K.C.; Xu, X. Responses of terrestrial ecosystem phosphorus cycling to nitrogen addition: A meta-analysis. Glob. Ecol. Biogeogr. 2017, 26, 713–728. [Google Scholar] [CrossRef]
- Wei, H.; Liu, Y.; Xiang, H.; Zhang, J.; Li, S.; Yang, J. Soil pH Responses to Simulated Acid Rain Leaching in Three Agricultural Soils. Sustainability 2019, 12, 280. [Google Scholar] [CrossRef] [Green Version]
- Yan, P.; Wu, L.; Wang, D.; Fu, J.; Shen, C.; Li, X.; Zhang, L.; Zhang, L.; Fan, L.; Wenyan, H. Soil acidification in Chinese tea plantations. Sci. Total Environ. 2020, 715, 136963. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, H.Y.; Searle, E.B.; Sardans, J.; Ciais, P.; Peñuelas, J.; Huang, Z. Whole soil acidification and base cation reduction across subtropical China. Geoderma 2020, 361, 114107. [Google Scholar] [CrossRef]
- Li, Q.; Li, A.; Yu, X.; Dai, T.; Peng, Y.; Yuan, D.; Zhao, B.; Tao, Q.; Wang, C.; Li, B.; et al. Soil acidification of the soil profile across Chengdu Plain of China from the 1980s to 2010s. Sci. Total Environ. 2020, 698, 134320. [Google Scholar] [CrossRef]
- Weaver, A.R.; Kissel, D.E.; Chen, F.; West, L.T.; Adkins, W.; Rickman, D.; Luvall, J.C. Mapping soil pH buffering capacity of selected fields in the coastal plain. Soil Sci. Soc. Am. J. 2004, 68, 662–668. [Google Scholar] [CrossRef]
- Roberts, T.M.; Ulrich, B.; Pankrath, J. Effects of the Accumulation of Air Pollutants in Forest Ecosystems. J. Appl. Ecol. 1984, 21, 735. [Google Scholar] [CrossRef]
- Zhao, D.; Arshad, M.; Li, N.; Triantafilis, J. Predicting soil physical and chemical properties using vis-NIR in Australian cotton areas. Catena 2021, 196, 104938. [Google Scholar] [CrossRef]
- Lee, S.; Sorensen, J.W.; Walker, R.L.; Emerson, J.B.; Nicol, G.W.; Hazard, C. Soil pH influences the structure of virus communities at local and global scales. Soil Biol. Biochem. 2022, 166, 108569. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Luo, Y. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Tian, D.; Zeng, H.; Li, Z.; Yi, C.; Niu, S. Global soil acidification impacts on belowground processes. Environ. Res. Lett. 2019, 14, 074003. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, J.; Vogt, R.D.; Mulder, J.; Wang, Y.; Qian, C.; Wang, J.; Zhang, X. Soil acidification as an additional driver to organic carbon accumulation in major Chinese croplands. Geoderma 2020, 366, 114234. [Google Scholar] [CrossRef]
- Wei, H.; Liu, W.; Zhang, J.; Qin, Z. Effects of simulated acid rain on soil fauna community composition and their ecological niches. Environ. Pollut. 2017, 220, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Sun, J.; Chen, Y.; Wang, J.; Shang, J.; Belshaw, N.; Shen, C.; Liu, J.; Li, H.; Linghu, W.; et al. Mechanism of uranium release from uranium mill tailings under long-term exposure to simulated acid rain: Geochemical evidence and environmental implication. Environ. Pollut. 2019, 244, 174–181. [Google Scholar] [CrossRef]
- Qureshi, S.; Richards, B.K.; Steenhuis, T.S.; McBride, M.B.; Baveye, P.; Dousset, S. Microbial acidification and pH effects on trace element release from sewage sludge. Environ. Pollut. 2004, 132, 61–71. [Google Scholar] [CrossRef]
- Xu, D.; Carswell, A.; Zhu, Q.; Zhang, F.; de Vries, W. Modelling long-term impacts of fertilization and liming on soil acidification at Rothamsted experimental station. Sci. Total Environ. 2019, 713, 136249. [Google Scholar] [CrossRef]
- Hamidi, N.H.; Ahmed, O.H.; Omar, L.; Ch’Ng, H.Y.; Johan, P.D.; Paramisparam, P.; Jalloh, M.B. Acid Soils Nitrogen Leaching and Buffering Capacity Mitigation Using Charcoal and Sago Bark Ash. Sustainability 2021, 13, 11808. [Google Scholar] [CrossRef]
- Bowman, W.D.; Cleveland, C.C.; Halada, Ĺ.; Hreško, J.; Baron, J.S. Negative impact of nitrogen deposition on soil buffering capacity. Nat. Geosci. 2008, 1, 767–770. [Google Scholar] [CrossRef]
- Nelson, P.N.; Su, N. Soil pH buffering capacity: A descriptive function and its application to some acidic tropical soils. Soil Res. 2010, 48, 201–207. [Google Scholar] [CrossRef]
- Xu, R.-K.; Zhao, A.-Z.; Yuan, J.-H.; Jiang, J. pH buffering capacity of acid soils from tropical and subtropical regions of China as influenced by incorporation of crop straw biochars. J. Soils Sedim. 2012, 12, 494–502. [Google Scholar] [CrossRef]
- Kissel, D.E.; Sonon, L.S.; Cabrera, M.L. Rapid Measurement of Soil pH Buffering Capacity. Soil Sci. Soc. Am. J. 2012, 76, 694–699. [Google Scholar] [CrossRef]
- Norton, S. Changes in chemical processes in soils caused by acid precipitation. Water Air Soil Pollut. 1977, 7, 389–400. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Wang, Y.; Yu, M.; Li, K.; Shao, Y.; Yan, J. Responses of soil buffering capacity to acid treatment in three typical subtropical forests. Sci. Total Environ. 2016, 563–564, 1068–1077. [Google Scholar] [CrossRef]
- Curtin, D.; Trolove, S. Predicting pH buffering capacity of New Zealand soils from organic matter content and mineral characteristics. Soil Res. 2013, 51, 494–502. [Google Scholar] [CrossRef]
- Luo, W.T.; Nelson, P.N.; Li, M.-H.; Cai, J.P.; Zhang, Y.Y.; Yang, S.; Wang, R.Z.; Wang, Z.W.; Wu, Y.N.; Han, X.G.; et al. Contrasting pH buffering patterns in neutral-alkaline soils along a 3600 km transect in northern China. Biogeosciences 2015, 12, 7047–7056. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Weiner, J.; Cavalieri, A.; Liu, H.; Li, T.; Cai, J.; Jiang, Y. The interaction between N and P addition on grassland soil acid buffering capacity is regulated by precipitation. Soil Sci. Plant Nutr. 2021, 67, 222–232. [Google Scholar] [CrossRef]
- Lu, X.; Mao, Q.; Mo, J.; Gilliam, F.S.; Zhou, G.; Luo, Y.; Zhang, W.; Huang, J. Divergent Responses of Soil Buffering Capacity to Long-Term N Deposition in Three Typical Tropical Forests with Different Land-Use History. Environ. Sci. Technol. 2015, 49, 4072–4080. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, L. The role of neutral salts in the ion exchange between acid precipitation and soil. Geoderma 1975, 14, 93–105. [Google Scholar] [CrossRef]
- Cai, J.; Luo, W.; Liu, H.; Feng, X.; Zhang, Y.; Wang, R.; Xu, Z.; Zhang, Y.; Jiang, Y. Precipitation-mediated responses of soil acid buffering capacity to long-term nitrogen addition in a semi-arid grassland. Atmos. Environ. 2017, 170, 312–318. [Google Scholar] [CrossRef]
- Zhang, Y.; de Vries, W.; Thomas, B.; Hao, X.; Shi, X. Impacts of long-term nitrogen fertilization on acid buffering rates and mechanisms of a slightly calcareous clay soil. Geoderma 2017, 305, 92–99. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Y.; Yu, M.; Cao, N.; Yan, J. Soil organic matter is important for acid buffering and reducing aluminum leaching from acidic forest soils. Chem. Geol. 2018, 501, 86–94. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using SPSS (Third Edition); SAGE Publications Ltd.: Los Angeles, CA, USA; London, UK; New Delhi, India; Singapore; Washington, DC, USA, 2009. [Google Scholar]
- Gruba, P.; Mulder, J. Tree species affect cation exchange capacity (CEC) and cation binding properties of organic matter in acid forest soils. Sci. Total Environ. 2015, 511, 655–662. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, J.; Zhao, X.; Triantafilis, J. Clay content mapping and uncertainty estimation using weighted model averaging. Catena 2021, 209, 105791. [Google Scholar] [CrossRef]
- Zhao, H.-L.; Yi, X.-Y.; Zhou, R.-L.; Zhao, X.-Y.; Zhang, T.-H.; Drake, S. Wind erosion and sand accumulation effects on soil properties in Horqin Sandy Farmland, Inner Mongolia. Catena 2006, 65, 71–79. [Google Scholar] [CrossRef]
- Wuepper, D.; Borrelli, P.; Finger, R. Countries and the global rate of soil erosion. Nat. Sustain. 2019, 3, 51–55. [Google Scholar] [CrossRef]
- Nguyen, T.; Tran, T.T.H. The Contribution of Various Components to pH Buffering Capacity of Acrisols in Southeastern Vietnam. Commun. Soil Sci. Plant Anal. 2019, 50, 1170–1177. [Google Scholar] [CrossRef]
- Daskalova, G.N.; Myers-Smith, I.H.; Bjorkman, A.D.; Blowes, S.A.; Supp, S.R.; Magurran, A.E.; Dornelas, M. Landscape-scale forest loss as a catalyst of population and biodiversity change. Science 2020, 368, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Trugman, A.T.; Badgley, G.; Anderson, C.M.; Bartuska, A.; Ciais, P.; Cullenward, D.; Field, C.B.; Freeman, J.; Goetz, S.J.; et al. Climate-driven risks to the climate mitigation potential of forests. Science 2020, 368, eaaz7005. [Google Scholar] [CrossRef] [PubMed]
- Díez, M.; Simón, M.; García, I.; Martín, F.; Peinado, F.J.M. Assessment of the Critical Load of Trace Elements in Soils Polluted by Pyrite tailings. A Laboratory Experiment. Water Air Soil Pollut. 2008, 199, 381–387. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Yang, J.; Liu, Z.; Zhang, J. Data Integration Analysis Indicates That Soil Texture and pH Greatly Influence the Acid Buffering Capacity of Global Surface Soils. Sustainability 2022, 14, 3017. https://doi.org/10.3390/su14053017
Wei H, Yang J, Liu Z, Zhang J. Data Integration Analysis Indicates That Soil Texture and pH Greatly Influence the Acid Buffering Capacity of Global Surface Soils. Sustainability. 2022; 14(5):3017. https://doi.org/10.3390/su14053017
Chicago/Turabian StyleWei, Hui, Jiayue Yang, Ziqiang Liu, and Jiaen Zhang. 2022. "Data Integration Analysis Indicates That Soil Texture and pH Greatly Influence the Acid Buffering Capacity of Global Surface Soils" Sustainability 14, no. 5: 3017. https://doi.org/10.3390/su14053017




