Sustainable Production of Tomato Plants (Solanum lycopersicum L.) under Low-Quality Irrigation Water as Affected by Bio-Nanofertilizers of Selenium and Copper
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Applied Nanofertilizers and Irrigation Water
2.3. Vegetative Growth Parameters and Harvesting
2.4. Measuring of Plant Enzymes
2.5. Soil Sampling, Chemical and Biological Analyses
2.6. Plant Analyses and Nutrient Uptake
2.7. Statistical Analyses
3. Results
3.1. Vegetative Growth under Bio-Nanofertilization
3.2. Tomato Yield and Its Quality
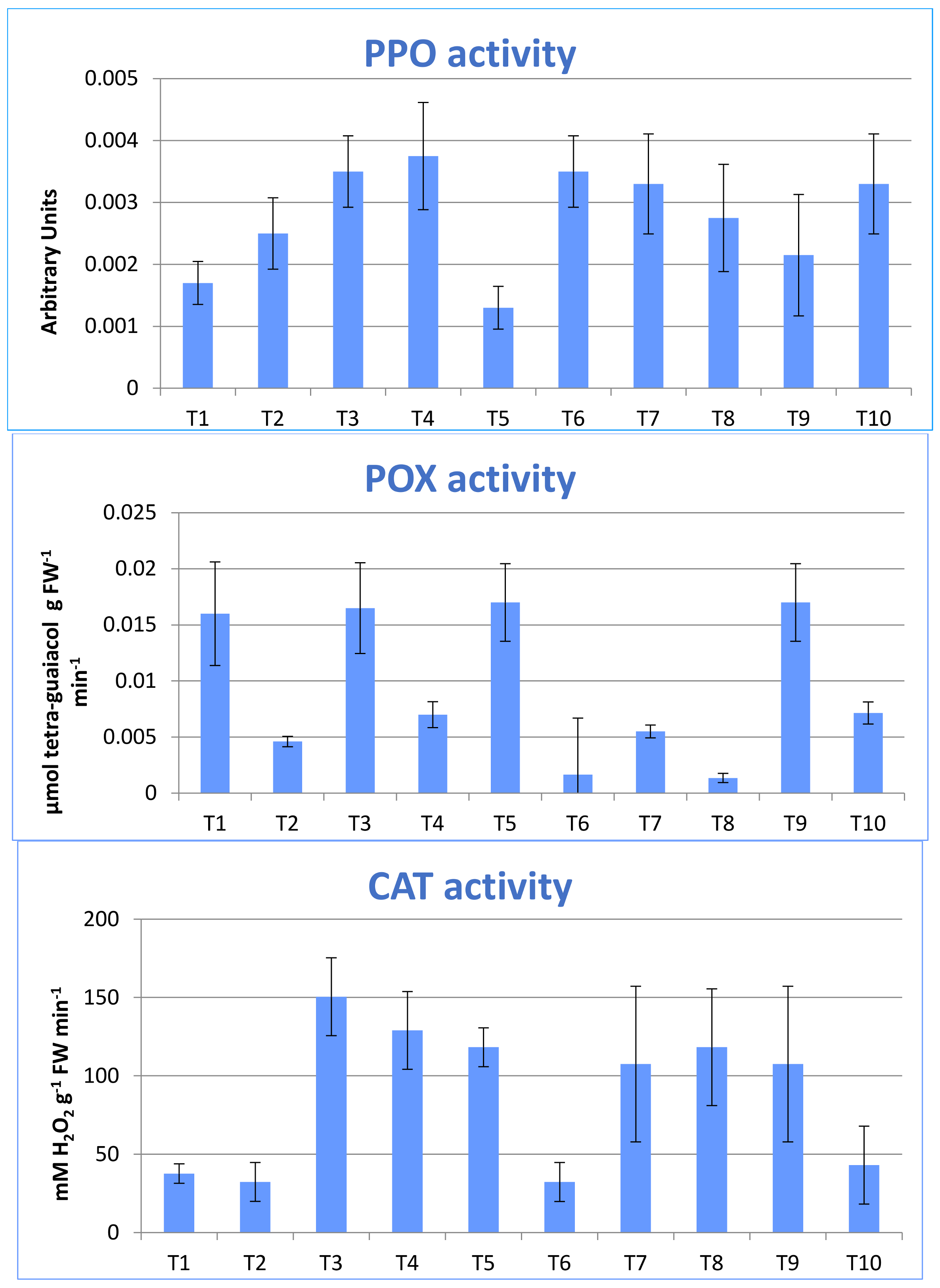
3.3. Enzymatic Antioxidants
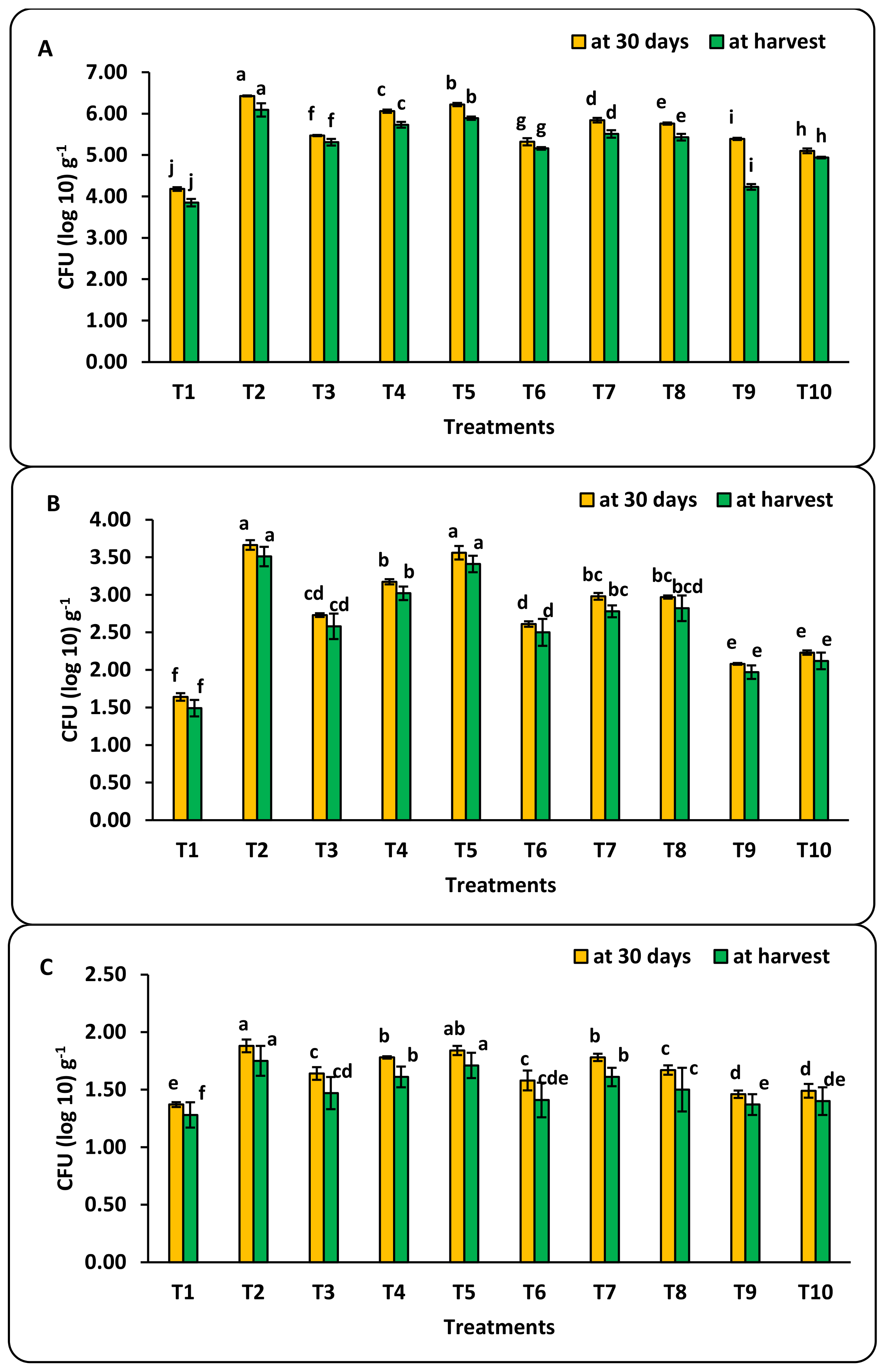
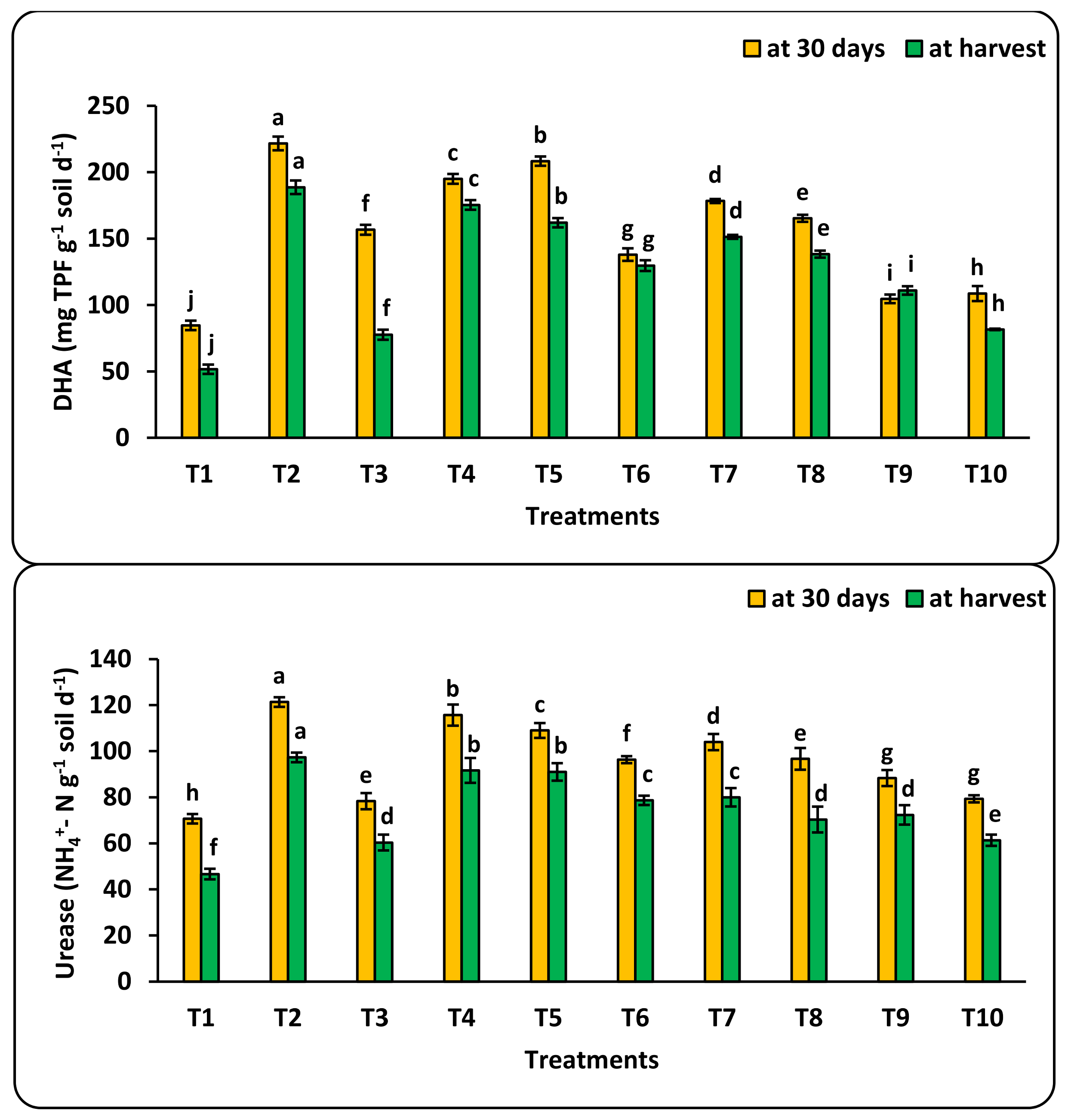
3.4. Bio-Nanofertilization and Soil Biological Activity
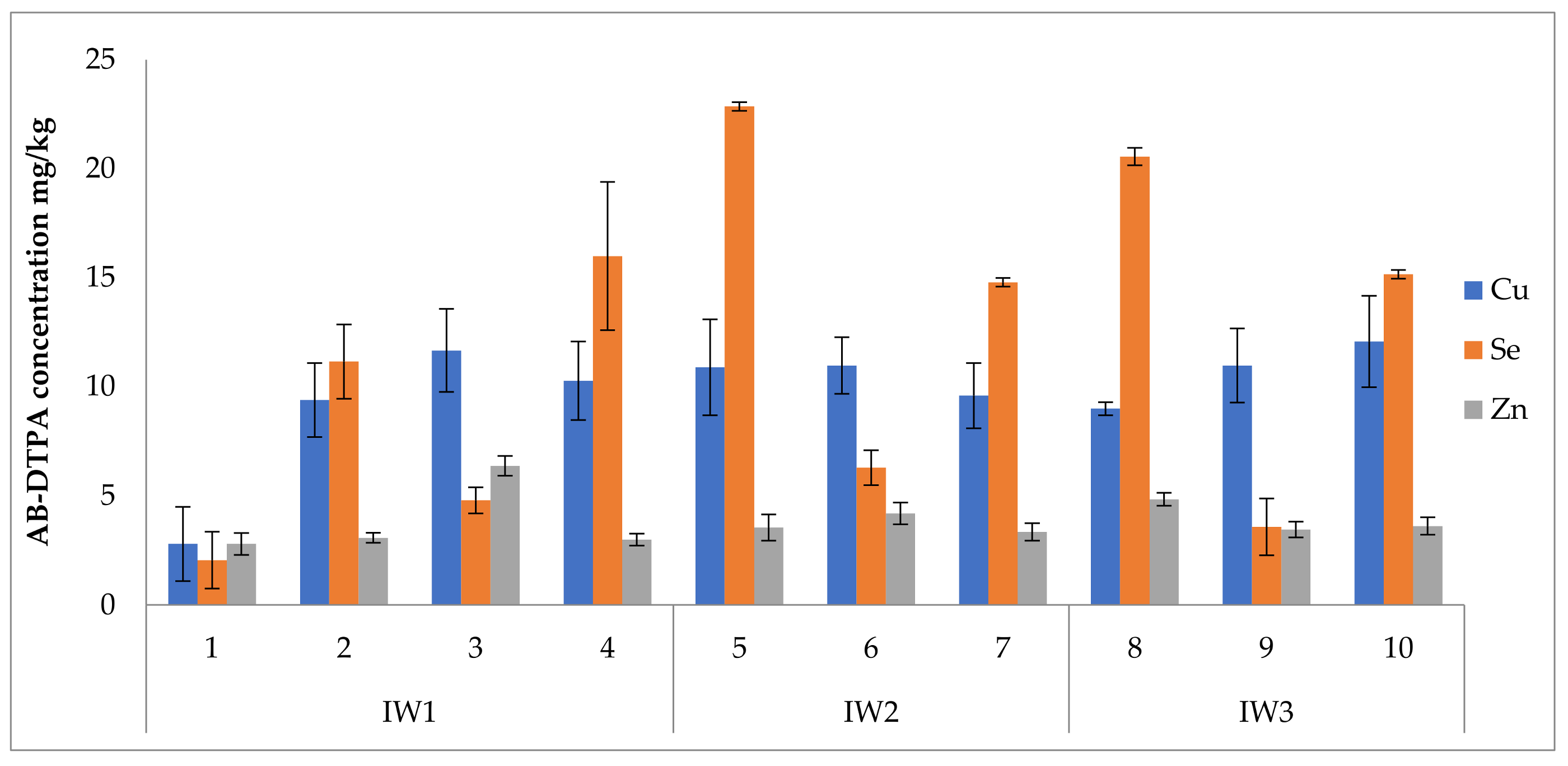
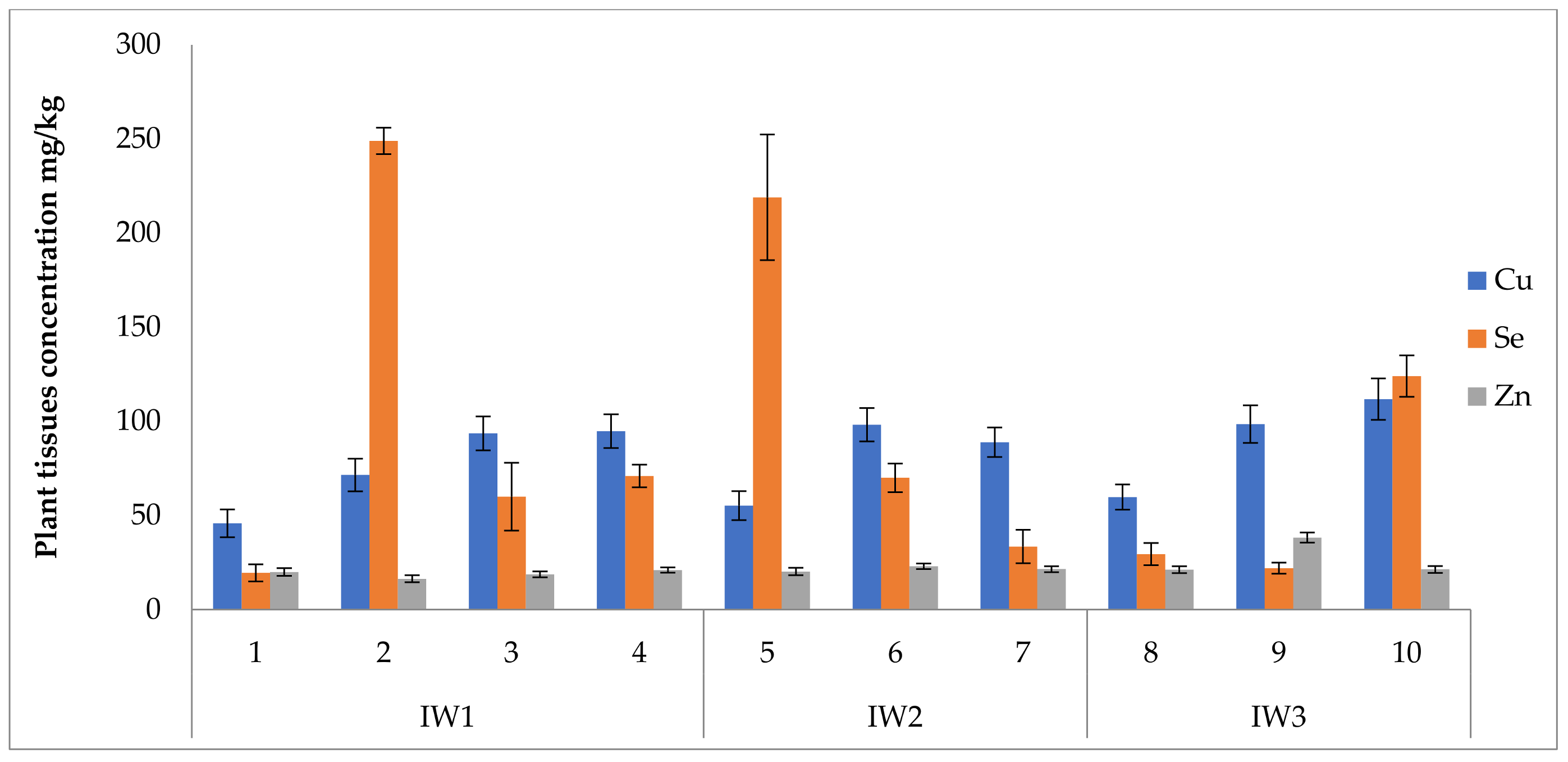
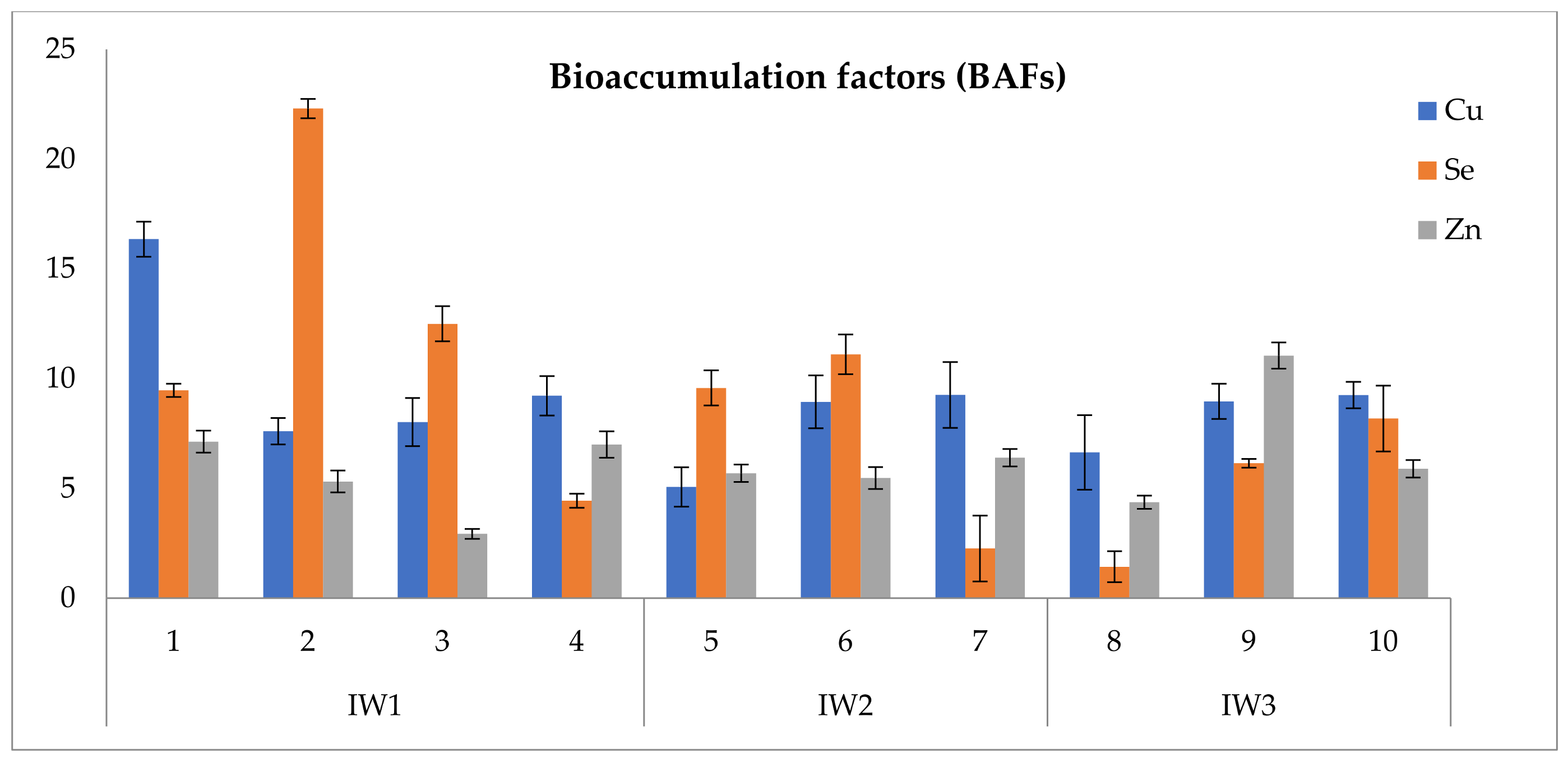
3.5. Uptake of Nutrients and Bioaccumulation
4. Discussion
4.1. Tomato Growth, Yield, and Its Quality
4.2. Irrigation Water Stress on Tomato Production
4.3. Soil Biology and Stress of Irrigation Water
4.4. Nutrient Bioaccumulation and Stress of Irrigation Water
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, S.; Rana, V.S.; Pawar, R.; Lakra, J.; Racchapannavar, V. Nanofertilizers for Sustainable Fruit Production: A Review. Environ. Chem. Lett. 2021, 19, 1693–1714. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Almutairi, K.F.; Alotaibi, M.; Shami, A.; Alhammad, B.A.; Battaglia, M.L. Nano-Fertilization as an Emerging Fertilization Technique: Why Can Modern Agriculture Benefit from Its Use? Plants 2020, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Jogaiah, S.; Singh, H.B.; Fraceto, L.F.; De Lima, R. Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; A Smart Delivery System for Crop Improvement; Woodhead Publishing: Kidlington, UK, 2020; ISBN 978-0-12-820092-6. [Google Scholar]
- Al-Mamun, R.; Hasan, R.; Ahommed, S.; Bacchu, S.; Ali, R.; Khan, Z.H. Nanofertilizers towards Sustainable Agriculture and Environment. Environ. Technol. Innov. 2021, 23, 101658. [Google Scholar] [CrossRef]
- Cota-Ruiz, K.; Ye, Y.; Valdes, C.; Deng, C.; Wang, Y.; Hernández-Viezcas, J.A.; Duarte-Gardea, M.; Gardea-Torresdey, J.L. Copper Nanowires as Nanofertilizers for Alfalfa Plants: Understanding Nano-Bio Systems Interactions from Microbial Genomics, Plant Molecular Responses and Spectroscopic Studies. Sci. Total Environ. 2020, 742, 140572. [Google Scholar] [CrossRef]
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Zinc Oxide Nanoparticles (ZnONPs) as a Novel Nanofertilizer: Influence on Seed Yield and Antioxidant Defense System in Soil Grown Soybean (Glycine Max Cv. Kowsar). Sci. Total Environ. 2020, 738, 140240. [Google Scholar] [CrossRef]
- Abd El-Azeim, M.M.; Sherif, M.A.; Hussien, M.S.; Tantawy, I.A.A.; Bashandy, S.O. Impacts of Nano- and Non-Nanofertilizers on Potato Quality and Productivity. Acta Ecol. Sin. 2020, 40, 388–397. [Google Scholar] [CrossRef]
- Abdulhameed, M.F.; Taha, A.A.; Ismail, R.A. Improvement of Cabbage Growth and Yield by Nanofertilizers and Nanoparticles. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100437. [Google Scholar] [CrossRef]
- Kumaraswamy, R.V.; Saharan, V.; Kumari, S.; Chandra Choudhary, R.; Pal, A.; Sharma, S.S.; Rakshit, S.; Raliya, R.; Biswas, P. Chitosan-Silicon Nanofertilizer to Enhance Plant Growth and Yield in Maize (Zea Mays L.). Plant Physiol. Biochem. 2021, 159, 53–66. [Google Scholar] [CrossRef]
- Sheoran, P.; Grewal, S.; Kumari, S.; Goel, S. Enhancement of Growth and Yield, Leaching Reduction in Triticum Aestivum Using Biogenic Synthesized Zinc Oxide Nanofertilizer. Biocatal. Agric. Biotechnol. 2021, 32, 101938. [Google Scholar] [CrossRef]
- Bonilla-Bird, N.J.; Ye, Y.; Akter, T.; Valdes-Bracamontes, C.; Darrouzet-Nardi, A.J.; Saupe, G.B.; Flores-Marges, J.P.; Ma, L.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; et al. Effect of Copper Oxide Nanoparticles on Two Varieties of Sweetpotato Plants. Plant Physiol. Biochem. 2020, 154, 277–286. [Google Scholar] [CrossRef]
- Tang, Q.; Xu, Z.; Hong, A.; Zhang, X.; Kah, M.; Li, L.; Wang, Y. Response of Soil Enzyme Activity and Bacterial Community to Copper Hydroxide Nanofertilizer and Its Ionic Analogue under Single versus Repeated Applications. Sci. Total Environ. 2021, 796, 148974. [Google Scholar] [CrossRef]
- Babajani, A.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Eslami, B. Differential Growth, Nutrition, Physiology, and Gene Expression in Melissa Officinalis Mediated by Zinc Oxide and Elemental Selenium Nanoparticles. Environ. Sci. Pollut. Res. 2019, 26, 24430–24444. [Google Scholar] [CrossRef]
- Moreno-Martín, G.; Sanz-Landaluze, J.; León-González, M.E.; Madrid, Y. Insights into the Accumulation and Transformation of Ch-SeNPs by Raphanus Sativus and Brassica Juncea: Effect on Essential Elements Uptake. Sci. Total Environ. 2020, 725, 138453. [Google Scholar] [CrossRef]
- Faizan, M.; Bhat, J.A.; Chen, C.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P.; Yu, F. Zinc Oxide Nanoparticles (ZnO-NPs) Induce Salt Tolerance by Improving the Antioxidant System and Photosynthetic Machinery in Tomato. Plant Physiol. Biochem. 2021, 161, 122–130. [Google Scholar] [CrossRef]
- Faizan, M.; Bhat, J.A.; Noureldeen, A.; Ahmad, P.; Yu, F. Zinc Oxide Nanoparticles and 24-Epibrassinolide Alleviates Cu Toxicity in Tomato by Regulating ROS Scavenging, Stomatal Movement and Photosynthesis. Ecotoxicol. Environ. Saf. 2021, 218, 112293. [Google Scholar] [CrossRef]
- Qadir, M.; Sposito, G.; Smith, C.J.; Oster, J.D. Reassessing Irrigation Water Quality Guidelines for Sodicity Hazard. Agric. Water Manag. 2021, 255, 107054. [Google Scholar] [CrossRef]
- Zakir, H.M.; Sharmin, S.; Akter, A.; Rahman, S. Assessment of Health Risk of Heavy Metals and Water Quality Indices for Irrigation and Drinking Suitability of Waters: A Case Study of Jamalpur Sadar Area, Bangladesh. Environ. Adv. 2020, 2, 100005. [Google Scholar] [CrossRef]
- El-Hassanin, A.S.; Samak, M.R.; Abdel-Rahman, G.N.; Abu-Sree, Y.H.; Saleh, E.M. Risk Assessment of Human Exposure to Lead and Cadmium in Maize Grains Cultivated in Soils Irrigated Either with Low-Quality Water or Freshwater. Toxicol. Rep. 2020, 7, 10–15. [Google Scholar] [CrossRef]
- Cakmakci, T.; Sahin, U. Productivity and Heavy Metal Pollution Management in a Silage Maize Field with Reduced Recycled Wastewater Applications with Different Irrigation Methods. J. Environ. Manag. 2021, 291, 112602. [Google Scholar] [CrossRef]
- Anjum, M.A.; Hussain, S.; Arshad, P.; Hassan, A. Irrigation Water of Different Sources Affects Fruit Quality Attributes and Heavy Metals Contents of Un-Grafted and Commercial Mango Cultivars. J. Environ. Manag. 2021, 281, 111895. [Google Scholar] [CrossRef]
- Kondash, A.J.; Redmon, J.H.; Lambertini, E.; Feinstein, L.; Weinthal, E.; Cabrales, L.; Vengosh, A. The Impact of Using Low-Saline Oilfield Produced Water for Irrigation on Water and Soil Quality in California. Sci. Total Environ. 2020, 733, 139392. [Google Scholar] [CrossRef]
- Oubane, M.; Khadra, A.; Ezzariai, A.; Kouisni, L.; Hafidi, M. Heavy Metal Accumulation and Genotoxic Effect of Long-Term Wastewater Irrigated Peri-Urban Agricultural Soils in Semiarid Climate. Sci. Total Environ. 2021, 794, 148611. [Google Scholar] [CrossRef]
- Dhiman, J.; Prasher, S.O.; ElSayed, E.; Patel, R.M.; Nzediegwu, C.; Mawof, A. Effect of Hydrogel Based Soil Amendments on Heavy Metal Uptake by Spinach Grown with Wastewater Irrigation. J. Clean. Prod. 2021, 311, 127644. [Google Scholar] [CrossRef]
- Xie, Z.; Yang, X.; Sun, X.; Huang, L.; Li, S.; Hu, Z. Effects of Biochar Application and Irrigation Rate on the Soil Phosphorus Leaching Risk of Fluvisol Profiles in Open Vegetable Fields. Sci. Total Environ. 2021, 789, 147973. [Google Scholar] [CrossRef]
- Mateus, A.; Torres, J.; Marimon-Bolivar, W.; Pulgarín, L. Implementation of Magnetic Bentonite in Food Industry Wastewater Treatment for Reuse in Agricultural Irrigation. Water Resour. Ind. 2021, 26, 100154. [Google Scholar] [CrossRef]
- Liu, J.; Williams, P.C.; Goodson, B.M.; Geisler-Lee, J.; Fakharifar, M.; Gemeinhardt, M.E. TiO2 Nanoparticles in Irrigation Water Mitigate Impacts of Aged Ag Nanoparticles on Soil Microorganisms, Arabidopsis Thaliana Plants, and Eisenia Fetida Earthworms. Environ. Res. 2019, 172, 202–215. [Google Scholar] [CrossRef]
- Alidokht, L.; Anastopoulos, I.; Ntarlagiannis, D.; Soupios, P.; Tawabini, B.; Kalderis, D.; Khataee, A. Recent Advances in the Application of Nanomaterials for the Remediation of Arsenic-Contaminated Water and Soil. J. Environ. Chem. Eng. 2021, 9, 105533. [Google Scholar] [CrossRef]
- Lal, S.; Singhal, A.; Kumari, P. Exploring Carbonaceous Nanomaterials for Arsenic and Chromium Removal from Wastewater. J. Water Process Eng. 2020, 36, 101276. [Google Scholar] [CrossRef]
- Samuel, M.S.; Selvarajan, E.; Chidambaram, R.; Patel, H.; Brindhadevi, K. Clean Approach for Chromium Removal in Aqueous Environments and Role of Nanomaterials in Bioremediation: Present Research and Future Perspective. Chemosphere 2021, 284, 131368. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Qi, M.; Huang, Z.; Xu, X.; Begum, N.; Qin, C.; Zhang, C.; Ahmad, N.; Mustafa, N.S.; Ashraf, M.; et al. Improving Growth and Photosynthetic Performance of Drought Stressed Tomato by Application of Nano-Organic Fertilizer Involves up-Regulation of Nitrogen, Antioxidant and Osmolyte Metabolism. Ecotoxicol. Environ. Saf. 2021, 216, 112195. [Google Scholar] [CrossRef] [PubMed]
- Alharby, H.F.; Metwali, E.M.R.; Fuller, M.P.; Aldhebiani, A.Y. The Alteration of MRNA Expression of SOD and GPX Genes, and Proteins in Tomato (Lycopersicon Esculentum Mill) under Stress of NaCl and/or ZnO Nanoparticles. Saudi J. Biol. Sci. 2016, 23, 773–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, S.; Gao, Y.; Tian, M.; Tian, Y.; Li, J. Comprehensive Evaluation of Effects of Various Carbon-Rich Amendments on Tomato Production under Continuous Saline Water Irrigation: Overall Soil Quality, Plant Nutrient Uptake, Crop Yields and Fruit Quality. Agric. Water Manag. 2021, 255, 106995. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Yusuf, M.; Khan, S.T.; Hayat, S. Zinc Oxide Nanoparticle-Mediated Changes in Photosynthetic Efficiency and Antioxidant System of Tomato Plants. Photosynthetica 2018, 56, 678–686. [Google Scholar] [CrossRef]
- Akanbi-Gada, M.A.; Ogunkunle, C.O.; Vishwakarma, V.; Viswanathan, K.; Fatoba, P.O. Phytotoxicity of Nano-Zinc Oxide to Tomato Plant (Solanum Lycopersicum L.): Zn Uptake, Stress Enzymes Response and Influence on Non-Enzymatic Antioxidants in Fruits. Environ. Technol. Innov. 2019, 14, 100325. [Google Scholar] [CrossRef]
- Younes, N.A.; Hassan, H.S.; Elkady, M.F.; Hamed, A.M.; Dawood, M.F.A. Impact of Synthesized Metal Oxide Nanomaterials on Seedlings Production of Three Solanaceae Crops. Heliyon 2020, 6, e03188. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, B.; Syed, A.; Rizvi, A.; Shahid, M.; Bahkali, A.H.; Khan, M.S.; Musarrat, J. Impact of Metal-Oxide Nanoparticles on Growth, Physiology and Yield of Tomato (Solanum Lycopersicum L.) Modulated by Azotobacter Salinestris Strain ASM. Environ. Pollut. 2021, 269, 116218. [Google Scholar] [CrossRef]
- Bakshi, M.; Liné, C.; Bedolla, D.E.; Stein, R.J.; Kaegi, R.; Sarret, G.; Pradas del Real, A.E.; Castillo-Michel, H.; Abhilash, P.C.; Larue, C. Assessing the Impacts of Sewage Sludge Amendment Containing Nano-TiO2 on Tomato Plants: A Life Cycle Study. J. Hazard. Mater. 2019, 369, 191–198. [Google Scholar] [CrossRef]
- Zaman, M.; Shahid, S.A.; Heng, L. Irrigation Water Quality. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer International Publishing: Cham, Switzerland, 2018; pp. 113–131. ISBN 978-3-319-96189-7. [Google Scholar]
- Hafez, Y.; Mourad, R.Y.; Mansour, M.; Abdelaal, K. Impact of Non-Traditional Compounds and Fungicides on Physiological and Biochemical Characters of Barely Infected with Blumeria Graminis f. Sp Hordei under Field Condtitions. Egypt. J. Biol. Pest Control. 2014, 24, 445–453. [Google Scholar]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Malik, C.P.; Singh, M.B. Plant Enzymology and Histo-Enzymology: A Text Manual; Kalyani Publishers: New Delhi, India, 1980. [Google Scholar]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Association of Enhanced Peroxidase Activity with Induced Systemic Resistance of Cucumber to Colletotrichum Lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis. Part 3, Chemical Methods; Soil Science Society of America, American Society of Agronomy, Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Allen, O.N. Experiments in Soil Bacteriology; Second printing; University of Wisconsin: Madison, WI, USA, 1959. [Google Scholar]
- Casida, L.E.J.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Science 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Pancholy, S.K.; Rice, E.L. Soil Enzymes in Relation to Old Succession: Amylase, Invertase, Cellulose, Dehydrogenase and Urease. Soil. Sci. Soc. Am. J. 1973, 37, 47–50. [Google Scholar] [CrossRef]
- Aitta, A.; El-Ramady, H.; Alshaal, T.; El-Henawy, A.; Shams, M.; Talha, N.; Elbehiry, F.; Brevik, E.C. Seasonal and Spatial Distribution of Soil Trace Elements around Kitchener Drain in the Northern Nile Delta, Egypt. Agriculture 2019, 9, 152. [Google Scholar] [CrossRef] [Green Version]
- Motoshita, M.; Ono, Y.; Pfister, S.; Boulay, A.-M.; Berger, M.; Nansai, K.; Tahara, K.; Itsubo, N.; Inaba, A. Consistent Characterisation Factors at Midpoint and Endpoint Relevant to Agricultural Water Scarcity Arising from Freshwater Consumption. Int. J. Life Cycle Assess 2018, 23, 2276–2287. [Google Scholar] [CrossRef] [Green Version]
- Elbehiry, F.; Elbasiouny, H.; Ali, R.; Brevik, E.C. Enhanced Immobilization and Phytoremediation of Heavy Metals in Landfill Contaminated Soils. Water Air Soil Pollut. 2020, 231, 204. [Google Scholar] [CrossRef]
- Radziemska, M. Study of Applying Naturally Occurring Mineral Sorbents of Poland (Dolomite Halloysite, Chalcedonite) for Aided Phytostabilization of Soil Polluted with Heavy Metals. Catena 2018, 163, 123–129. [Google Scholar] [CrossRef]
- Álvarez-Mateos, P.; Alés-Álvarez, F.-J.; García-Martín, J.F. Phytoremediation of Highly Contaminated Mining Soils by Jatropha Curcas L. and Production of Catalytic Carbons from the Generated Biomass. J. Environ. Manag. 2019, 231, 886–895. [Google Scholar] [CrossRef]
- Zhou, B.; Zhou, H.; Puig-Bargués, J.; Li, Y. Using an Anti-Clogging Relative Index (CRI) to Assess Emitters Rapidly for Drip Irrigation Systems with Multiple Low-Quality Water Sources. Agric. Water Manag. 2019, 221, 270–278. [Google Scholar] [CrossRef]
- Ma, S.-C.; Zhang, H.-B.; Ma, S.-T.; Wang, R.; Wang, G.-X.; Shao, Y.; Li, C.-X. Effects of Mine Wastewater Irrigation on Activities of Soil Enzymes and Physiological Properties, Heavy Metal Uptake and Grain Yield in Winter Wheat. Ecotoxicol. Environ. Saf. 2015, 113, 483–490. [Google Scholar] [CrossRef]
- Shoaib, A.; Meraj, S.; Nafisa; Khan, K.A.; Javaid, M.A. Influence of Salinity and Fusarium Oxysporum as the Stress Factors on Morpho-Physiological and Yield Attributes in Onion. Physiol. Mol. Biol. Plants 2018, 24, 1093–1101. [Google Scholar] [CrossRef]
- Morales-Espinoza, M.C.; Cadenas-Pliego, G.; Pérez-Alvarez, M.; Hernández-Fuentes, A.D.; Cabrera de la Fuente, M.; Benavides-Mendoza, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Se Nanoparticles Induce Changes in the Growth, Antioxidant Responses, and Fruit Quality of Tomato Developed under NaCl Stress. Molecules 2019, 24, 3030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Hernández, H.; Juárez-Maldonado, A.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Sánchez-Aspeytia, D.; González-Morales, S. Chitosan-PVA and Copper Nanoparticles Improve Growth and Overexpress the SOD and JA Genes in Tomato Plants under Salt Stress. Agronomy 2018, 8, 175. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Hernández, H.; González-Morales, S.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Effects of Chitosan–PVA and Cu Nanoparticles on the Growth and Antioxidant Capacity of Tomato under Saline Stress. Molecules 2018, 23, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Fuentes, A.D.; López-Vargas, E.R.; Pinedo-Espinoza, J.M.; Campos-Montiel, R.G.; Valdés-Reyna, J.; Juárez-Maldonado, A. Postharvest Behavior of Bioactive Compounds in Tomato Fruits Treated with Cu Nanoparticles and NaCl Stress. Appl. Sci. 2017, 7, 980. [Google Scholar] [CrossRef] [Green Version]
- Quiterio-Gutiérrez, T.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Hernández-Fuentes, A.D.; Sandoval-Rangel, A.; Benavides-Mendoza, A.; Cabrera-de la Fuente, M.; Juárez-Maldonado, A. The Application of Selenium and Copper Nanoparticles Modifies the Biochemical Responses of Tomato Plants under Stress by Alternaria Solani. Int. J. Mol. Sci. 2019, 20, 1950. [Google Scholar] [CrossRef] [Green Version]
- Samuel, A.; Brejea, R.; Domuta, C.; Bungau, S.; Cenusa, N.; Tit, D.M. Enzymatic Indicators of Soil Quality. J. Environ. Prot. Ecol. 2017, 18, 871–878. [Google Scholar]
- Samuel, A.D.; Tit, D.M.; Melinte (Frunzulica), C.E.; Iovan, C.; Purza, L.; Gitea, M.; Bungau, S. Enzymological and Physicochemical Evaluation of the Effects of Soil Management Practices. Rev. Chim. 2017, 68, 2243–2247. [Google Scholar] [CrossRef]
- Bungau, S.; Behl, T.; Aleya, L.; Bourgeade, P.; Aloui-Sossé, B.; Purza, A.L.; Abid, A.; Samuel, A.D. Expatiating the Impact of Anthropogenic Aspects and Climatic Factors on Long-Term Soil Monitoring and Management. Environ. Sci. Pollut. Res. 2021, 28, 30528–30550. [Google Scholar] [CrossRef]
- Akbar, S.; Ali, Z.; Hussain, S.; Mohammad, A.; Riaz, Y.; Shakeel, A.; Ahmad, I.; Mussarat, M.; Malik, R.N.; Khan, K.Y.; et al. Metal Accumulation Potential, Human Health Risks, and Yield Attributes of Hundred Bread Wheat Genotypes on Irrigation with Municipal and Remediated Wastewater. Environ. Sci. Pollut. Res. 2021, 28, 35023–35037. [Google Scholar] [CrossRef]
- Waheed, H.; Ilyas, N.; Iqbal Raja, N.; Mahmood, T.; Ali, Z. Heavy Metal Phyto-Accumulation in Leafy Vegetables Irrigated with Municipal Wastewater and Human Health Risk Repercussions. Int. J. Phytoremediation 2019, 21, 170–179. [Google Scholar] [CrossRef]

| Soil and Water Samples from Three Locations | pH (1:2.5) | EC (dS m−1) | Ca+2 | Mg+2 | CO3−2 | HCO3−1 | Cl− |
|---|---|---|---|---|---|---|---|
| mmolc L−1 | |||||||
| Soil used (1 soil: 3 sand w/w) | 8.44 | 0.413 | 3 | 10 | 0 | 5 | 2 |
| Kafr El-Sheikh location (IW1) | 7.5 | 0.599 | 3 | 3 | 0 | 4 | 2 |
| El-Hamoul location (IW2) | 8.2 | 1.44 | 5 | 3.4 | 0 | 4.2 | 5 |
| Baltim location (IW3) | 8.4 | 2.84 | 6 | 9.4 | 0 | 5 | 18 |
| Treatments | Definition of Treatment | Nanofertilizer Type | Source of Irrigation Water | |
|---|---|---|---|---|
| T1 | Control | No Adding Nanofertilizers | Irrigation Using Tap Water | |
| T2 | IW1 | Good water quality | Nano-Se | Irrigation from Meet Yazid canal at Kafr El-Sheikh location |
| T3 | Nano-Cu | |||
| T4 | Nano Se + Cu | |||
| T5 | IW2 | Moderate water quality | Nano-Se | Irrigation from Kitchener drain at El-Hamoul location |
| T6 | Nano-Cu | |||
| T7 | Nano Se + Cu | |||
| T8 | IW3 | Low water quality | Nano-Se | Irrigation from Kitchener drain at Baltim location |
| T9 | Nano-Cu | |||
| T10 | Nano Se + Cu | |||
| Treatments | Plant Stem Length (cm) | Plant Fresh Weight (g/Plant) | Leaf Area (cm2/Plant) | Branch No./Plant | No. of Leaves/Plant | Chlorophyll Content (SPAD) | |
|---|---|---|---|---|---|---|---|
| T1 | Control | 65.3 b | 250.5 a | 64.92 b | 4.0 a | 15.3 a | 50.5 f |
| T2 | IW1 | 61.3 cd | 211.9 b | 71.50 a | 2.5 a | 16.3 a | 61.6 e |
| T3 | 65.2 b | 211.4 b | 65.71 b | 3.0 a | 15.2 a | 63.5 e | |
| T4 | 54.3 e | 186.0 c | 60.43 cd | 2.4 a | 13.3 bc | 54.1 f | |
| T5 | IW2 | 69.8 a | 172.2 d | 52.64 e | 3.5 a | 13.4 bc | 66.4 d |
| T6 | 63.2 c | 184.4 c | 52.68 e | 2.9 a | 14.6 b | 71.2 c | |
| T7 | 58.7 d | 159.8 e | 51.52 e | 2.1 a | 12.7 bc | 76.2 b | |
| T8 | IW3 | 59.8 d | 169.6 d | 61.90 c | 2.1 a | 14.2 b | 79.7 a |
| T9 | 59.7 d | 203.9 b | 67.65 b | 2.3 a | 13.5 bc | 65.5 d | |
| T10 | 67.8 ab | 163.4 e | 61.97 c | 3.0 a | 12.8 bc | 67.8 d | |
| F-test | ** | ** | ** | NS | * | ** | |
| Treatments | Fruit pH | SSC (%) | Vitamin C (mg 100 g−1 FW) | Firmness (kg cm−2) | Fruit Yield (kg Plant−1) | |
|---|---|---|---|---|---|---|
| T1 | Control | 4.45 a | 5.38 d | 41.6 b | 430.0 c | 1.82 bc |
| T2 | IW1 | 4.54 a | 7.44 b | 33.2 d | 389.0 d | 1.83 bc |
| T3 | 4.48 a | 8.70 ab | 34.9 d | 511.0 a | 1.55 d | |
| T4 | 4.60 a | 8.84 ab | 38.4 c | 396.7 d | 1.90 b | |
| T5 | IW2 | 4.06 a | 7.95 b | 42.7 b | 494.7 ab | 2.05 a |
| T6 | 4.31 a | 5.78 cd | 39.6 c | 383.7 d | 1.75 c | |
| T7 | 4.48 a | 6.11 c | 45.9 a | 482.3 b | 1.62 d | |
| T8 | IW3 | 4.33 a | 7.18 b | 41.6 b | 444.3 c | 2.00 a |
| T9 | 4.31 a | 9.24 a | 44.8 a | 350.7 e | 2.07 a | |
| T10 | 4.42 a | 8.46 ab | 46.0 a | 335.0 e | 0.85 e | |
| F-test | NS | ** | ** | ** | ** | |
| Soil Treatments | pH | EC (dS m−1) | TDS (mg kg−1) | CaCO3 (g kg−1) | OM (g kg−1) | |
|---|---|---|---|---|---|---|
| T1 | Control | 8.41 | 0.482 | 236 | 98 | 9.9 |
| T2 | IW1 | 8.55 | 0.430 | 231 | 109 | 8.6 |
| T3 | 8.41 | 0.578 | 294 | 95 | 8.1 | |
| T4 | 8.50 | 0.498 | 250 | 111 | 9.9 | |
| T5 | IW2 | 8.39 | 0.610 | 305 | 111 | 11.1 |
| T6 | 8.23 | 0.649 | 323 | 99 | 9.6 | |
| T7 | 8.38 | 0.808 | 404 | 105 | 7.9 | |
| T8 | IW3 | 8.30 | 0.830 | 415 | 103 | 0.64 |
| T9 | 8.37 | 0.788 | 400 | 112 | 10.1 | |
| T10 | 8.33 | 0.892 | 450 | 116 | 13.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saffan, M.M.; Koriem, M.A.; El-Henawy, A.; El-Mahdy, S.; El-Ramady, H.; Elbehiry, F.; Omara, A.E.-D.; Bayoumi, Y.; Badgar, K.; Prokisch, J. Sustainable Production of Tomato Plants (Solanum lycopersicum L.) under Low-Quality Irrigation Water as Affected by Bio-Nanofertilizers of Selenium and Copper. Sustainability 2022, 14, 3236. https://doi.org/10.3390/su14063236
Saffan MM, Koriem MA, El-Henawy A, El-Mahdy S, El-Ramady H, Elbehiry F, Omara AE-D, Bayoumi Y, Badgar K, Prokisch J. Sustainable Production of Tomato Plants (Solanum lycopersicum L.) under Low-Quality Irrigation Water as Affected by Bio-Nanofertilizers of Selenium and Copper. Sustainability. 2022; 14(6):3236. https://doi.org/10.3390/su14063236
Chicago/Turabian StyleSaffan, Mohamed M., Mohamed A. Koriem, Ahmed El-Henawy, Shimaa El-Mahdy, Hassan El-Ramady, Fathy Elbehiry, Alaa El-Dein Omara, Yousry Bayoumi, Khandsuren Badgar, and József Prokisch. 2022. "Sustainable Production of Tomato Plants (Solanum lycopersicum L.) under Low-Quality Irrigation Water as Affected by Bio-Nanofertilizers of Selenium and Copper" Sustainability 14, no. 6: 3236. https://doi.org/10.3390/su14063236








