Essential Oils of Three Aromatic Plant Species as Natural Herbicides for Environmentally Friendly Agriculture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Tested Essential Oils
2.2. Effects on Seed Germination
2.3. Effects on Plant Growth
2.4. Statistical Analysis
3. Results
3.1. Effects of Essential Oils on Seed Germination
3.2. Effects of Essential Oils on Plant Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Preston, C. Plant biotic stress: Weeds. In Encyclopedia of Agriculture and Food Systems; Van Alfen, N.K., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 343–348. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: www.weedscience.org (accessed on 22 February 2022).
- Duke, S.; Pan, Z.; Bajsa-Hirschel, J.; Boyette, C.D. The potential future roles of natural compounds and microbial bioherbicides in weed management in crops. Adv. Weed Sci. 2022, 40, e020210054. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.S.; Kaufman, E. Natural product pesticides: Their development, delivery and use against insect vectors. Mini-Rev. Org. Chem. 2012, 9, 185–202. [Google Scholar] [CrossRef]
- De Mastro, G.; El Mahdi, J.; Ruta, C. Bioherbicidal potential of the essential oils from Mediterranean Lamiaceae for weed control in organic farming. Plants 2021, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, S.; Cioni, P.L.; Flamini, G.; Pardossi, A. Weeds for weed control: Asteraceae essential oils as natural herbicides. Weed Res. 2017, 57, 342–353. [Google Scholar] [CrossRef]
- Rehman, R.; Hanif, M.A.; Mushtaq, Z.; Mochona, B.; Qi, X. Biosynthetic factories of essential oils: The aromatic plants. Nat. Prod. Chem. Res. 2016, 4, 1000227. [Google Scholar] [CrossRef] [Green Version]
- Nikolova, M.T.; Berkov, S.H. Use of essential oils as natural herbicide. Ecol. Balk. 2018, 10, 259–265. [Google Scholar]
- Chou, C.H. The role of allelopathy in the diversity of plant communities in Taiwan. Bot. Bull. Acad. Sin. 1993, 34, 211–221. [Google Scholar]
- Aslam, F.; Khaliq, A.; Matloob, A.; Tanveer, A.; Hussain, S.; Zahir, Z.A. Allelopathy in agro-ecosystems: A critical review of wheat allelopathy-concepts and implications. Chemoecology 2017, 27, 1–24. [Google Scholar] [CrossRef]
- Li, Z.-H.; Wang, Q.; Ruan, X.; Pan, C.-D.; Jiang, D.-A. Phenolics and plant allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [Green Version]
- Scavo, A.; Restuccia, A.; Mauromicale, G. Allelopathy: Principles and basic aspects for agroecosystem control. In Sustainable Agriculture Reviews; Gaba, S., Smith, B., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2018; Volume 28. [Google Scholar] [CrossRef]
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic effects and mechanism of action of essential oils and terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef]
- Singh, A.A.; Rajeswari, G.; Nirmal, L.A.; Jacob, S. Synthesis and extraction routes of allelochemicals from plants and microbes: A review. Rev. Anal. Chem. 2021, 40, 293–311. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Kohli, R.K.; Kaur, S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manag. 2008, 256, 2166–2174. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Setia, N.; Kaur, S.; Ravinder, K.K. Chemical composition and phytotoxicity of volatile essential oil from intact and fallen leaves of Eucalyptus citriodora. Z. Für Nat. C 2006, 61, 465–471. [Google Scholar] [CrossRef]
- Fagodia, S.K.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Phytotoxicity and cytotoxicity of Citrus aurantiifolia essential oil and its major constituents: Limonene and citral. Ind. Crops Prod. 2017, 108, 708–715. [Google Scholar] [CrossRef]
- Dudai, N.; Poljakoff-Mayber, A.; Mayer, A.M.; Putievsky, E.; Lerner, H.R. Essential oils as allelochemicals and their potential use as bioherbicides. J. Chem. Ecol. 1999, 25, 1079–1089. [Google Scholar] [CrossRef]
- Angelini, L.G.; Carpanese, G.; Cioni, P.L.; Morelli, I.; Macchia, M.; Flamini, G. Essential oils from Mediterranean Lamiaceae as weed germination inhibitors. J. Agric. Food Chem. 2003, 51, 6158–6164. [Google Scholar] [CrossRef]
- Araniti, F.; Lupini, A.; Sorgonà, A.; Statti, G.A.; Abenavoli, M.R. Phytotoxic activity of foliar volatiles and essential oils of Calamintha nepeta (L.) Savi. Nat. Prod. Res. 2013, 27, 1651–1656. [Google Scholar] [CrossRef]
- Islam, A.K.M.; Kato-Noguchi, H. Allelopathic potential of five Labiatae plant species on barnyard grass (’Echinochloa crus-galli’). Aust. J. Crop Sci. 2013, 7, 1369–1374. [Google Scholar]
- Jassbi, A.R.; Zare, S.; Firuzi, O.; Xiao, J. Bioactive phytochemicals from shoots and roots of Salvia species. Phytochem. Rev. 2016, 15, 829–867. [Google Scholar] [CrossRef]
- Atak, M.; Mavi, K.; Uremis, I. Bioherbicidal effects of oregano and rosemary essential oils on germination and seedling growth of bread wheat cultivars and weeds. Rom. Biotechnol. Lett. 2016, 21, 11149–11158. [Google Scholar]
- Della Pepa, T.; Elshafie, H.S.; Capasso, R.; De Feo, V.; Camele, I.; Nazzaro, F.; Scognamiglio, M.R.; Caputo, L. Antimicrobial, and phytotoxic activity of Origanum heracleoticum and O. majorana essential oils growing in Cilento (Southern Italy). Molecules 2019, 24, 2576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouini, A.; Verdeguer, M.; Pinton, S.; Araniti, F.; Palazzolo, E.; Badalucco, L.; Laudicina, V.A. Potential effects of essential oils extracted from Mediterranean aromatic plants on target weeds and soil microorganisms. Plants 2020, 9, 1289. [Google Scholar] [CrossRef] [PubMed]
- Khare, P.; Srivastava, S.; Nigam, N.; Singh, A.K.; Singh, S. Impact of essential oils of E. citriodora, O. basilicum and M. arvensis on three different weeds and soil microbial activities. Environ. Technol. Innov. 2019, 14, 100343. [Google Scholar] [CrossRef]
- Singh, N.; Singh, H.P.; Batish, D.R.; Kohli, R.K.; Yadav, S.S. Chemical characterization, phytotoxic, and cytotoxic activities of essential oil of Mentha longifolia. Environ. Sci. Pollut. Res. Int. 2020, 27, 13512–13523. [Google Scholar] [CrossRef]
- Murray, M.T. 94-Mentha piperita (Peppermint). In Textbook of Natural Medicine, 5th ed.; Pizzorno, J.E., Murray, M.T., Eds.; Elseviere: Amsterdam, The Netherlands, 2020; pp. 713–715. [Google Scholar] [CrossRef]
- Bandoni, A. Los Recursos Vegetales Aromáticos en Latinoamérica; Ciencia y Tecnología para el Desarrollo CYTED; University of Buenos Aires: Buenos Aires, Argentina, 2002; pp. 27–29. Available online: https://es.scribd.com/doc/7959699/Los-Recursos-Vegetales-Aromaticos-en-America-Latina (accessed on 17 February 2022).
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef]
- Abdi, G.; Shokrpour, M.; Salami, S.A. Essential oil composition at different plant growth development of peppermint (Mentha x piperita L.) under water deficit stress. J. Essent. Oil Bear. Plants 2019, 22, 431–440. [Google Scholar] [CrossRef]
- Wu, Z.; Tan, B.; Liu, Y.; Dunn, J.; Martorell Guerola, P.; Tortajada, M.; Cao, Z.; Ji, P. Chemical composition and antioxidant properties of essential oils from peppermint, native spearmint and Scotch spearmint. Molecules 2019, 24, 2825. [Google Scholar] [CrossRef] [Green Version]
- Morales, R. Thymbra L. In Flora iberica. Plantas vasculares de la Península Ibérica e Islas Baleares; Morales, R., Quintanar, A., Cabezas, F., Pujadas, A.J., Cirujano, S., Eds.; Real Jardín Botánico, CSIC: Madrid, Spain, 2010; Volume 12, pp. 1–3. [Google Scholar]
- Blanco, Y. La utilización de la alelopatía y sus efectos en diferentes cultivos agrícolas. Cult. Trop. 2006, 27, 5–16. Available online: http://www.redalyc.org/pdf/1932/193217731003.pdf (accessed on 18 January 2022).
- Miceli, A.; Negro, C.; Tommasi, L. Essential oil variability in Thymbra capitata (L.) Cav. growing wild in Southern Apulia (Italy). Biochem. Syst. Ecol. 2006, 34, 528–535. [Google Scholar] [CrossRef]
- Hanana, M.; Mansour, M.B.; Algabr, M.; Amri, I.; Gargouri, S.; Romane, A.; Jamoussi, B.; Hamrouni, L. Potential use of essential oils from four Tunisian species of Lamiaceae: Biological alternative for fungal and weed control. Rec. Nat. Prod. 2017, 11, 258–269. [Google Scholar]
- Verdeguer, M.; Torres-Pagan, N.; Muñoz, M.; Jouini, A.; García-Plasencia, S.; Chinchilla, P.; Berbegal, M.; Salamone, A.; Agnello, S.; Carrubba, A.; et al. Herbicidal activity of Thymbra capitata (L.) Cav. essential oil. Molecules 2020, 25, 2832. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R. A Review of the traditional uses, phytochemistry and biological activities of the genus Santolina. Planta Med. 2018, 84, 627–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbajal, R.; Ortiz, S.; Sáez, L.; Santolina, L. Flora Iberica. Plantas vasculares de la Península Ibérica e Islas Baleares; Benedí, C., Buira, A., Rico, E., Crespo Villalba, M.B., Quintanar, A., Aedo, C., Eds.; Real Jardín Botánico, CSIC: Madrid, Spain, 2019; Volume 16, pp. 1938–1968. [Google Scholar]
- Demirci, B.; Özek, T.; Baser, K.H.C. Chemical composition of Santolina chamaecyparissus L. essential oil. J. Essent. Oil Res. 2000, 12, 625–627. [Google Scholar] [CrossRef]
- Garg, S.N.; Gupta, D.; Mehta, V.K.; Kumar, S. Volatile constituents of the essential oil of Santolina chamaecyparissus Linn. from the Southern Hills of India. J. Essent. Oil Res. 2001, 13, 234–235. [Google Scholar] [CrossRef]
- Michael, P.W. Some weedy species of Amaranthus (amaranths) and Conyza/Erigeron (fleabanes) naturalized in the Asian-Pacific region. In Proceedings of the 6th Asian-Pacific Weed Science Society Conference, Jakarta, Indonesia, 11–17 July 1977; Volume 1, pp. 87–95. [Google Scholar]
- Negrean, G.; Ioana, C. Conyza bonariensis, a new plant with invasive character in Romanian flora. Ann. Univ. Craiova-Biol. 2012, 17, 743–748. [Google Scholar]
- Wirth, T.; Csiky, J. Contributions to the Hungarian alien flora: Erigeron bonariensis L. and E. sumatrensis Retz. (Asteraceae) in Hungary. Bot. Közlemények 2020, 107, 33–43. [Google Scholar] [CrossRef]
- Somervaille, A.; McLennan, B. The 2nd Fallow Weed Management Guide; Conservation Farmers: Toowoomba, Australia, 2003. [Google Scholar]
- Wu, H.; Walker, S.; Rollin, M.J.; Tan, D.K.Y.; Robinson, G.; Werth, J. Germination, persistence, and emergence of flaxleaf fleabane (Conyza bonariensis [L.] Cronquist). Weed Biol. Manag. 2007, 7, 192–199. [Google Scholar] [CrossRef]
- Kleinman, Z.; Rubin, B. Non-target-site glyphosate resistance in Conyza bonariensis is based on modified subcellular distribution of the herbicide. Pest. Manag. Sci. 2017, 73, 246–253. [Google Scholar] [CrossRef]
- HRAC (Herbicide Resistance Action Committee). Available online: https://hracglobal.com/tools/classification-lookup (accessed on 22 February 2022).
- Champion, P.; James, T.; Dawson, M. New names for New Zealand weeds. N. Z. Plant Prot. 2010, 63, 72–77. [Google Scholar] [CrossRef]
- Santa Cruz, J.; Cordero, S. First record of Araujia sericifera (Apocynaceae: Asclepiadoideae) for Chile, a new alien climbing species from South America. B. Soc. Argent. Bot. 2018, 53, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Calle Fernandez, M. Control de la germinación in vitro de Araujia sericifera con aceites esenciales de Laurus nobilis, Myrtus communis, Citrus sinensis y Citrus limon. Master’s Thesis, Universitat Politècnica de València, Valencia, Spain, 2010. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil; California Agriculture Experiment Station, College of Agriculture, University of California: Berkeley, CA, USA, 1950; Volume 347, pp. 1–32. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 4–9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 25 February 2022).
- Xavier, V.; Finimundy, T.C.; Heleno, S.A.; Amaral, J.S.; Calhelha, R.C.; Vaz, J.; Pires, T.C.S.P.; Mediavilla, I.; Esteban, L.S.; Ferreira, I.C.F.R.; et al. Chemical and bioactive characterization of the essential oils obtained from three Mediterranean plants. Molecules 2021, 26, 7472. [Google Scholar] [CrossRef]
- Anwar, S.; Naseem, S.; Karimi, S.; Asi, M.R.; Akrem, A.; Ali, Z. Bioherbicidal activity and metabolic profiling of potent allelopathic plant fractions against major weeds of wheat—Way forward to low the risk of synthetic herbicides. Front. Plant Sci. 2021, 12, 632390. [Google Scholar] [CrossRef]
- O’Bryan, C.A.; Pendleton, S.J.; Crandall, P.G.; Ricke, S.C. Potential of plant essential oils and their components in animal agriculture—In vitro studies on antibacterial mode of action. Front. Vet. Sci. 2015, 2, 35. [Google Scholar] [CrossRef] [Green Version]
- El-Beltagi, H.; Dhawii, F.; Aly, A.A.; El-Ansary, A.E. Chemical compositions and biological activities of the essential oils the essential oils from gamma irradiated celery (Apium graveolens L.) seeds L. Not. Bot. Horti Agrobot. 2020, 48, 2114–2133. [Google Scholar] [CrossRef]
- Mkaddem Guedri, M.; Romdhane, M.; Lebrihi, A.; Mathieu, F.; Bouajila, J. Chemical composition and antimicrobial and antioxidant activities of Tunisian, France and Austrian Laurus nobilis (Lauraceae) essential oils. Not. Bot. Horti Agrobot. 2020, 48, 1929–1940. [Google Scholar] [CrossRef]
- Mani-López, E.; Cortés-Zavaleta, O.; López-Malo, A. A review of the methods used to determine the target site or the mechanism of action of essential oils and their components against fungi. SN Appl. Sci. 2021, 3, 44. [Google Scholar] [CrossRef]
- Cadena, M.B.; Preston, G.M.; Van der Hoorn, R.A.L.; Townley, H.E.; Thompson, I.P. Species-specific antimicrobial activity of essential oils and enhancement by encapsulation in mesoporous silica nanoparticles. Ind. Crops Prod. 2018, 122, 582–590. [Google Scholar] [CrossRef]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular targets for components of essential oils in the insect nervous system—A review. Molecules 2018, 23, 34. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, A.K.; Singh, V.K.; Kedia, A.; Das, S.; Dubey, N.K. Essential oils and their bioactive compounds as eco-friendly novel green pesticides for management of storage insect pests: Prospects and retrospects. Environ. Sci. Pollut. Res. 2021, 28, 18918–18940. [Google Scholar] [CrossRef]
- Werrie, P.-Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.-L. Phytotoxicity of essential oils: Opportunities and constraints for the development of biopesticides. A review. Foods 2020, 9, 1291. [Google Scholar] [CrossRef]
- de Souza Barros, V.M.; Ferreira Pedrosa, J.L.; Ribeiro Gonçalves, D.; Carvalho Lopes de Medeiros, F.; Rodrigues Carvalho, G.; Gonçalves, A.H.; Quinute Teixeira, P.V.V. Herbicides of biological origin: A review. J. Hort. Sci. Biotechnol. 2021, 96, 288–296. [Google Scholar] [CrossRef]
- Jorritsma-Wienk, L.D.; Ameloot, E.; Lenssen, J.P.; de Kroon, H. Differential responses of germination and seedling establishment in populations of Tragopogon pratensis (Asteraceae). Plant Biol. 2007, 9, 109–115. [Google Scholar] [CrossRef]
- Jiménez-Alfaro, B.; Silveira, F.A.O.; Fidelis, A.; Poschlod, P.; Commander, L.E. Seed germination traits can contribute better to plant community ecology. J. Veg. Sci. 2016, 27, 637–645. [Google Scholar] [CrossRef]
- Donohue, K.; De Casas, R.R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, post-germination adaptation, and species ecological ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Mariano, V.; Christianini, A.V. Effects of anthropogenic disturbance on seed germination under field conditions: A meta-analysis. Acta Oecol. 2021, 113, 103791. [Google Scholar] [CrossRef]
- Putnam, A.R.; Duke, W.O. Allelopathy in agroecosystems. Annu. Rev. Phytopathol. 1978, 16, 431–451. [Google Scholar] [CrossRef]
- Wetson, L.A. Utilization of allelopathy for weed management in agroecosystems. Agron. J. 1996, 88, 860–866. [Google Scholar]
- Colas, F.; Cordeau, S.; Granger, S.; Jeuffroy, M.-H.; Pointurier, O.; Queyrel, W.; Rodriguez, A.; Villerd, J.; Colbach, N. Co-development of a decision support system for integrated weed management: Contribution from future users. Eur. J. Agron. 2020, 114, 126010. [Google Scholar] [CrossRef]
- Verschwele, A. Integrated weed management in cropping systems: Principles, methods and experience of field trials. In Exploring and Optimizing Agricultural Landscapes. Innovations in Landscape Research; Mueller, L., Sychev, V.G., Dronin, N.M., Eulenstein, F., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Arista, M.; Ortiz, P.L. Araujia Brot. In Flora iberica. Plantas vasculares de la Península Ibérica e Islas Baleares; Talavera, S., Andrés, C., Arista, M., Fernández Piedra, M.P., Gallego, M.J., Ortiz, P.L., Romero Zarco, C., Salgueiro, F.J., Silvestre, S., Quintanar, A., Eds.; Real Jardín Botánico, CSIC: Madrid, Spain, 2012; Volume 11, pp. 117–119. [Google Scholar]
- Al Hassan, M.; Pacurar, A.; López-Gresa, M.P.; Donat-Torres, M.P.; Llinares, J.V.; Boscaiu, M.; Vicente, O. Effects of salt stress on three ecologically distinct Plantago species. PLoS ONE 2016, 11, e0160236. [Google Scholar] [CrossRef]
- Plazas, M.; González-Orenga, S.; Nguyen, H.T.; Morar, I.M.; Fita, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Growth and antioxidant responses triggered by water stress in wild relatives of eggplant. Sci. Hort. 2022, 293, 110685. [Google Scholar] [CrossRef]
- Johnson, D.W.; Smith, S.E.; Dobrenz, A.K. Genetic and phenotypic relationships in response to NaCl at different developmental stages in alfalfa. Theor. Appl. Genet. 1992, 83, 833–838. [Google Scholar] [CrossRef]
- Vicente, O.; Boscaiu, M.; Nranjo, M.A.; Estrelles, E.; Bellés, J.M.; Soriano, P. Responses to salt stress in the halophyte Plantago crassifolia (Plantaginaceae). J. Arid Environ. 2004, 58, 463–481. [Google Scholar] [CrossRef]
- Vokou, D.; Douvli, P.; Blionis, G.J.; Halley, J.M. Effects of monoterpenoids, acting alone or in pairs, on seed germination and subsequent seedling growth. J. Chem. Ecol. 2003, 29, 2281–2301. [Google Scholar] [CrossRef]
- Chang, Y.; Harmon, P.F.; Treadwell, D.D.; Carrillo, D.; Sarkhosh, A.; Brecht, J. Biocontrol potential of essential oils in organic horticulture systems: From farm to fork. Front. Nutr. 2022, 8, 805138. [Google Scholar] [CrossRef]
- Verdeguer, M.; Castañeda, L.G.; Torres-Pagan, N.; Llorens-Molina, J.A.; Carrubba, A. Control of Erigeron bonariensis with Thymbra capitata, Mentha piperita, Eucalyptus camaldulensis, and Santolina chamaecyparissus essential oils. Molecules 2020, 25, 562. [Google Scholar] [CrossRef] [Green Version]
- Saoud, I.; Hamrouni, L.; Gargouri, S.; Amri, I.; Hanana, M.; Fezzani, T. Chemical composition, weed killer and antifungal activities of Tunisian thyme (Thymus capitatus Hoff. et Link.) essential oils. Acta Aliment. Hung. 2013, 42, 417–427. [Google Scholar] [CrossRef]
- Gagliano Candela, R.; Maggi, F.; Lazzara, G.; Rosselli, S.; Bruno, M. The essential oil of Thymbra capitata and its application as a biocide on stone and derived surfaces. Plants 2019, 8, 300. [Google Scholar] [CrossRef] [Green Version]
- Cavalieri, A.; Caporali, F. Effects of essential oils of cinnamon, lavender and peppermint on germination of Mediterranean weeds. Allelopath. J. 2010, 25, 441–452. [Google Scholar]
- Mahdavikia, F.; Saharkhiz, M.J. Phytotoxic activity of essential oil and water extract of peppermint (Mentha× piperita L. CV. Mitcham). J. Appl. Res. Med. Aromat. Plants 2015, 2, 146–153. [Google Scholar] [CrossRef]
- Grosso, C.; Coelho, J.A.; Urieta, J.S.; Palabra, A.M.; Barroso, J.G. Herbicidal activity of volatiles from coriander, winter savory, cotton lavender, and thyme isolated by hydrodistillation and supercritical fluid extraction. J. Agric. Food Chem. 2010, 27, 11007–11013. [Google Scholar] [CrossRef] [PubMed]
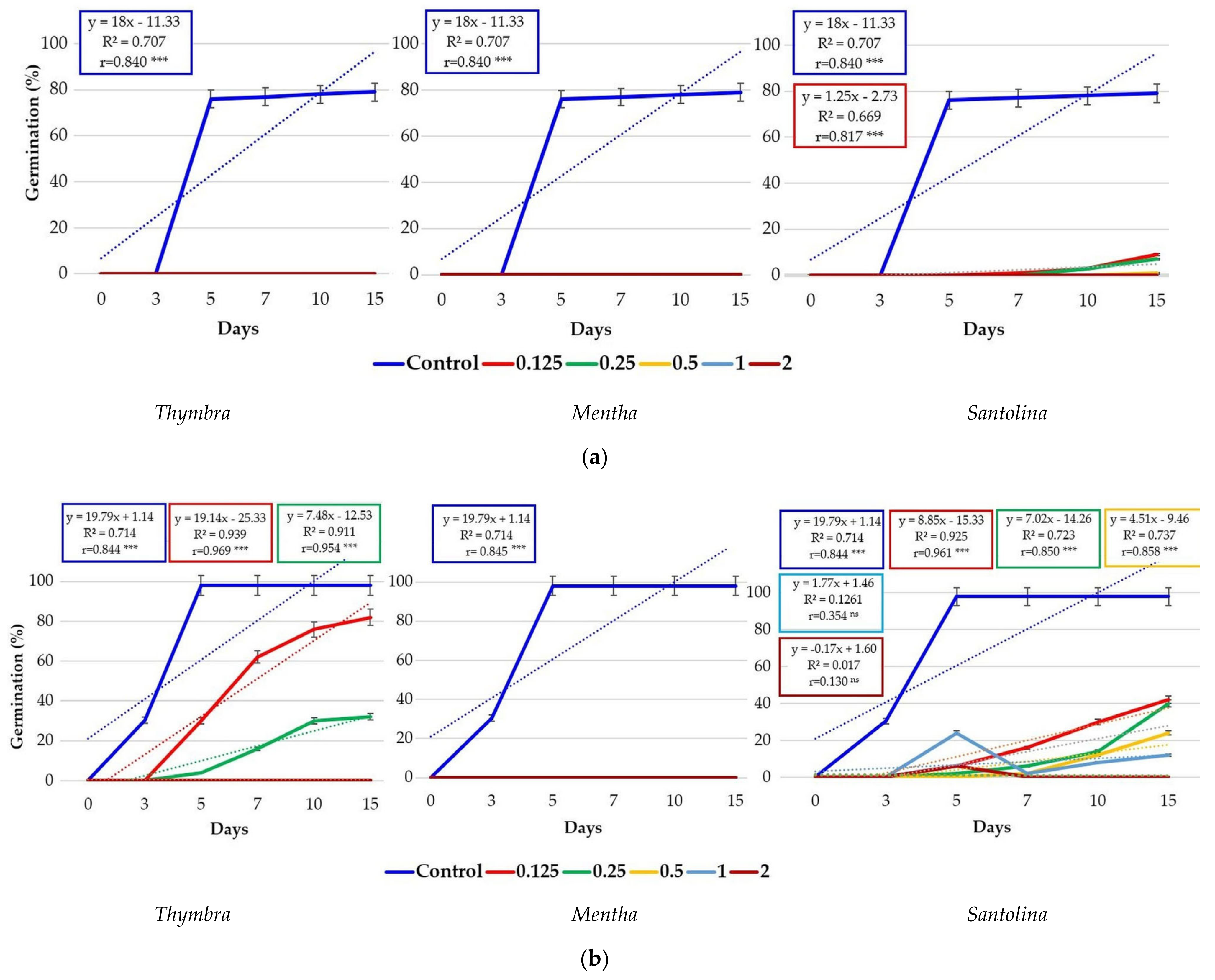
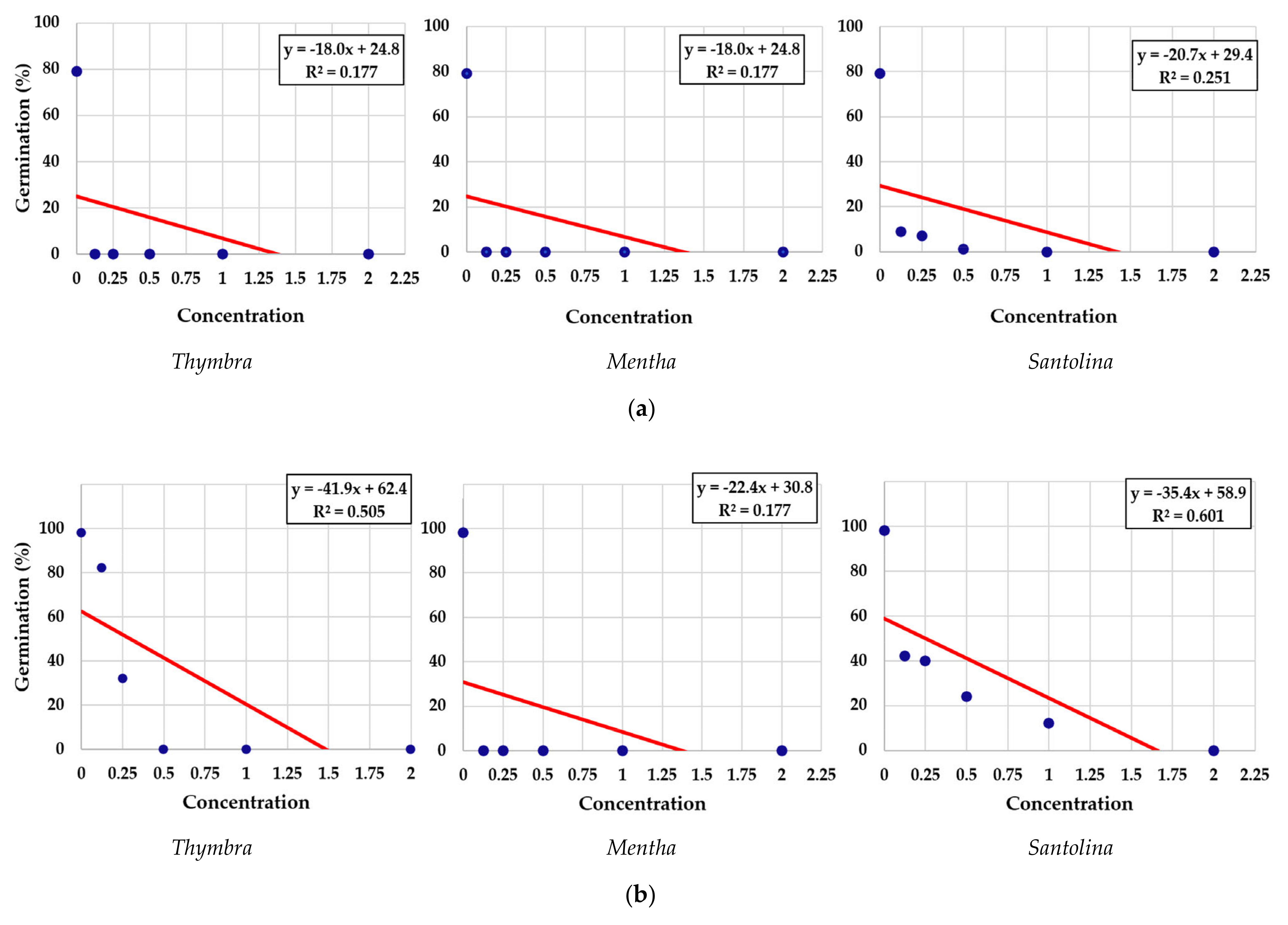
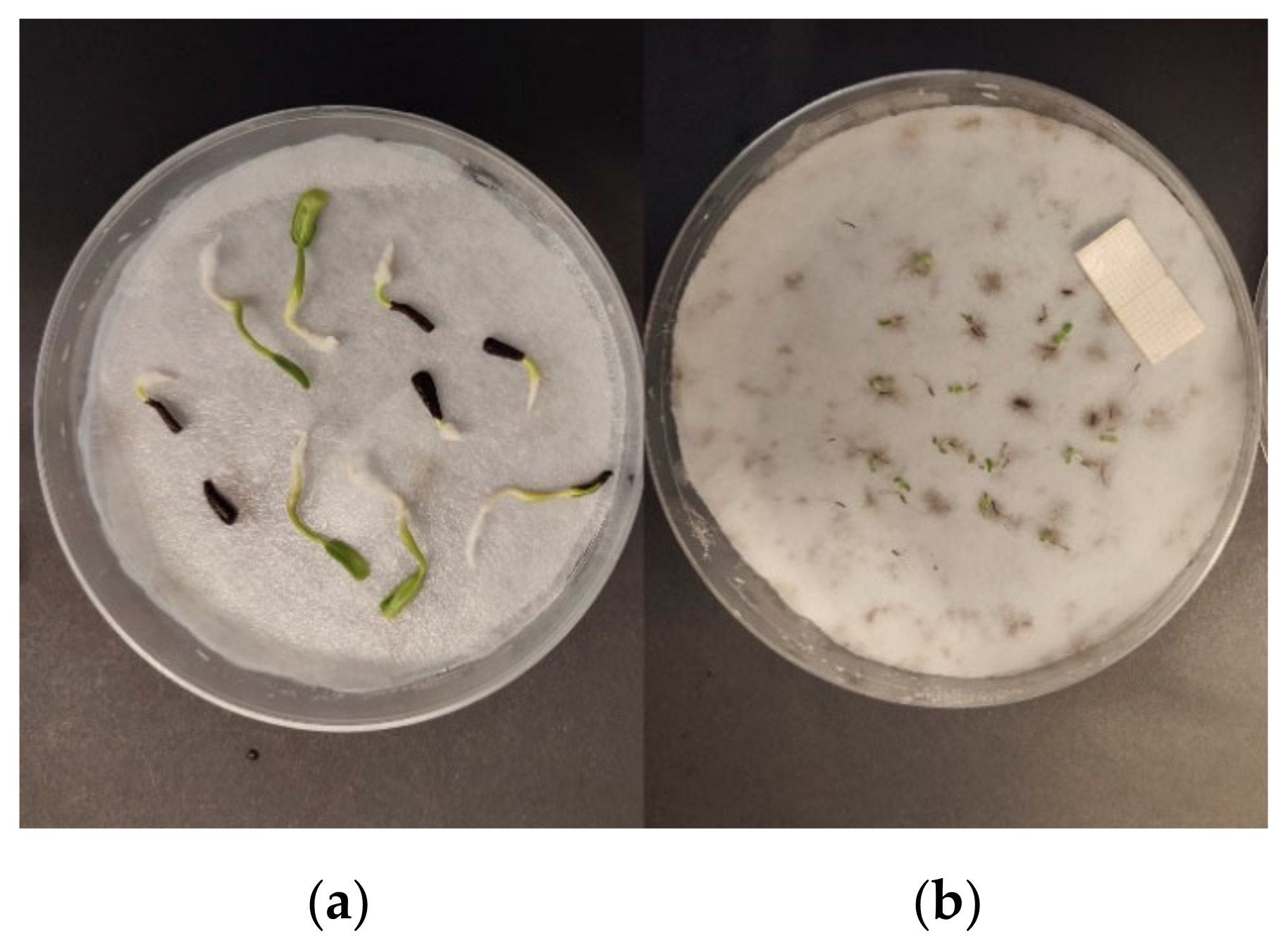
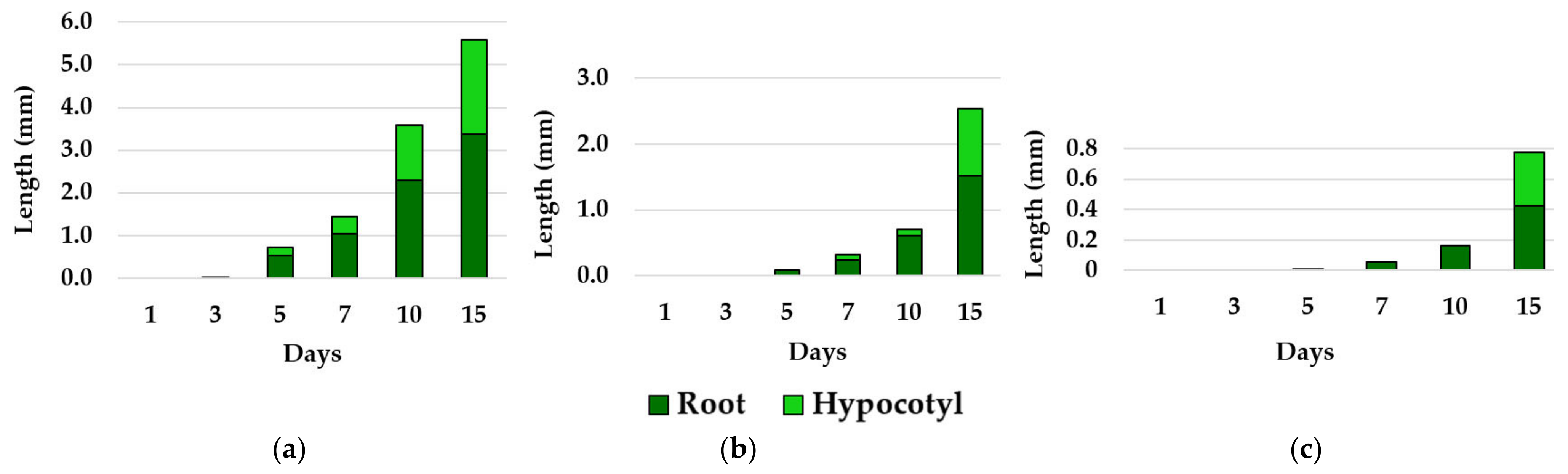

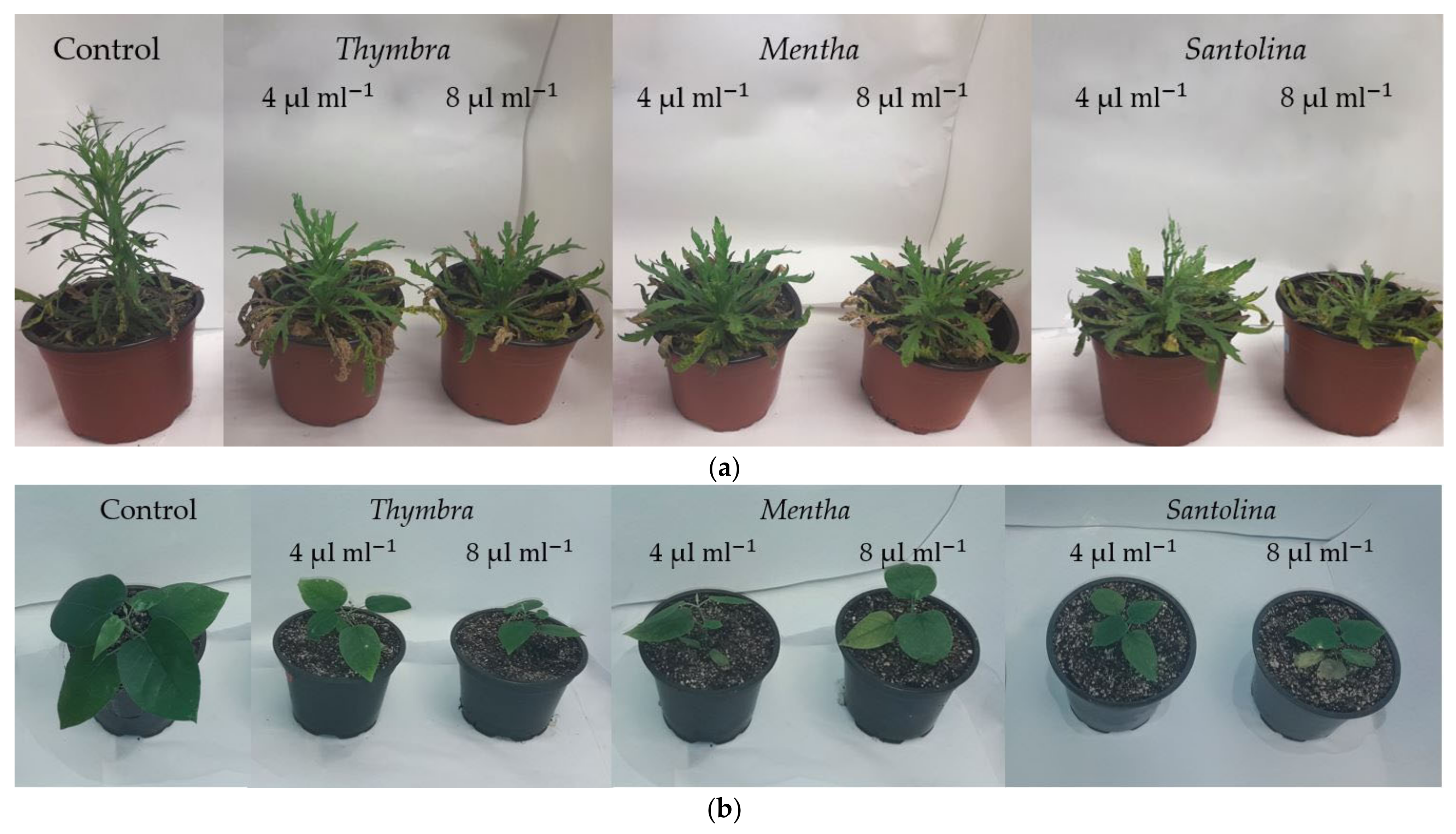
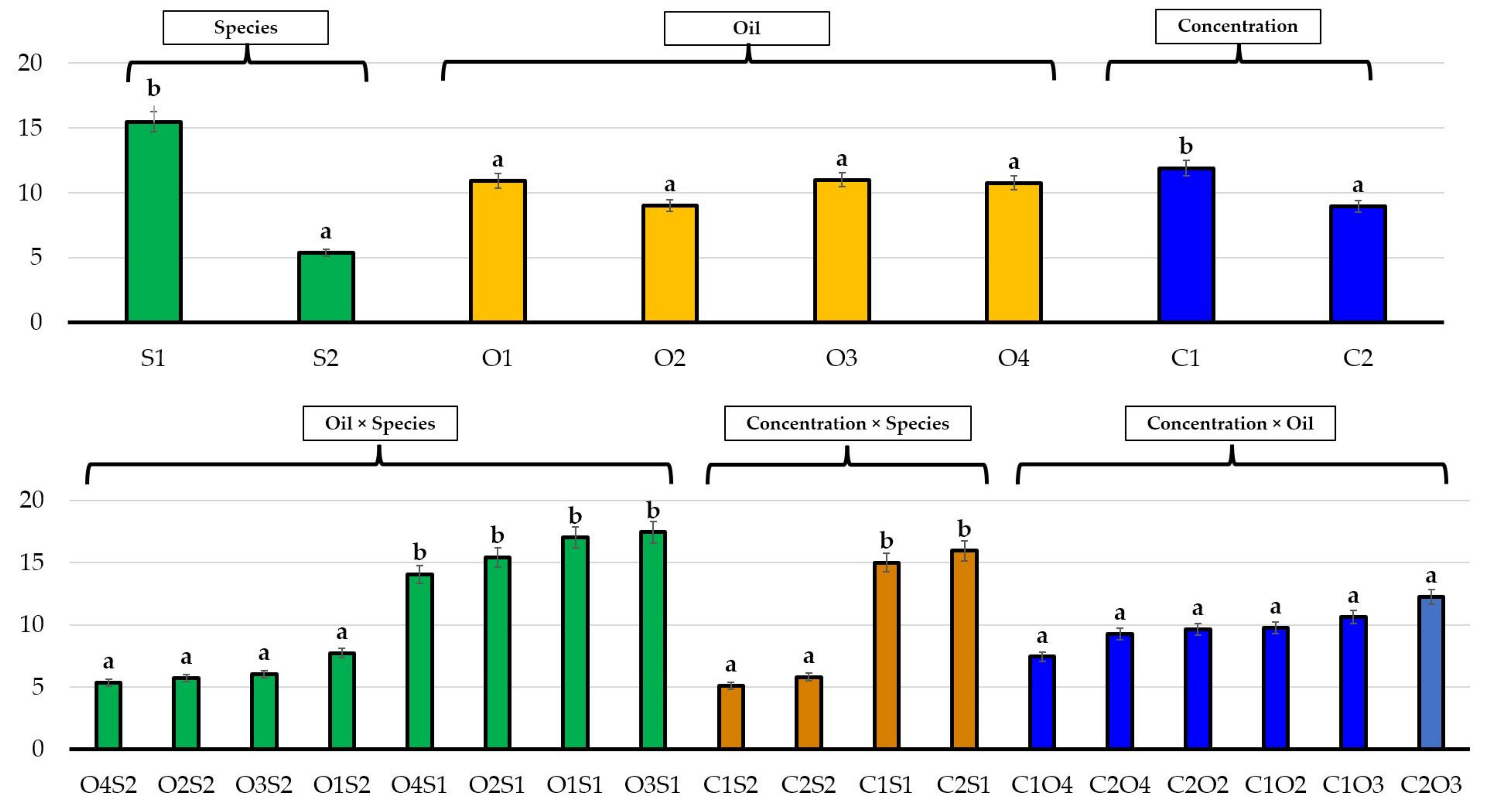
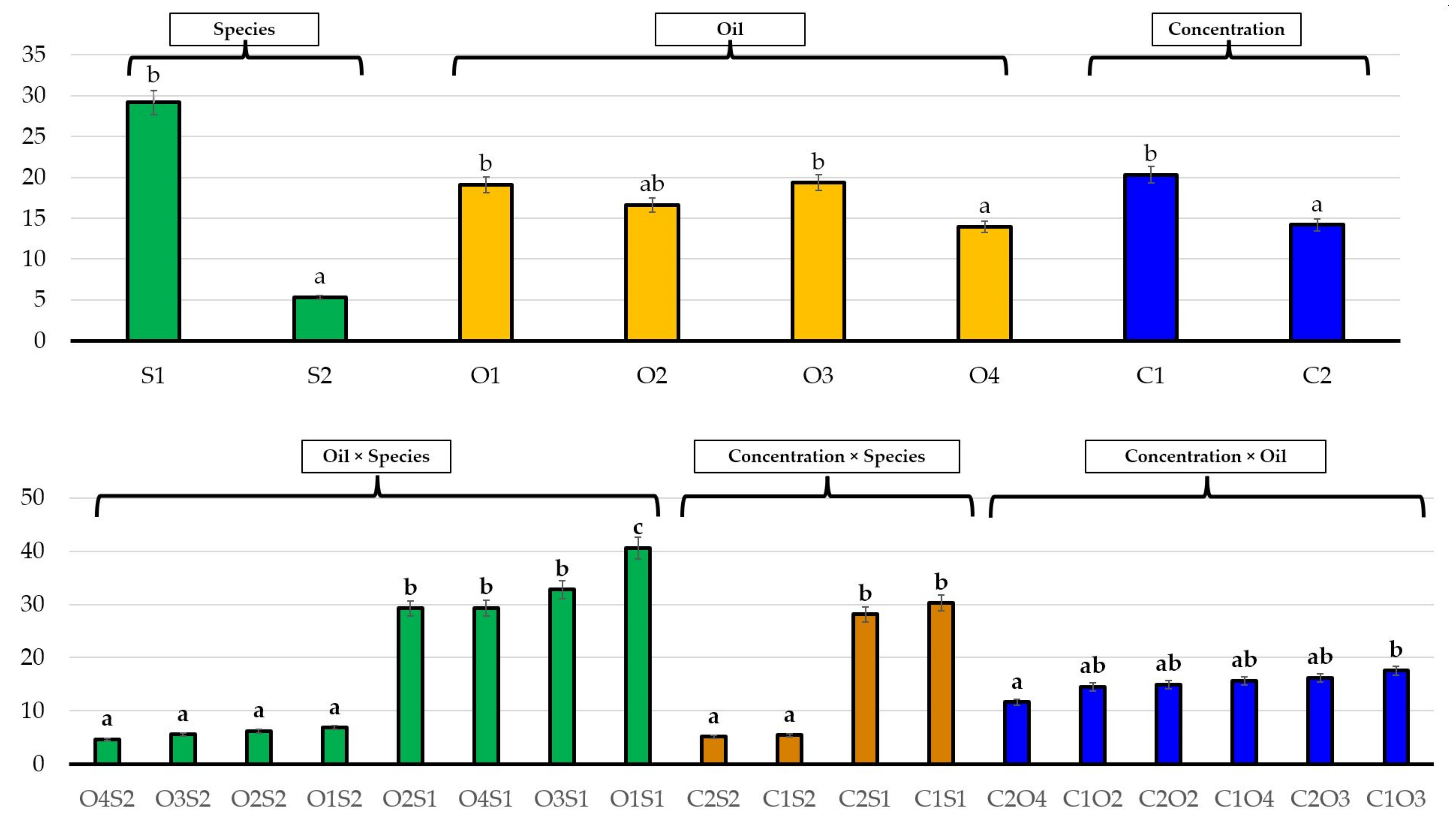


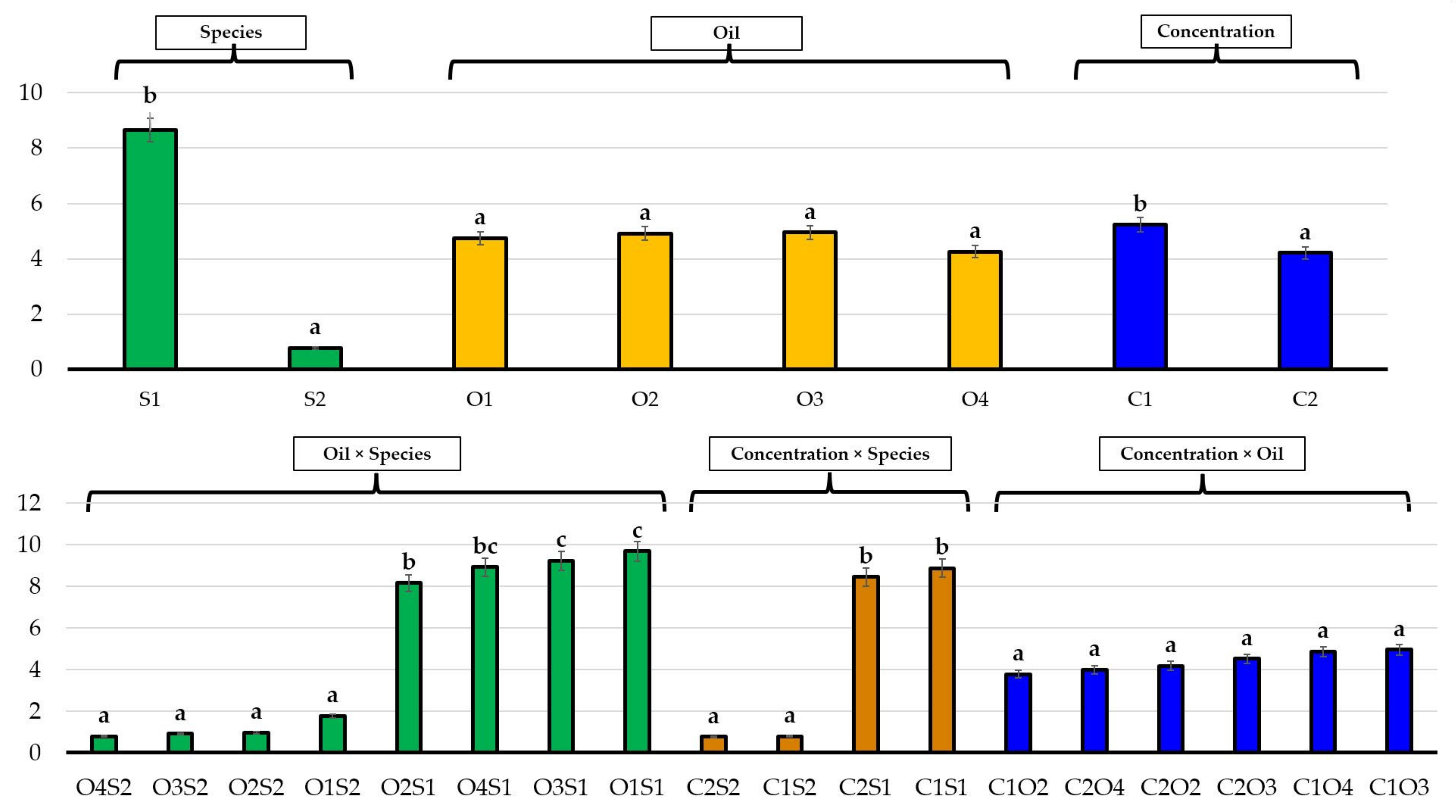
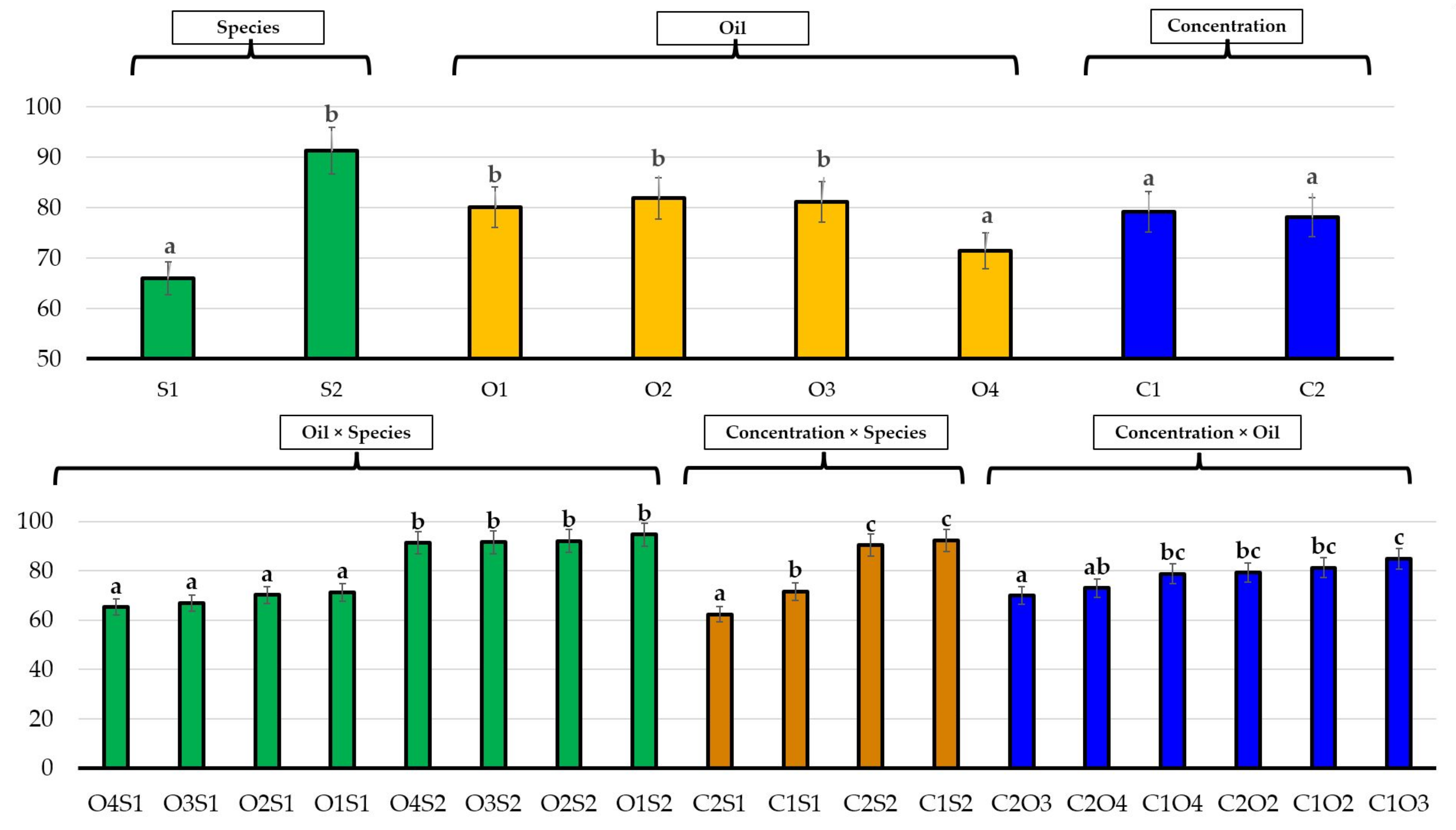
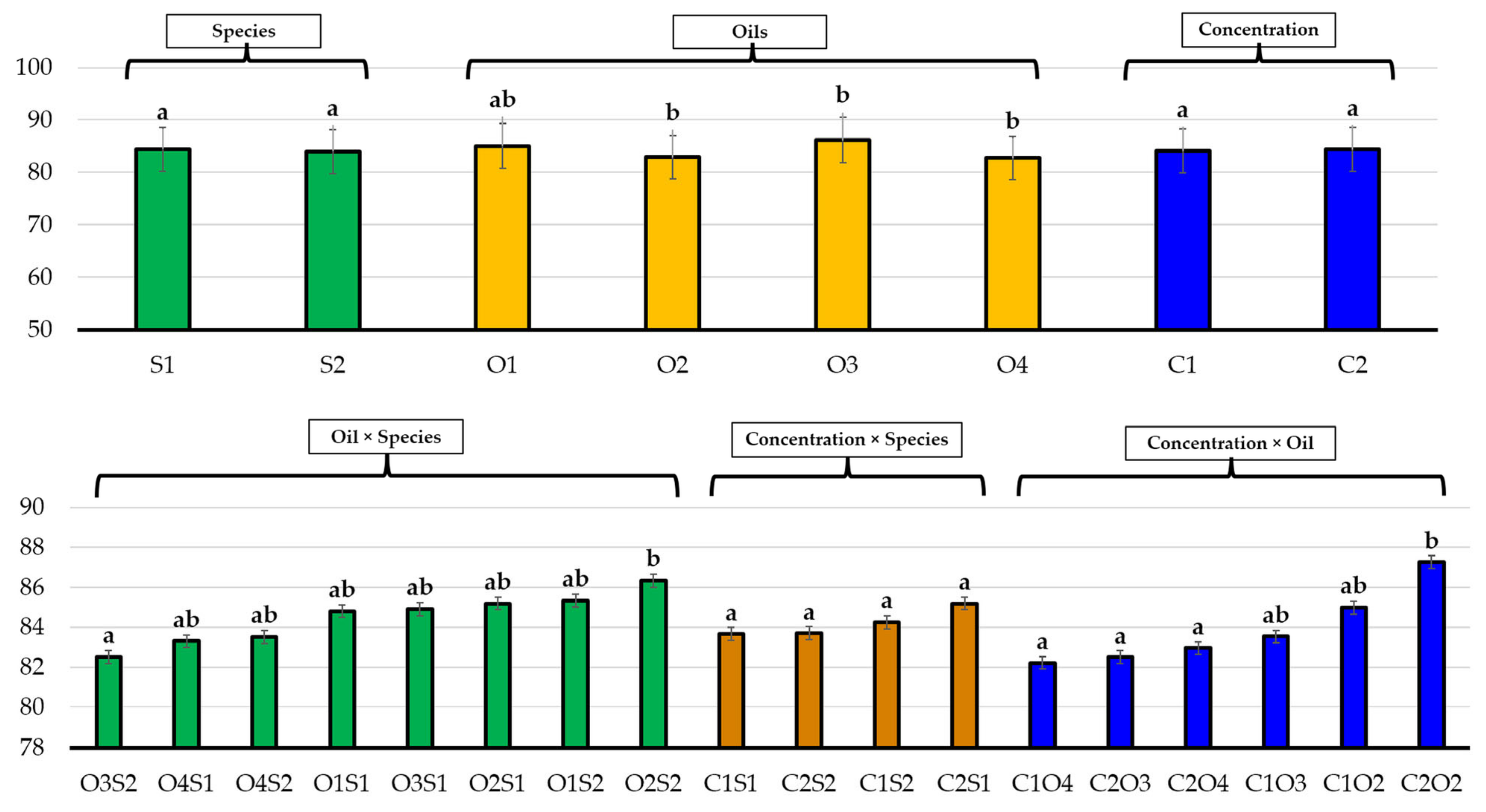
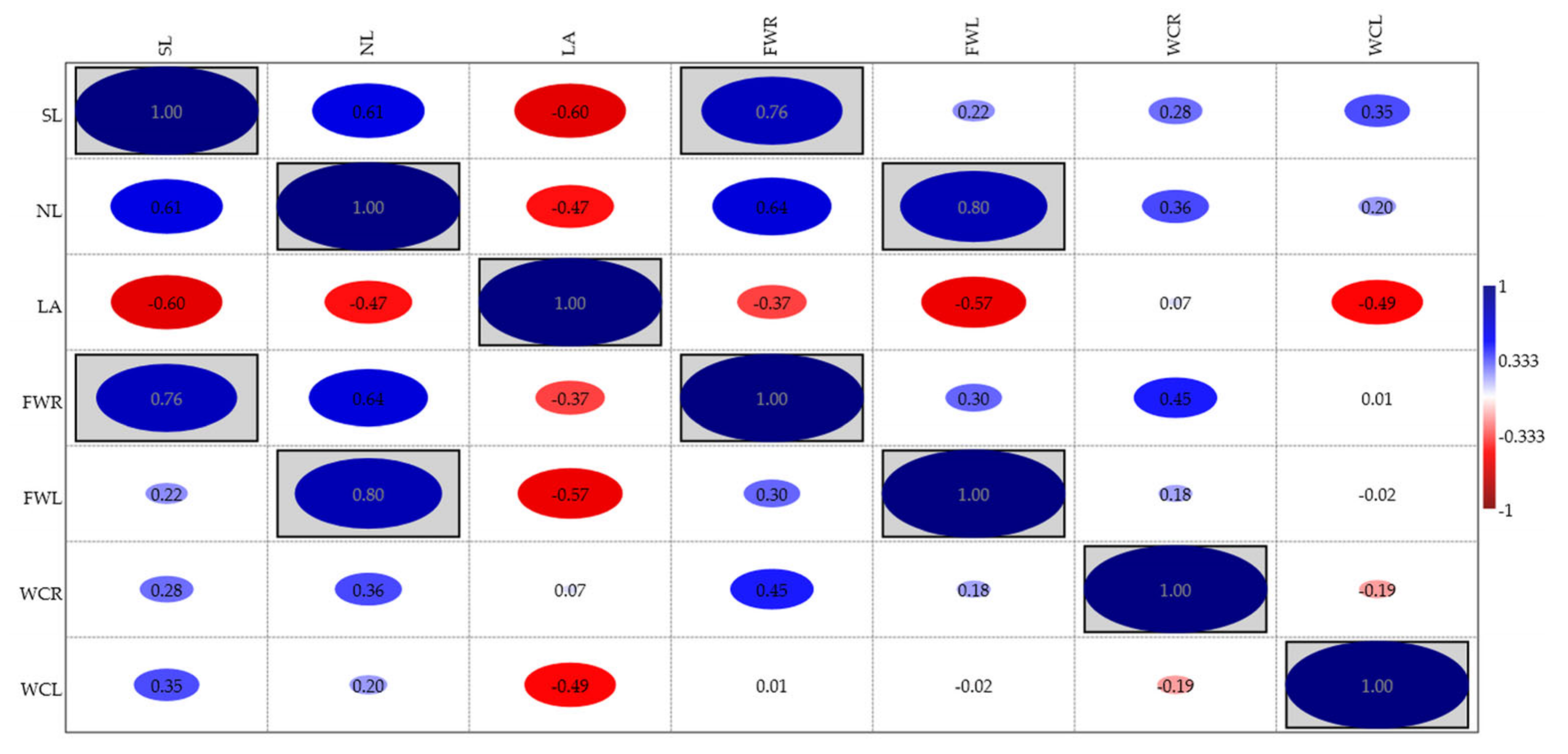

| No. | EO Treatment (Concentration) | E. bonariensis | A. sericifera | ||||
|---|---|---|---|---|---|---|---|
| Thymbra | Mentha | Santolina | Thymbra | Mentha | Santolina | ||
| 1. | Control | 0.28 a ± 0.02 | 0.28 a ± 0.02 | 0.28 a ± 0.02 | 5.58 a ± 0.18 | 5.58 a ± 0.02 | 5.58 a ± 0.02 |
| 2. | 0.125 | 0.00 b ± 0.00 | 0.00 b ± 0.00 | 0.02 b ± 0.01 | 2.53 b ± 0.22 | 0.00 b ± 0.00 | 1.00 b ± 0.012 |
| 3. | 0.25 | 0.00 b ± 0.00 | 0.00 b ± 0.00 | 0.02 b ± 0.01 | 0.75 c ± 0.17 | 0.00 b ± 0.00 | 0.64 b ± 0.16 |
| 4. | 0.5 | 0.00 b ± 0.00 | 0.00 b ± 0.00 | 0.00 c ± 0.00 | 0.00 d ± 0.13 | 0.00 b ± 0.00 | 0.40 c ± 0.13 |
| 5. | 1 | 0.00 b ± 0.00 | 0.00 b ± 0.00 | 0.00 c ± 0.00 | 0.00 d ± 0.09 | 0.00 b ± 0.00 | 0.20 c ± 0.09 |
| 6. | 2 | 0.00 b ± 0.00 | 0.00 b ± 0.00 | 0.00 c ± 0.00 | 0.00 d ± 0.00 | 0.00 b ± 0.00 | 0.00 d ± 0.00 |
| Treatment */ The Combination of Factors | Stem Length (cm) | Number of Leaves | Leaf Area (cm2) | Fresh Weight Roots (g) | Fresh Weight Leaves (g) | Water Content Roots (%) | Water Content Leaves (%) |
|---|---|---|---|---|---|---|---|
| S1O1 | 17.01 d | 40.57 d | 6.38 a | 2.87 d | 9.68 d | 71.25 b | 84.80 bc |
| S1O2C1 | 15.16 cd | 23.14 bc | 8.28 ab | 2.77 d | 7.07 b | 74.13 bc | 83.10 abc |
| S1O2C2 | 14.16 cd | 24.00 bc | 6.79 a | 1.59 abcd | 7.72 bc | 65.25 ab | 87.67 c |
| S1O3C1 | 16.06 d | 29.71 c | 4.83 a | 2.46 cd | 9.40 cd | 74.76 bc | 83.90 abc |
| S1O3C2 | 15.89 d | 28.00 c | 4.94 a | 2.61 cd | 8.60 bcd | 54.76 a | 85.99 bc |
| S1O4C1 | 11.77 bcd | 27.57 bc | 7.61 ab | 1.92 bcd | 9.32 cd | 66.49 ab | 82.80 abc |
| S1O4C2 | 13.37 cd | 19.86 b | 8.00 ab | 1.45 abc | 7.76 bc | 57.97 a | 82.29 abc |
| S2O1 | 7.73 abc | 6.86 a | 28.16 c | 1.10 ab | 1.77 a | 94.67 d | 85.33 bc |
| S2O2C1 | 4.37 ab | 5.86 a | 13.08 b | 0.56 a | 0.49 a | 88.34 d | 86.87 bc |
| S2O2C2 | 5.11 ab | 5.86 a | 11.16 ab | 0.54 a | 0.65 a | 93.41 d | 86.82 bc |
| S2O3C1 | 5.19 ab | 5.43 a | 11.09 ab | 0.61 ab | 0.51 a | 95.04 d | 83.16 abc |
| S2O3C2 | 5.20 ab | 4.43 a | 10.88 ab | 0.58 ab | 0.44 a | 85.14 cd | 78.99 a |
| S2O4C1 | 3.11 a | 3.71 a | 6.51 a | 0.36 a | 0.38 a | 91.15 d | 81.62 ab |
| S2O4C2 | 5.20 ab | 3.43 a | 6.59 a | 0.34 a | 0.23 a | 88.09 d | 83.61 abc |
| S1 | 14.77 B | 27.55 B | 6.69 A | 2.24 B | 8.51 B | 66.37 A | 84.36 A |
| S2 | 5.13 A | 5.08 A | 12.50 B | 0.58 A | 0.64 A | 90.83 B | 83.77 A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellache, M.; Torres-Pagan, N.; Verdeguer, M.; Benfekih, L.A.; Vicente, O.; Sestras, R.E.; Sestras, A.F.; Boscaiu, M. Essential Oils of Three Aromatic Plant Species as Natural Herbicides for Environmentally Friendly Agriculture. Sustainability 2022, 14, 3596. https://doi.org/10.3390/su14063596
Bellache M, Torres-Pagan N, Verdeguer M, Benfekih LA, Vicente O, Sestras RE, Sestras AF, Boscaiu M. Essential Oils of Three Aromatic Plant Species as Natural Herbicides for Environmentally Friendly Agriculture. Sustainability. 2022; 14(6):3596. https://doi.org/10.3390/su14063596
Chicago/Turabian StyleBellache, Manel, Natalia Torres-Pagan, Mercedes Verdeguer, Leila Allal Benfekih, Oscar Vicente, Radu E. Sestras, Adriana F. Sestras, and Monica Boscaiu. 2022. "Essential Oils of Three Aromatic Plant Species as Natural Herbicides for Environmentally Friendly Agriculture" Sustainability 14, no. 6: 3596. https://doi.org/10.3390/su14063596
APA StyleBellache, M., Torres-Pagan, N., Verdeguer, M., Benfekih, L. A., Vicente, O., Sestras, R. E., Sestras, A. F., & Boscaiu, M. (2022). Essential Oils of Three Aromatic Plant Species as Natural Herbicides for Environmentally Friendly Agriculture. Sustainability, 14(6), 3596. https://doi.org/10.3390/su14063596












