Abstract
Root system architecture plays a vital role in plant growth, development, and adaptation by absorbing water and nutrients and providing mechanical support for growing plants. Unfortunately, little information is available in the literature on the root dynamics of summer mung bean under conservation agriculture conditions. In this study, field experiments were conducted during the summer seasons of two consecutive years (2020 and 2021) to investigate the root system dynamics of summer mung bean under different conservation agriculture practices. The highest stem and system width, depth to width length, number of nodal roots, taproot diameter, secondary root length (both right and left) of summer mung bean were recorded in the Soybean (permanent bed; PB)-Wheat(PB)-Summer mung (PB)(+Residual; +R) based cropping systems, followed by Maize(PB)-Wheat(PB)-Summer mung (PB)(+R), while, the lowest values of above parameters were recorded in the Puddled Transplanted Rice–Conventional till (PTR-CT)Wheat-Summer mung (-R). Further, the pod length, number of seeds per pod, number of pods per plant, seed yield and symbiotic parameters (including number of nodules per plant, leghaemoglobin content) and root dry weight were recorded highest in Soybean (PB)-Wheat (PB)-Summer mung (PB)(+R). Interestingly, the yield of summer mung bean increased around 13.4–29.5% when residues were retained on the soil surface with treatments involving residual removal. The soil dehydrogenase enzyme activity increased significantly under Soybean (PB)-Wheat (PB)-Summer mung (PB)(+R) based cropping system as compared to PTR-CT Wheat-Summer mung (-R). In addition, the number of pods per plant exhibited a significantly positive correlation with yield during both crop seasons. Overall, this study suggests that the inclusion of summer mung in soybean-based cropping systems may substantially improve the root architecture and soil quality and increase crop yield under conservation agriculture.
1. Introduction
The root system architecture is a critical trait that plays a vital role in soil evaporation and water transport, and improves productivity in water-stressed conditions. Under drought and heat stress conditions, the deep and proliferating root system helps extract adequate water and nutrients from the deeper layers of the soil [,]. Shovelomics is an easy, robust, and inexpensive method for assessing plant roots and their efficient responses to different stresses. An excellent example represents the roots of legumes with extensive thickening able to extract soil water from deep grounds []. Significant differences exist between the legumes and grasses in secondary growth and the embryonic root system. In the case of crops grown in the field, the primary root is not developed properly; hence, the hypocotyls (nodal) roots take up nutrients from the deeper soil profile []. The root design of field-excavated root crown was recently measured using a Digital Imaging of Root Traits automated image processing method (DIRT). DIRT is a computer-assisted image processing program designed to quantify and differentiate crop root morphologies. For over 70 DIRT traits, it may be unbiased and automated. The key aim of this analysis was to consider the effects of various crop residues on previous crops and see RSA’s effects on various cropping systems.
Legume inclusion in dominant cropping systems and adding residues lower the soil moisture loss while also improving soil organic carbon (SOC) and biophysical characteristics can have several advantages including those associated with life present or those associated with legume residues effects [,]. Therefore, it is obligatory to identify the sustainable intensification options of the rice-wheat cropping system (RWCS) using short duration summer legumes, i.e., summer mung or to diversify the RWCS with alternative cropping systems []. The legume pulses have been chosen for production in Asian and African countries for decades because they have the natural ability to restore soil fertility and maintain soil consistency. They are quite potent in soil minerals uptake and soil fertility improvement []. Crop leftovers, particularly legume residues, should be used to the fullest extent possible to improve soil fertility []. Since the dawn of civilisation, grain legumes (pulse crops) have been used in agricultural systems []. Summer mung (Vigna radiata L.), often known as summer green gram, is a good choice for RWCS because of its short growing season. The high protein edible seeds of the mungbean are advantageous over other crops. Their high nutrition value is advantageous over other pulses because of its high nutritional quality, digestion, and anti behaviour []. Summer mung integration in RWCS increases overall system productivity as well as cereal component crop productivity (rice and wheat), primarily when used for a long time []. Summer mung in the RWCS had a comparable beneficial impact on SOC in Mollisols and Inceptisols of Indo-Gangetic plains (IGP) []. Planting a short-duration mung following wheat and absorbing their residues in the next rice, according to [], rendered the RWCS highly profitable, favourable, soil-restorative, and more sustainable than conventional systems.
A cropping system is defined as the cropping pattern and management to derive benefits from a given resource base under a specific environmental condition. These include crop area, crop biomass, economic yield, crop rotation, crop calendar, time, and spread of sowing and harvest. Crop production systems have shifted to cereal-based farming systems; however, this has raised concerns about soil degradation and crop yield reductions [,]. The declining yield in cereal-based cropping systems is the utmost important factor to be considered. It has caused nutritional imbalances in the soil environment, rendering crop production systems unsustainable []. The rice-wheat cropping system is one of the most important cereal-cereal cycles for satisfying South Asia’s food needs. However, years of using the technique along the same farm have resulted in soil health loss, raising concerns about its long-term viability [,].
Furthermore, the biological nitrogen fixation inputs boost soil microbiology while reducing N2O emissions []. A protocol is recommended based on these findings. Despite the fact that many studies have been published on the impact of crop residues management on agronomic productivity and economic profitability, a comprehensive evaluation of conservation-agriculture-based intensification systems in general and lacking info on summer mung root system architecture (RSA) in various cropping systems. Therefore, the objective of this research was to investigate root dynamic, symbiotic parameters and yield of summer mung bean under conservation agriculture-based practices in irrigated sandy loam elliptic soil in north-western India. A final research was undertaken to evaluate visual trait scores and manual traits.
2. Material Method
2.1. Experimental Site
The field experiments were conducted for two consecutive years (2020 and 2021) under normal conditions at the Punjab Agricultural University, Ludhiana, India. Ludhiana is situated at 30°54 N latitude and 75°56 E longitude, at 247 metres above sea level. During the summer season of the year 2020, temperature ranged from 11.14 to 42.09 °C, whereas it ranged from 5.8 to 39.4 °C during the summer season of the year 2021 (recorded during 16th and 25th standard meteorological week). Total rainfall received during crop season was 72.4 mm for 2020 and 126.8 mm for 2021. The soil of the experimental field was sandy loam in texture, low in available N (181.9 kg/ha), medium in available P (21.2 kg/ ha) and K (208.6 kg/ha) and alkaline in reaction (pH 7.31).
2.2. Experimental Design and Treatments Details
The field experiments were conducted in a randomized complete block design (RCBD) with four replications and six treatments. The details of treatments are given in Table 1.

Table 1.
An overview of treatments.
2.3. Crop Management
The experiment was started in the Rabi season of the year 2018–2019. Wheat, and summer mung were used as zero cycle crops, and then all treatments were applied in the next crop, kharif. A week before planting, vigorous pre-sowing irrigation (75 mm depth) was applied as per recommended irrigation package for the crop. The summer mung variety (i.e., SML 832) used for the present study has erect plants, determinate growth habit with medium stature and matures in around 61 days. The crop geometry for summer mung was 22.5 cm × 7 cm (showing a plant density of 6.34 lakhs plants ha−1). The irrigation was applied at critical growth phases through flood method in conventional till treatments and soil matric potential (−40 ±1 kpa) based irrigation scheduling for the permanent bed treatments. The Summer mung bean seeds were treated with the captan (fungicide) @ 3 kg ha−1 seed to protect the crop against fungal diseases. The fertiliser application involved 12.5 kg ha−1 N and 40 kg ha−1 P2O5, the entire amount of nitrogen and phosphorus was applied as basal application. The supply of nitrogen was made through urea (46% N) and phosphorus was supplied through single super phosphate (16% P2O5). For weed control, a pre-emergence herbicide (pendimethalin) @ 2.5 l ha−1 was applied, and one hand weeding was performed 20 days after sowing. The crop was sprayed with 3.75 L of Dursban 20 EC (chlorpyriphos) per acre by dissolving in 250 litres of water to suppress the tobacco caterpillar. All the recommended cultural operations other than the treatments were followed to raise the crop.
2.4. Data Recording
The number of nodules per plant was counted on six plants chosen at random in different treatments. Plants were gently removed from the soil using a sieve and large earthen ball sand. The roots were gently rinsed in running water, the nodules were removed, and the number of nodules per plant was counted. Wilson’s and Reisenauer’s method (1963) [] was used to determine the content of leghaemoglobin, bold, and pink nodules at 50 days after sowing (DAS). Roots from six randomly selected plants in each plot were collected and sun-dried after washing in running tap water using a sieve. Then they were oven-dried at 60 °C until they reached a consistent weight and then their weights (mg/plant) were recorded. Crops were harvested manually according to residues protocols, and data was recorded on the following parameters: pod length, number of seeds per pod, number of pods per plant, grain, and straw yield.
2.5. Root Phenotyping following the Shovelomics Techniques
Field root phenotyping using Shovelomics techniques: Excavate and wash roots; the root crowns are excavated in approximately two stages 30 DAS and before harvest. Photograph root crowns using a Canon EOS 70D DSLR camera mounted to an aluminium frame wrapped in black cloth. Still, the root crowns were imaged with an open front to eliminate directional and optimize diffuse illumination. Roots should be placed with a circular scale of 42 mm on a matt black vinyl backdrop and a white sample ID sticker. First, the whole root crown was photographed, then divided into the primary shoot and tillers. Take measurements (ImageJ). Images are evaluated using an ObjectJ plugin project for ImageJ and all characteristics are analysed, i.e., stem distance, system width, depth to width length, taproot diameter, calculated number of roots and nodal root lengths and the attributing characteristics of the yield (Table 2).

Table 2.
Description of different roots recorded.
2.6. Statistical Analysis
The data were analysed in randomized complete block design with the help of a statistical analysis system (SAS Institute, Cary, NC, USA) [] and R software to determine the significance among different treatments and image analysed with the help of Imagej and DIRT (Digital Imaging of Root Traits) software for roots characters. The differences between treatment means were composed using Tukey’s HSD test at p < 0.05%. To eliminate biases, Principal Component Analysis (PCA) was used. Variables with high factor loading values in each Principal Component (PC) were regarded as the best representations of system attributes.
3. Result
Effect of conservation agriculture (CA) practices on root system architecture (RSA).
3.1. Effect of CA on Root Parameters at 30 DAS
The highest stem width (135.8–155.8 mm), system width (2504.5–2704.5 mm), depth to width length (1324.8–1524.8 mm), number of nodal roots (20–22), taproot diameter (68.4–78.3 mm), secondary root length right (1233.4–1433.4 mm) and secondary root length left (1749.5–1999.5) were recorded in T6 (Table 3). Stem width and taproot diameter during the year 2020, T4 and T2 remained at par with T6, whereas in the year 2021, T2, T3, T4, and T5 were significantly lower than T6 for stem width, while during the year 2021, T2, T4, and T5 were significantly lower than T6 for tap root diameter. The system width ranged from 730 to2704 mm showing significant differences among the treatments. The increment was 1.86 and 1.71 folds for depth to width under T6 compared to T1 during 2020 and 2021, respectively. The nodal root number ranged from 9 to 22 with the highest observed in T6 followed by T4. The secondary root length right ranged from 816 to 1433 mm with no significant differences among the treatments (except T6). The secondary root length left ranged from 584 to 1999 during both years.

Table 3.
Effect of different conservation agriculture and conventional till management practices on root parameters of summer mung bean (at 30 days after sowing) in different cropping systems.
3.2. Effect of CA on Root Parameters Recorded before Harvesting
Data on different root traits (Table 4) indicate that conservation agriculture practices significantly increased the root traits viz., stem width, system width, number of nodal roots, taproot diameter, secondary root length right and left than conventional practices (T1). The highest stem width (122.1–212.8 mm), system width (2212.4–2339.4 mm), depth to width length (1305.1–1505 mm), number of nodal roots (25–30), taproot diameter (71.7–81.7 mm), secondary root length right (1616.6–1816.6 mm) and secondary root length left (1717.6–1917.6) were recorded in T6. In the case of stem width, during the year 2020, T4 remained at par with T6, whereas in 2021, T4 was significantly different from T6. The system width ranged from 1338 to 2339 mm, and T2, T3, T4, and T5 did not differ substantially during the year 2020, while in the year 2021, T6 remained at par with each other T4. The increment in-depth to width under T6 compared to control (T1) was 133% and 120% during 2020 and 2021, respectively. The nodal root number ranged from 8 to 30 with the highest nodal root number observed in T6 followed by T4 and T2. The increment in taproot diameter under T6 compared to control was the tune of 478% and 264% during 2020 and 2021, respectively. The secondary root length right ranged from 610 to 1816 mm and T6 remained at par with T4 during both years. The secondary root length left ranged from 693 to 1917 mm.

Table 4.
Effect of different conservation agriculture and conventional till management practices on root parameters of summer mung bean (before harvesting) in different cropping systems.
3.3. Yield and Component Traits
The data seed yield and other component traits were recorded at harvest time (Table 5). T6 exhibited the highest pod length (8.03–8.22 cm), number of seeds per pod (10.75–12) and number of pods per plant (32–35). In the year 2020, the highest pod length, no. of seeds/pod and no of pod per plants were observed in T6, which remained statistically on par with T2 and T4, whereas, in the year 2021, the highest pod length, no. of seeds per pod and no of pod per plants were recorded in T6 which was significantly different from T2. The increment in number of pods per plant and number of seeds per pod were 64.10 & 71.4% and 61.29 & 74.6% for T6 compared to control (T1) during 2020 and 2021, respectively.

Table 5.
Effect of different conservation agriculture and conventional till management practices on yield attributes and yield of summer mung bean during 2020 and 2021 in different cropping systems.
Significant improvement in summer mung yield was observed when residues from the previous crop were retained in the soil (Figure 1). The highest seed yield was 978 kg/ha, and 1012 kg/ha was recorded in T6, which was a statistically significant difference with T2 (888 kg/ha and 918 kg/ha) and T4 (905 and 950 kg/ha) during both the year but treatment T2 was at par with T4. The increment in seed yield under T6 compared to control (T1) was 29.5% and 24.6% during 2020 and 2021, respectively. The minimum seed yield was noticed in T1 (755 and 812 kg/ha) for both seasons. The treatment T6 showed maximum seed yield, which was significantly superior to the remaining treatments.
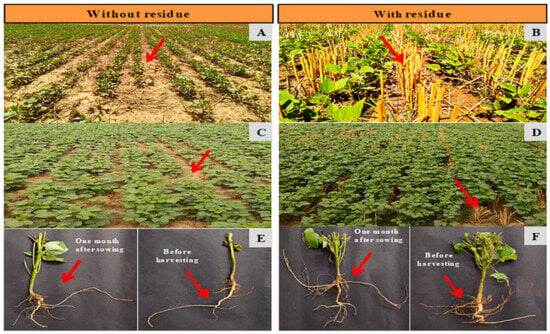
Figure 1.
Different growth stage of summer mung without residue (A,C,E) and with residue (B,D,F): Early growth stage 15 DAS (A,B), crop growth stage at 30 DAS (C,D) and root study at 30 DAS and before harvesting (E,F).
3.4. Symbiotic Parameters and Root Dry Weight
Data on number of nodules per plant, the weight content of fresh leghaemoglobin nodule, and the root dry weight of summer mung recorded before harvesting are presented in Table 6. In both the years, treatment T6 showed the highest number of nodules per plant of summer mung, which was statistically significant with treatment T4 but statistically at par with T2. In the second year, the content of leghaemoglobin of summer mung was recorded highest in treatment T6, which was significantly different from the treatments T4 and T2. Still treatment T2 was statistically on par with T4, and recorded the lowest leghaemoglobin content in T5. Dry root weight increased to 50 DAS in the second year, then declined somewhat at maturity. The highest dry root weight was observed in T6, followed by T4, T2, T3, and T1, whereas the lowest was observed in T5 (Table 6). The DHA ranged from 29.4 to 51.3 μg TPF g−1 soil 24 h−1, highest DHA was recorded in T6, followed by T2 and T4. Compared to T1, DHA was 58.84% higher in T6 and 31.29% higher in T4.

Table 6.
Effect of different conservation agriculture and conventional till management practices on symbiotic parameters and root dry weight of summer mung bean (before harvesting) in different cropping systems.
3.5. Principal Component Analysis
The principal component analysis is a technique for identifying minimum data sets where only the variables with high loading factors were selected from each PC. This analysis may be used to choose the optimal genotypes for breeding. Figure 2 represents the variance on each axis, the percentage of total variance representing the coefficients used in weighted sum (eigenvectors or loadings) []. The first two principal components had more than 1 eigenvalue. These two principal components explained 97.4 and 1.6%, respectively, constituting 98.9% of the total variation for 15 different traits recorded in the year 2020. Whereas, in the year 2021, the first two principal components explained 92.9 and 5.45%, respectively, constituting 98.3% of the total variation for 15 different traits (Figure 2 and Table 7).
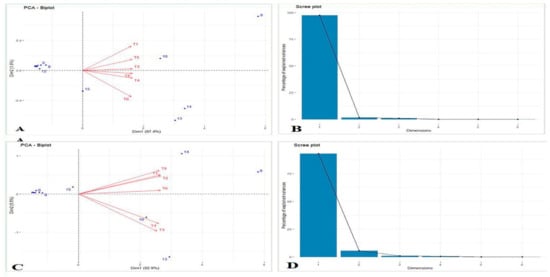
Figure 2.
Principal component analysis during 2020 (A,B) and 2021 (C,D). Note: 1 = yield, 2 = pod length, 3 = no. of seeds/pod, 4 = root dry wt., 5 = leghaemoglobin content, 6 = no of pod per plant, 7 = stem width, 8 = system width, 9 = depth to width, 10 = nodal root number, 11 = tap root diameter, 12 = tap root diameter, 13 = SLR (Secondary root length left), 14 = SLL (Secondary root length left), 15 = seminal root length. T1 = puddled transplanted rice (PTR)-conventional till (CT) Wheat-summer mung) (−R or completely removal residues), T2 = PTR- Happy seeder Wheat-zero till summer mung (ZT) (+R or residues retention on the soil surface), T3 = Fresh bed Maize (FB)-CT Wheat -summer mung (−R),T4 = Permanent bed Maize (PB)- Wheat (PB)-summer mung (PB) (+R),T5 = Soybean (FB)-Wheat (CT)-summer mung(−R) and T6 = Soybean(PB)-Wheat(PB)-summer mung (PB) (+R).

Table 7.
Eigenvalues, variance percentages, cumulative variances (%), and principal component analysis standard errors for both years.
3.6. Correlation of Different Components with Yield
A correlation study helps to understand the relationship between major yield component traits. Correlations among the different traits are presented in Table 8 and Figure 3. The pod length (0.821), stem width (0.815) and number of pods per plant (0.798) had significant positive correlation with yield during the year 2020. Similarly in the year 2021, number of pods per plant (0.886), number of seeds per pod (0.785), and the nodal root number (0.753) showed a significant positive correlation with seed yield. The number of pods per plant exhibited substantial correlation with seed yield in both the years (Table 8 and Figure 3).

Table 8.
Correlation coefficients among all possible combinations of different yield component traits recorded on summer mung before harvesting.
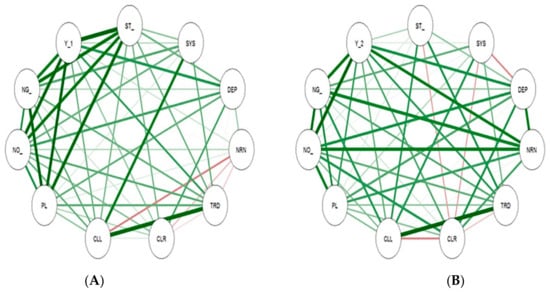
Figure 3.
Correlation analysis of summer mung during 2020-21 before harvest (A,B). Note: ST_ = Stem width, SYS = System width, DEP = Depth to width, NRN = Nodal root number, TRD = Tap root diameter, CLR/SLR = Secondary root length right, CLL/ SLL = Secondary root length left, PL = Pod length, NO_ = No. of pods/plant, NG = No. of seeds/pod and Y_1 and Y_2 = Yield (kg/ha).
Dark green colour shaded shows the strongly positively correlated to each other and shows significant difference for both the year. In light green colour shaded the positively correlated and it show no significant difference to each other and red colour line shows negative correlated and no significant difference each other.
4. Discussion
4.1. Effect of Conservation Agriculture Practices on Different Root Parameters
Improved root system architecture observed under conservation agriculture-based practices indicates that the retention of legume (summer mung and soybean) improved the soil organic matter, nitrogen, and phosphorus, and decreased the immobilization of nitrogen owing to lower carbon to nitrogen ratio and increased availability of nutrients to the plants. The straw application boosts the availability of macro and micronutrients and increases the soil organic matter and nitrogen stocks [,]. In the second year, T6 showed the most considerable increase in stem width (102.19%), depth to width length (39.80%), and nodal root (100%). The retention of crop residues under CA improved microbial biomass carbon and microbial biomass nitrogen, enhancing soil biological activities compared to conventional practices. []. In the second year to increase the 96.19% secondary root length (SRL) right, 44.07% secondary root length (SRL) left and 230.7% taproot diameter was recorded in T6 concerning T5 treatment to cropping systems with pulses having more exceptional soil carbon sequestration ability than mono-cropping. Previous studies have shown under conservation agriculture practices that roots mainly increase the number of soil macropores and promote the connectivity between soil pores, leading to changes in soil structure, soil macropores, soil water holding capacity, and water conductivity. Plant residues of pulses have a narrow C:N ratio, which helps in easy decomposition, mineralization, and increased wheat grain yield, as compared to no mulch [,]. Mulch created 40% larger root length densities in lower layers (>0.15 m) than no-mulch, owing to increased soil moisture conservation in deeper layers. Root exudates contain amino acids, sugars, and carboxylic acids, which act as attractants for beneficial microorganisms [].
4.2. Seed Yield and Component Traits
The highest yield attributes of summer mung were recorded in conservation agriculture practices (T6, T4, and T2) due to pulses content mineralizable-nitrogen being higher than the non-pulse crops or under uncultivated fallows. The yield of summer mung increased around 13.4–29.5% in residues retention (T2, T4 and T6) on the soil surface as compared to without residues treatments (T1, T3 and T5). It may be attributed to the retention of pulse crop residues on soil surfaces, leading to greater nutrient availability and increasing morphological traits. It could be because legumes provide a number of advantages, including N supply through biotic N fixation, improved nutrient availability through the deeper rooting, decreased compaction, higher SOC [,]. According to Sharma and Prasad [], combined application of wheat residues and green manure (sesbania or mung bean) boosted crops grain output and agronomic N efficacy and improved the normally negative apparent N balances.
4.3. Symbiotic Parameters and Root Dry Weight and Dehydrogenase Activity
Symbiotic parameters and root dry weight being recorded at their highest values in conservation agriculture-based treatment might be due to the residues of pulse content being three times more nitrogen-rich than the cereal residues []. The retention of residues may increase soil quality, hydraulic conductivity, crop water supply capacities, nutrient availability, exchangeable cations, soil responsiveness, carbon sequestration, microbial biomass nitrogen and carbon activities, and species diving properties [,,,,]. DHA was 58.84% in the present study, and 31.29% was higher in T6 and T4 than T1, respectively. Soil enzyme activity is positively connected with zero tillage and inversely correlated with CT [,] and it might be due to mixing of previous crops residue which was retained on the soil surface which increases the availability of labile carbon produced after decomposition of the previous year [].
4.4. Principal Component Analysis (PCA) and Correlation
Most of the assessed variables were highest with treatment T6 followed by T4 (Figure 2). The most influential variables for the PC1 were varied yield, pod length and no. of seeds/pod and for the second principal component were secondary root length left seminal root length (Table 6). This suggests that continuous inclusion of C sources through preceding crop residue increased microbial activity as well as the availability of various microbial communities in the soil, nutrient availability, and rhizodeposition, all of which could be factors in increased soil carbon pools but also hydrolytic enzymatic activities. In the orthogonal space of PCA, []. Most of the studied variables (microbial biomass carbon and basal soil respiration) were more closely conjugated with organically managed soils than inorganically managed soils. This supports [] an earlier finding that PCA clearly distinguished zero tillage with crop residues from CT without residue treatments. Correlation analysis revealed that the number of pods per plant correlated highly with yield during both seasons. Similar results were also reported by []. Dark green colour shaded shows the strong positive correllations to each other and the significant difference for both the seasons (Table 8 and Figure 3).
5. Conclusions
The current study revealed that Soybean (PB)-Wheat (PB)-Summer mung (PB)(+R) increased seed yield, symbiotic parameters, and root traits. The seed yield of summer mung under T6 was significantly 8.0–9.5% and 24.6–29.5% higher as compared to T4 and T1, respectively. Pod length, number of seeds/pod and secondary root length left were dominant indicators for assessing crop yield in this study under conservation agricultural-based sustainable practice than the conventional.
Author Contributions
Writing—original draft preparation, A.K., P.K. and K.S.S.; Conceptualization, K.S.S., A.K.; Methodology, K.S.S. and A.K.; Resources, L.K.S.; Supervision, P.K. and K.S.S.; Formal analysis, A.K.; Software, A.K. and L.K.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available on request.
Acknowledgments
Authors wish to thank the anonymous reviewers for their careful reading and corrections. The authors are also thankful to the Punjab Agricultural University, Ludhiana, India and Department of Science and Technology (DST) New Delhi, India for providing facilities for conducting these studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Forotaghe, Z.A.; Souri, M.K.; Jahromi, M.G.; Torkashvand, A.M. Physiological and biochemical responses of onion plants to deficit irrigation and humic acid application. Open Agric. 2021, 6, 728–737. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Souri, M.K.; Mousavi, A.; Sahebani, N. Biochar and vermicompost improve growth and physiological traits of eggplant (Solanum melongena L.) under deficit irrigation. Chem. Biol. Technol. Agric. 2021, 8, 19. [Google Scholar] [CrossRef]
- Basu, P.; Brown, K.; Lynch, J. Ethylene modulates genetic, positional, and nutritional regulation of root plagiogravitropism. Funct. Plant Biol. 2007, 34, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Saengwilai, P.; Tian, X.; Lynch, J.P. Low Crown Root Number Enhances Nitrogen Acquisition from Low-Nitrogen Soils in Maize. Plant Physiol. 2014, 166, 581–589. [Google Scholar] [CrossRef]
- Souri, M.K.; Sooraki, F.Y.; Moghadamyar, M. Growth and quality of cucumber, tomato, and green bean under foliar and soil applications of an aminochelate fertilizer. Hortic. Environ. Biotechnol. 2017, 58, 530–536. [Google Scholar] [CrossRef]
- Souri, M.K.; Naiji, M.; Aslani, M. Effect of Fe-Glycine Aminochelate on Pod Quality and Iron Concentrations of Bean (Phaseolus vulgaris L.) Under Lime Soil Conditions. Commun. Soil Sci. Plant Anal. 2018, 49, 215–224. [Google Scholar] [CrossRef]
- Bana, R.S.; Shivay, Y.S.; Sepat, S.; Rana, K.S.; Pooniya, V. Effect of summer forage crops and phosphogypsum-enriched urea on productivity of basmati rice-wheat cropping system. Res. Crops. 2013, 14, 649–653. [Google Scholar]
- Souri, M.K.; Aslani, M. Beneficial effects of foliar application of organic chelate fertilizers on French bean production under field conditions in a calcareous soil. Adv. Hortic. Sci. 2018, 32, 265–272. [Google Scholar]
- Jasdan, M.; Hutchaon, S.S. Utilization of green manure for raising soil fertilityin China. Soil Sci. 1996, 135, 65–69. [Google Scholar]
- Ghosh, P.K.; Bandyopadhyay, K.K.; Wanjari, R.H.; Manna, M.C.; Misra, A.K.; Mohanty, M.; Rao, A.S. Legume Effect for Enhancing Productivity and Nutrient Use-Efficiency in Major Cropping Systems–An Indian Perspective: A Review. J. Sustain. Agric. 2007, 30, 59–86. [Google Scholar] [CrossRef]
- Hazra, K.K.; Venkatesh, M.S.; Ghosh, P.K.; Ganeshamurthy, A.N.; Kumar, N.; Nadarajan, N.; Singh, A.B. Long-term effect of pulse crops inclusion on soil–plant nutrient dynamics in puddled rice (Oryza sativa L.)-wheat (Triticum aestivum L.) cropping system on an Inceptisol of Indo-Gangeticplain zone of India. Nutr. Cycl. Agroecosyst. 2014, 100, 95–110. [Google Scholar] [CrossRef]
- Hazra, K.K.; Ghosh, P.K.; Venkatesh, M.S.; Nath, C.P.; Kumar, N.; Singh, M.; Singh, J.; Nadarajan, N. Improving soil organic carbon pools through inclusion of summer mung bean in cereal–cereal cropping systems in Indo-Gangetic plain. Arch. Agron. Soil Sci. 2018, 340, 1690–1704. [Google Scholar] [CrossRef]
- Sharma, S.; Prasad, R. Effects of Sesbania green manuring and mungbean residue incorporation of productivity and nitrogen uptake of a rice-wheat cropping system. Bioresour. Technol. 1999, 67, 171–175. [Google Scholar] [CrossRef]
- Haq, A. Studies on the Yield and Related Morphological Characters of Some New Mungbean Genotypes in Irrigated Environment. Master’s Thesis, University of Agriculture, Faisalabad, Punjab, Pakistan, 1989. [Google Scholar]
- Parayil, G. The Green Revolution in India: A Case Study of Technological Change. Technol. Cult. 1992, 33, 737–756. [Google Scholar] [CrossRef]
- Srivastava, J.P.; Mukhopadhyay, M. Sustainable Intensification of Rice-Wheat Cropping Systems in India; Sustainable Intensification of Agricultural Production Systems, Learning and Leadership Center; The World Bank: Washington, DC, USA, 1997. [Google Scholar]
- Swaroop, A.; Ganeshamurthy, A.N. Emerging nutrient deficiencies in intensive cropping systems and their remediation. Fertil. News 1998, 43, 37–50. [Google Scholar]
- Duxbury, K.G.; Gupta, B.R. Effect of farmyard manure, chemical and biofertilizers on yiield and quality of rice (Oryza sativa L.) and soil properties. J. Indian Soc. Soil Sci. 2000, 48, 773–780. [Google Scholar]
- Prasad, R. Organic farming vis-à-vis modern agriculture. Curr. Sci. 2005, 89, 252–254. [Google Scholar]
- Peoples, M.B.; Brockwell, J.; Herridge, D.F.; Rochester, I.J.; Alves, B.J.; Urquiaga, S.; Boddey, R.M.; Dakora, F.D.; Bhattarai, S.; Maskey, S.L.; et al. The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 2009, 48, 1–17. [Google Scholar] [CrossRef]
- Wilson, D.O.; Reisenauer, H.M. Detremination of leghemoglobin in legume nodules. Anal. Biochem. 1963, 6, 27–30. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT® 9.4 Interface to North Carolina State University-SFA-T: Reference; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Hotelling, H. Relations between two sets of varieties. Biometrica 1936, 28, 321–377. [Google Scholar] [CrossRef]
- Yadvinder, S.; Bijay, S.; Meelu, O.P.; Khind, C.S. Long-term effects of organic manuring and crop residues on the productivity and sustainability of rice-wheat cropping system in Northwest India. In Long-Term Soil Fertility Experiments in Rice-Wheat Cropping Systems; RWCIGP: New Delhi, India, 2010. [Google Scholar]
- Yadvinder, S.; Bijay, S.; Timsina, J. Crop residue management for nutrient cycling and improving soil productivity in rice-based cropping systems in the tropics. Adv. Agron. 2005, 85, 269–407. [Google Scholar]
- Ghosh, P.K.; Venkatesh, M.S.; Hazra, K.K.; Kumar, N. Long-term effect of pulses and nutrientmanagement on soil organic carbon dynamics and sustainability on an Inceptisol of Indo-Gangeticplains of India. Exp. Agric. 2012, 48, 473–487. [Google Scholar] [CrossRef]
- Chakraborty, D.; Nagarajan, S.; Aggarwal, P.; Gupta, V.K.; Tomar, R.K.; Garg, R.N.; Sahoo, R.N.; Sarkar, A.; Chopra, U.K.; Sarma, K.S.S.; et al. Effect of mulching on soil and plant water status, and the growth and yield of wheat (Triticumaestivum L.) in a semi-arid environment. Agric. Water Manag. 2008, 95, 1323–1334. [Google Scholar] [CrossRef]
- Chakraborty, D.; Garg, R.N.; Tomar, R.K.; Singh, R.; Sharma, S.K.; Singh, R.K.; Trivedi, S.M.; Mittal, R.B.; Sharma, P.K.; Kamble, K.H. Synthetic and organic mulching and nitrogen effect on winter wheat (Triticumaestivum L.) in a semi-arid environment. Agric. Water Manag. 2010, 97, 738–748. [Google Scholar] [CrossRef]
- Sugiyama, A.; Yazaki, K. Root Exudates of Legume Plants and Their Involvement in Interactions with Soil Microbes. Available online: http://www.springer.com/978-3-642-23046-2 (accessed on 15 July 2021).
- Wani, S.P.; Rupela, O.P.; Lee, K.K. Sustainable agriculture in the semi arid tropics through biological N2 fixation in grain legumes. Plant Soil 1995, 174, 29–49. [Google Scholar] [CrossRef]
- Singh, V.K.; Dwivedi, B.S.; Shukla, A.K.; Chauhan, Y.S.; Yadav, R.L. Diversification of rice with pigeon pea in a rice–wheat cropping system on a Typic Ustochrept: Effect on soil fertility, yield and nutrient use efficiency. Field Crops Res. 2005, 92, 85–105. [Google Scholar] [CrossRef]
- Sharma, S.; Prasad, R. Effect of crop-residue management on the production and agronomic nitrogen efficiency in a rice-wheat cropping system. J. Plant Nutr. Soil Sci. 2008, 171, 295–302. [Google Scholar] [CrossRef]
- Ganeshamurthy, A.N.; Ali, M.; Srinivasarao, C.H. Role of pulses in soil health and sustainable crop production. Indian J. Fert. 2004, 49, 71–86. [Google Scholar]
- Beri, V.; Sidhu, B.S.; Bahl, G.S.; Bhat, A.K. Nitrogen and phosphorus transformations as affected by crop residue management practices and their influence on crop yield. Soil Use Manag. 1995, 11, 51–54. [Google Scholar] [CrossRef]
- Beri, V.; Sidhu, B.S.; Bahl, G.S.; Bhat, A.K.; Singh, B.P. Nutrient balance and soil properties as affected by management of crop residues. In Proceedings of the International Symposium on Nutrient Management for Sustained Productivity, Punjab, India, 15–19 July 1992; pp. 133–135. [Google Scholar]
- Bijay, S.; Shan, Y.H.; Johnson-beeebout, S.E.; Yadvinder, S.; Buresh, R.J. Crop residue management for lowland rice-based cropping systems in Asia. Adv. Agron. 2008, 98, 118–199. [Google Scholar]
- Power, J.F.; Doran, J.W.; Wilhelm, W.W. Crop residue effects on soil environment and dryland maize and soybean production. Soil Tillage Res. 1986, 8, 101–111. [Google Scholar] [CrossRef]
- Singh, G.; Jalota, S.K.; Sidhu, B.S. Soil physical and hydraulic properties in a rice-wheat cropping system in India: Effects of rice-straw management. Soil Use Manag. 2005, 21, 17–21. [Google Scholar] [CrossRef]
- Chaudury, J.; Mandal, U.K.; Sharma, K.L.; Ghosh, H.; Mandal, B. Assessing soil quality under long term rice based cropping system. Commun. Soil Sci. Plant Anal. 2005, 36, 1141–1161. [Google Scholar] [CrossRef]
- Roldan, J.R.; Salinas-Garcia, J.R.; Alguacil, M.M.; Diaz, E.; Caravaca, F. Soil enzyme activity suggests advantages of conservation tillage practices in sorghum cultivation under subtropical condition. Geoderma 2005, 129, 178–185. [Google Scholar] [CrossRef]
- Piñera-Chavez, F.J.; Berry, P.M.; Foulkes, M.J.; Molero, G.; Reynolds, M.P. Avoiding lodging in irrigated spring wheat. II. Genetic variation of stem and root structural properties. Field Crops Res. 2016, 196, 64–74. [Google Scholar] [CrossRef]
- Tamilselvi, S.M.; Chinnadurai, C.; Ilamurugua, K.; Arulmozhiselvan, K.; Balachandar, D. Effect of long-term nutrient managements on biological and biochemical properties of semi-arid tropical Alfisol during maize crop development stages. Ecol. Indic. 2015, 48, 76–87. [Google Scholar] [CrossRef]
- Bera, T.; Sharma, S.; Thind, H.S.; Singh, Y.; Sidhu, H.S.; Jat, M.L. Soil biochemical changes at different wheat growth stages in response to conservation agriculture practices in rice-wheat system of north-western India. Soil Res. 2017, 56, 91–104. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).