Influence of Organic Amendments and Moisture Regime on Soil CO2-C Efflux and Polycyclic Aromatic Hydrocarbons (PAHs) Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solvents
2.2. PAHs Standards
2.3. Incubation Experiment and Determination of CO2-C Efflux, pH and EC
2.4. PAHs Extraction, Fractionation and Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect of pH and EC
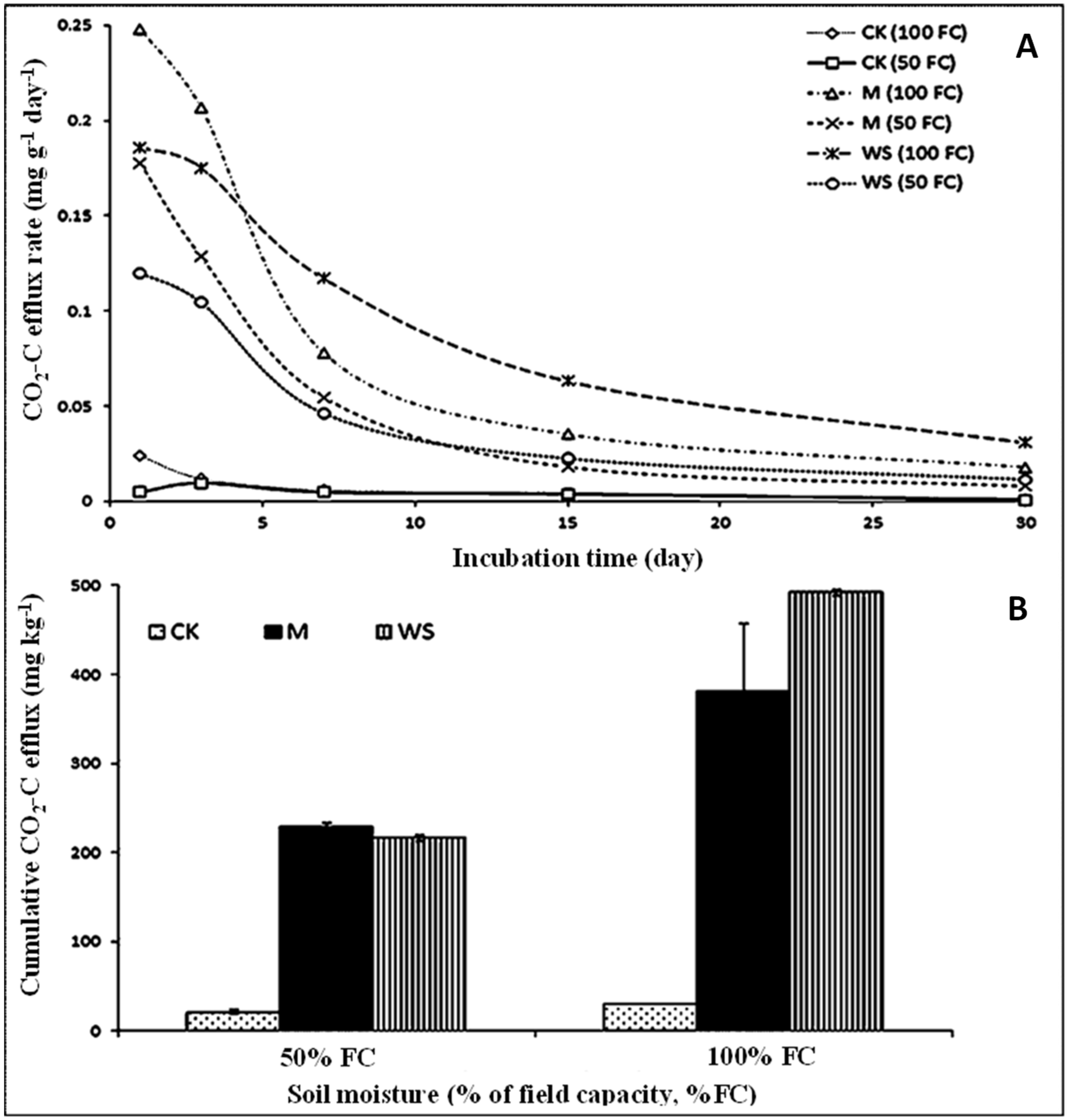
3.2. Treatment Effects on Organic C Mineralization as CO2 Efflux Rate
3.3. Treatment Effects on PAHs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guarino, C.; Zuzolo, D.; Marziano, M.; Conte, B.; Baiamonte, G.; Morra, L.; Benotti, D.; Gresia, D.; Stacul, E.R.; Cicchella, D.; et al. Investigation and assessment for an efective approach to the reclamation of polycyclic aromatic hydrocarbon (PAHs) contaminated site: SIN Bagnoli, Italy. Sci. Rep. 2019, 9, 11522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Zhang, J.; Zhang, F.; Liu, X.; Zhou, M. Contamination and health risk assessment of PAHs in farmland soils of the Yinma River Basin, China. Ecotoxicol. Environ. Saf. 2018, 156, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, M.; Trably, E.; Bernet, N.; Patureau, D. Biodegradation of polycyclic aromatic hydrocarbons: Using microbial bioelectrochemical systems to overcome an impasse. Environ. Pollut. 2017, 231 Pt 1, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Jain, R.; Srivastava, N.; Borthakur, A.; Pal, D.B.; Singh, R.; Madhav, S.; Srivastava, P.; Tiwary, D.; Mishra, P.K. Current and emerging trends in bioremediation of petrochemical waste: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 155–201. [Google Scholar] [CrossRef]
- Davie-Martin, C.L.; Stratton, K.G.; Teeguarden, J.G.; Waters, K.M.; Simonich, S.L.M. Implications of Bioremediation of Polycyclic Aromatic Hydrocarbon-Contaminated Soils for Human Health and Cancer Risk. Environ. Sci. Technol. 2017, 51, 9458–9468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lin, X.; Liu, W.; Wang, Y.; Zeng, J.; Chen, H. Effect of organic wastes on the plant-microbe remediation for removal of aged PAHs in soils. J. Environ. Sci. 2012, 24, 1476–1482. [Google Scholar] [CrossRef]
- Han, X.; Hu, H.; Shi, X.; Zhang, L.; He, J. Effects of different agricultural wastes on the dissipation of PAHs and the PAH-degrading genes in a PAH-contaminated soil. Chemosphere 2017, 172, 286–293. [Google Scholar] [CrossRef]
- Wan, C.K.; Wong, J.W.C.; Fang, M.; Ye, D.Y. Effect of organic waste amendments on degradation of PAHs in soil using thermophillic composting. Environ. Technol. 2003, 24, 23–30. [Google Scholar] [CrossRef]
- Kobayashi, T.; Murai, Y.; Tatsumi, K.; Iimura, Y. Biodegradation of polycyclic aromatic hydrocarbons by Sphingomonas sp. enhanced by water-extractable organic matter from manure compost. Sci. Total Environ. 2009, 407, 5805–5810. [Google Scholar] [CrossRef]
- Pawar, R.M. The Effect of Soil pH on Bioremediation of Polycyclic Aromatic Hydrocarbons (PAHS). J. Bioremed. Biodeg. 2015, 6, 291. [Google Scholar] [CrossRef]
- Kästner, M.; Mahro, B. Microbial degradation of polycyclic aromatic hydrocarbons in soils affected by the organic matrix of compost. Appl. Microbiol. Biotechnol. 1996, 44, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Lukić, B.; Huguenot, D.; Panico, A.; Fabbricino, M.; van Hullebusch, E.D.; Esposito, G. Importance of organic amendment characteristics on bioremediation of PAH-contaminated soil. Environ. Sci. Pollut. Res. Int. 2016, 23, 5041–5052. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Wu, Y.; Zeng, J.; Zhang, T.; Lin, X. Influence of organic amendments used for benz[a]anthracene remediation in a farmland soil: Pollutant distribution and bacterial changes. J. Soils Sediments 2020, 20, 32–41. [Google Scholar] [CrossRef]
- Ahuja, L.R.; Nachabe, M.H.; Rockiki, R. Soils: Field Capacity. Encycl. Water Sci. Second Ed. 2008, 2, 1128–1131. [Google Scholar]
- Black, C.A. Methods of Soil Analysis; American Society of Agronomy, Inc.: Madison, WI, USA, 1965; pp. 1562–1565. [Google Scholar]
- Leifeld, J.; Siebert, S.; Kögel-Knabner, I. Biological activity and organic matter mineralization of soil amended with biowaste composts. J. Plant Nutr. Soil Sci. 2002, 165, 151–159. [Google Scholar] [CrossRef]
- Alotaibi, H.S.; Usman, A.R.; Abduljabbar, A.S.; Ok, Y.S.; Al-Faraj, A.I.; Sallam, A.S.; Al-Wabel, M.I. Carbon mineralization and biochemical effects of short-term wheat straw in crude oil contaminated sandy soil. Appl. Geochem. 2018, 88 Pt B, 276–287. [Google Scholar] [CrossRef]
- EL-Saeid, M.H.; Al-Turki, A.M.; Nadeem, M.E.A.; Hassanin, A.S.; Al-Wabel, M.I. Photolysis degradation of polyaromatic hydrocarbons (PAHs) on surface sandy soil. Environ. Sci. Pollut. Res. 2015, 22, 9603–9616. [Google Scholar] [CrossRef] [PubMed]
- Angelova, V.R.; Akova, V.I.; Artinova, N.S.; Ivanov, K.I. The effect of organic amendments on soil chemical characteristics. Bulg. J. Agric. Sci. 2013, 19, 958–971. [Google Scholar]
- Walker, D.J.; Clemente, R.; Roig, A.; Bernal, M.P. The effects of soil amendments on heavy metal bioavailability in two contaminated Mediterranean soils. Environ. Pollut. 2003, 122, 303–312. [Google Scholar] [CrossRef]
- Badía, D.; Martí, C.; Aguirre, A.J. Straw management effects on CO2 efflux and C storage in different Mediterranean agricultural soils. Sci. Total Environ. 2013, 465, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.R.A.; Almaroai, Y.A.; Ahmad, M.; Vithanage, M.; Ok, Y.S. Toxicity of synthetic chelators and metal availability in poultry manure amended Cd, Pb and As contaminated agricultural soil. J. Hazard Mater. 2013, 262, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Sagrilo, E.; Jeffery, S.; Hoffland, E.; Kuyper, T.W. Emission of CO2 from biochar-amended soils and implications for soil organic carbon. GCB Bioenergy 2014, 7, 1294–1304. [Google Scholar] [CrossRef] [Green Version]
- Duong, T.T.T.; Baumann, K.; Marschner, P. Frequent addition of wheat straw residues to soil enhances carbon mineralization rate. Soil Biol. Biochem. 2009, 41, 1475–1482. [Google Scholar] [CrossRef]
- Yang, S.; Xiao, Y.; Xu, J.; Liu, X. Effect of straw return on soil respiration and NEE of paddy fields under water-saving irrigation. PLoS ONE 2018, 13, e0204597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Włóka, D.; Placek, A.; Rorat, A.; Smol, M.; Kacprzak, M. The evaluation of polycyclic aromatic hydrocarbons (PAHs) biodegradation kinetics in soil amended with organic fertilizers and bulking agents. Ecotoxicol. Environ. Saf. 2017, 145, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Yu, J. The effect of pH value on the polycyclic aromatic hydrocarbons degradation in sludge during biological aerobic fermentation process. Adv. Mater. Res. 2013, 664, 72–76. [Google Scholar] [CrossRef]
- Kästner, M.; Breuer-Jammali, M.; Mahro, B. Impact of inoculation protocols, salinity, and pH on the degradation of polycyclic aromatic hydrocarbons (PAHs) and survival of PAH-degrading bacteria introduced into soil. Appl. Environ. Microbiol. 1998, 64, 359–362. [Google Scholar] [CrossRef] [Green Version]
| PAHs | No. of Rings | MW (g/mol) | Solubility in Water at 25 °C (mg L−1) | Vapor Pressure (Pa) | Log Kow | Log Koc | LOD (ng/mL) | LQD (ng/mL) | Recovery % |
|---|---|---|---|---|---|---|---|---|---|
| Pyrene (Pyr) | 4 | 202 | 1.3 × 10−1 | 3.3 × 10−4 | 4.90 | 4.58 | 1.33 | 3.99 | 99.65 ± 2.68 |
| Fluoranthene (Flt) | 4 | 202 | 2.1 × 10−1 | 6.7 × 10−4 | 4.90 | 4.58 | 1.21 | 3.63 | 97.58 ± 2.66 |
| Benzo[a]pyrene (BaP) | 5 | 252 | 3.8 × 10−3 | 7.5 × 10−7 | 6.06 | 6.74 | 1.41 | 4.23 | 98.44 ± 2.12 |
| Benzo[k]fluoranthene (BkF) | 5 | 252 | 4.3 × 10−3 | 6.7 × 10−5 | 6.06 | 5.74 | 1.63 | 4.89 | 98.48 ± 2.39 |
| Benzo[g,h,i]perylene (BghiP) | 6 | 276 | 2.6 × 10−4 | 1.4 × 10−8 | 6.50 | 6.20 | 2.11 | 6.33 | 99.18 ± 2.09 |
| Indeno[1,2,3-cd]pyrene (IP) | 6 | 276 | 5.3 × 10−4 | 1.3 × 10−8 | 6.50 | 6.20 | 2.14 | 6.42 | 98.11 ± 2.34 |
| Soil pH | ||||||
| Incubation Time, Day | 50% FC | 100% FC | ||||
| CK | M | WS | CK | M | WS | |
| 0 | 7.82 ± 0.01 | 7.22 ± 0.07 | 7.40 ± 0.02 | 7.83 ± 0.0 | 7.18 ± 0.02 | 7.33 ± 0.01 |
| 1 | 7.85 ± 0.02 | 7.25 ± 0.03 | 7.34 ± 0.0 | 7.91 ± 0.01 | 7.34 ± 0.02 | 7.32 ± 0.06 |
| 3 | 8.01 ± 0.02 | 7.37 ± 0.05 | 7.30 ± 0.02 | 7.93 ± 0.03 | 7.28 ± 0.01 | 7.36 ± 0.02 |
| 30 | 8.21 ± 0.08 | 7.40 ± 0.04 | 7.44 ± 0.01 | 8.25 ± 0.04 | 7.38 ± 0.03 | 7.65 ± 0.12 |
| Analysis of variance | ||||||
| F value | 21.00 | 3.73 | 22.65 | 59.99 | 21.16 | 5.55 |
| p value | 0.007 | 0.118 | 0.006 | 0.001 | 0.006 | 0.066 |
| Soil EC | ||||||
| Incubation Time, Day | 50% FC | 100% FC | ||||
| CK | M | WS | CK | M | WS | |
| 0 | 0.47 ± 0.0 | 0.62 ± 0.02 | 0.73 ± 0.01 | 0.47 ± 0.0 | 0.68 ± 0.02 | 0.75 ± 0.0 |
| 1 | 0.45 ± 0.0 | 0.65 ± 0.03 | 0.72 ± 0.01 | 0.51 ± 0.0 | 0.74 ± 0.04 | 0.70 ± 0.0 |
| 3 | 0.46 ± 0.0 | 0.61 ± 0.02 | 0.69 ± 0.0 | 0.48 ± 0.02 | 0.65 ± 0.02 | 0.73 ± 0.01 |
| 30 | 0.44 ± 0.0 | 0.61 ± 0.02 | 0.67 ± 0.01 | 0.48 ± 0.02 | 0.61 ± 0.0 | 0.56 ± 0.02 |
| Analysis of variance | ||||||
| F value | 19.67 | 0.60 | 30.33 | 3.28 | 6.83 | 39.48 |
| p value | 0.007 | 0.649 | 0.003 | 0.141 | 0.047 | 0.002 |
| Day 1 | ||||||||||||
| PAHs | 50% FC | 100% FC | ||||||||||
| CK | ±SD | M | ±SD | WS | ±SD | CK | ±SD | M | ±SD | WS | ±SD | |
| Pyr | 99.5 | 0.10 | 97.1 | 0.01 | 97.2 | 0.08 | 99.4 | 0.05 | 97.7 | 0.16 | 97.1 | 0.01 |
| Flt | 99.6 | 0.06 | 97.3 | 0.05 | 97.3 | 0.05 | 99.6 | 0.06 | 97.3 | 0.05 | 97.3 | 0.05 |
| BaP | 99.6 | 0.04 | 98.5 | 0.02 | 98.3 | 0.01 | 99.6 | 0.04 | 98.5 | 0.02 | 98.3 | 0.01 |
| BgP | 99.7 | 0.20 | 97.2 | 0.01 | 97.2 | 0.01 | 99.7 | 0.20 | 97.2 | 0.01 | 97.2 | 0.01 |
| BkF | 99.6 | 0.09 | 98.3 | 0.11 | 98.3 | 0.11 | 99.5 | 0.02 | 98.3 | 0.11 | 98.3 | 0.11 |
| IP | 99.6 | 0.05 | 97.4 | 0.06 | 97.1 | 0.03 | 99.6 | 0.04 | 97.7 | 0.11 | 97.2 | 0.02 |
| Day 15 | ||||||||||||
| PAHs | 50% FC | 100% FC | ||||||||||
| CK | ±SD | M | ±SD | WS | ±SD | CK | ±SD | M | ±SD | WS | ±SD | |
| Pyr | 98.2 | 0.05 | 73.8 | 0.05 | 77.7 | 0.10 | 98.2 | 0.05 | 73.6 | 0.24 | 77.9 | 0.01 |
| Flt | 97.5 | 0.04 | 75.2 | 0.06 | 78.2 | 0.04 | 97.5 | 0.04 | 75.2 | 0.06 | 78.2 | 0.04 |
| BaP | 99.1 | 0.01 | 82.5 | 0.05 | 83.2 | 0.01 | 99.1 | 0.01 | 83.4 | 0.12 | 85.1 | 0.02 |
| BgP | 97.4 | 0.04 | 73.2 | 0.03 | 76.1 | 0.05 | 97.4 | 0.04 | 73.2 | 0.03 | 76.1 | 0.05 |
| BkF | 97.5 | 0.02 | 73.1 | 0.03 | 76.1 | 0.01 | 97.5 | 0.02 | 73.1 | 0.03 | 76.1 | 0.01 |
| IP | 98.9 | 0.54 | 76.3 | 0.01 | 77.3 | 0.01 | 98.9 | 0.54 | 76.6 | 0.27 | 78.4 | 0.01 |
| Day 30 | ||||||||||||
| PAHs | 50% FC | 100% FC | ||||||||||
| CK | ±SD | M | ±SD | WS | ±SD | CK | ±SD | M | ±SD | WS | ±SD | |
| Pyr | 97.4 | 0.02 | 50.2 | 0.02 | 51.0 | 0.00 | 97.2 | 0.01 | 52.8 | 0.09 | 55.4 | 0.09 |
| Flt | 95.3 | 0.02 | 50.3 | 0.06 | 52.2 | 0.05 | 95.4 | 0.07 | 51.4 | 0.06 | 54.0 | 0.01 |
| BaP | 97.6 | 0.04 | 60.3 | 0.12 | 63.2 | 0.02 | 97.1 | 0.02 | 62.5 | 0.18 | 67.2 | 0.05 |
| BghiP | 95.6 | 0.05 | 51.7 | 0.01 | 52.2 | 0.08 | 95.4 | 0.04 | 53.8 | 0.04 | 55.2 | 0.11 |
| BkF | 95.1 | 0.02 | 51.3 | 0.11 | 53.2 | 0.09 | 95.5 | 0.02 | 53.3 | 0.02 | 56.2 | 0.15 |
| IP | 96.1 | 0.05 | 52.4 | 0.03 | 54.5 | 0.04 | 96.4 | 0.04 | 54.1 | 0.02 | 56.3 | 0.05 |
| p Values among Treatments at Each Incubation Time | ||||||
| Pyr | Flt | BaP | BghiP | BkF | IP | |
| 50% FC | ||||||
| 1 | 0.000 | 0.000 | <0.0001 | 0.001 | 0.005 | <0.0001 |
| 15 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| 30 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| 100% FC | ||||||
| 1 | 0.001 | 0.000 | <0.0001 | 0.001 | 0.004 | 0.000 |
| 15 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| 30 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| p Values among Incubation Time at Each Treatment | ||||||
| Pyr | Flt | BaP | BghiP | BkF | IP | |
| 50% FC | ||||||
| CK | <0.0001 | <0.0001 | <0.0001 | 0.000 | <0.0001 | 0.009 |
| M | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| WS | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| 100% FC | ||||||
| CK | <0.0001 | <0.0001 | <0.0001 | 0.000 | <0.0001 | 0.011 |
| M | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| WS | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Variables | pH | Cum. CO2-C | Pyr | Flt | BaP | BghiP | BkF | IP |
|---|---|---|---|---|---|---|---|---|
| pH | — | |||||||
| Cum. CO2-C | −0.722 | — | ||||||
| Pyr | 0.983 *** | −0.794 | — | |||||
| Flt | 0.981 *** | −0.814 * | 0.999 *** | — | ||||
| BaP | 0.99 *** | −0.776 | 0.998 *** | 0.997 *** | — | |||
| BghiP | 0.979 *** | −0.806 | 1 *** | 0.999 *** | 0.997 *** | — | ||
| BkF | 0.984 *** | −0.802 | 1 *** | 1 *** | 0.998 *** | 1 *** | — | |
| IP | 0.981 *** | −0.812 * | 0.999 *** | 1 *** | 0.998 *** | 1 *** | 1 *** | — |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
EL-Saeid, M.H.; Usman, A.R.A. Influence of Organic Amendments and Moisture Regime on Soil CO2-C Efflux and Polycyclic Aromatic Hydrocarbons (PAHs) Degradation. Sustainability 2022, 14, 4116. https://doi.org/10.3390/su14074116
EL-Saeid MH, Usman ARA. Influence of Organic Amendments and Moisture Regime on Soil CO2-C Efflux and Polycyclic Aromatic Hydrocarbons (PAHs) Degradation. Sustainability. 2022; 14(7):4116. https://doi.org/10.3390/su14074116
Chicago/Turabian StyleEL-Saeid, Mohamed Hamza, and Adel R. A. Usman. 2022. "Influence of Organic Amendments and Moisture Regime on Soil CO2-C Efflux and Polycyclic Aromatic Hydrocarbons (PAHs) Degradation" Sustainability 14, no. 7: 4116. https://doi.org/10.3390/su14074116
APA StyleEL-Saeid, M. H., & Usman, A. R. A. (2022). Influence of Organic Amendments and Moisture Regime on Soil CO2-C Efflux and Polycyclic Aromatic Hydrocarbons (PAHs) Degradation. Sustainability, 14(7), 4116. https://doi.org/10.3390/su14074116







