Abstract
Dipole–Dipole interactions (DDI) constitute an effective mechanism by which two physical entities can interact with each other. DDI processes can occur in a resonance framework if the energies of the two dipoles are very close. In this case, an energy transfer can occur without the need to emit a photon, taking the name of Förster Resonance Energy Transfer (FRET). Given their large dependence on the distance and orientation between the two dipoles, as well as on the electromagnetic properties of the surrounding environment, DDIs are exceptional for sensing applications. There are two main ways to carry out FRET-based sensing: (i) enhancing or (ii) inhibiting it. Interaction with resonant environments such as plasmonic, optical cavities, and/or metamaterials promotes the former while acting on the distance between the FRET molecules favors the latter. In this review, we browse both the two ways, pointing the spotlight to the intrinsic interdisciplinarity these two sensing routes imply. We showcase FRET-based sensing mechanisms in a variety of contexts, from pH sensors to molecular structure measurements on a nano-metrical scale, with a particular accent on the central and still mostly overlooked role played between a nano-photonically structured environment and photoluminescent molecules.
1. Introduction
Photoluminescence-based sensing mechanisms lie at the basis of countless high-resolution investigations. It is enough to mention an important technique called “immunofluorescence”, which is currently gaining celebrity under the name of “third-generation swab test” for SARS-CoV-2 virus detection. In such a technique, a photoemissive molecule called “fluorophore” is linked to an antibody that can selectively be functionalized to a targeted entity called “antigen” that is supposed to be present and fixed in a specimen. Simple fluorescence microscopy analysis can then reveal the presence of the antigen through quick optical investigation. Apart from the simple yet effective detection of static fluorescence, the time evolution of the spontaneous emission offers broader possibilities for far more sophisticated analyses. One of the most used and perhaps the most studied photodynamical process for sensing applications is the so-called “Förster Resonant Energy Transfer” (FRET). FRET constitutes a particular kind of long-range dipole–dipole interaction occurring between an initially excited “donor” (D) to an “acceptor” (A) in its ground state [1,2,3].
Before diving into an attempt to unravel all the physics hidden behind the puzzle of the acronym, it is wise to give a quick explanation of what is usually meant by the term “dipole-dipole” interaction.
A molecule in its excited state manifests a certain degree of charge delocalization all across its length. Such a charge configuration is characterized by a spatial displacement “d” between the center of gravity of the positive and the negative charge. It is reasonable to expect that such a spatial charge delocalization causes the onset of an electrical dipole moment, expressed in terms of the product “qr” between the charge displacement “r” and the charge value “q” [3]. Since this charge displacement is associated with an electronic transition, it turns natural the concept of a “transition dipole moment”. The transition dipole moment associated with the transition between a precise initial ⟩ and final ⟩ state can be expressed as , being µ the electric dipole moment operator. Once the molecule is brought into its excited state, it can relax by emitting a photon. Even though such a sentence usually creates no discomforts in the scientist’s conscience, it should. The reason why, indeed, a molecule in its excited state should spontaneously collapse back to its ground state is one of the most controverted physics phenomena, called “spontaneous emission”. If indeed nothing occurs to perturb the molecule in its new state, the molecule should tend to preserve its status forever. It is easy to understand such a mechanism when a photon with suitable energy comes to perturb the molecule, making it jump back to its ground state (the so-called “stimulated” emission), but figuring out the mechanism that leads the molecule to spontaneously relax by emitting a photon is a far more complicated task, that can be solved only through a quantum mechanical treatment [4]. To what concerns this review, it is enough to say that the probability for a two-level system in its excited state to spontaneously relax by emitting a photon is given by the so-called “Fermi golden rule”, which relates to the spontaneous decay rate () of an atom with a quantity denominated “local density of states” (LDOS), , through the relation expressed in Equation (1) [2,4]:
The LDOS can be briefly understood as the total number of electromagnetic modes accessible per unit volume and unit frequency at a given location r0 [5].
When two molecules with close-enough transition energies are placed in proximity of each other, they can interact. Let us consider the case in which the first molecule features slightly higher transition energy than the second one. We will call this molecule “donor” (D), to distinguish it from the second one that is called “acceptor” (A). If a photon with suitable energy is absorbed by the donor, a transition from the ground () to the excited () state is provoked in this molecule, and an induced electric dipole appears in the donor, with an associated transition dipole moment calculated as shown before. As a consequence, an induced dipole can be generated in the acceptor. If the orientation of these two transition dipoles is favorable and their energies are close enough, the two dipoles lie in a “quasi-resonance” condition that allows an energy transfer from the “donor” D to the “acceptor” A without the need for photon emission. Such a process takes the name of “Resonant Energy Transfer” (RET) [3].
The name FRET tells a lot about the phenomenon. The term “Resonant” is used to highlight that the interacting dipoles are in almost perfect resonance condition. FRET configures as an additional relaxation channel for the donor, to which a proper “rate” can be associated. Such a quantity, denominated “FRET rate” (, determines the energy exchange rate between the D and the A through non-radiative channels, mediated by the resonance condition between the acceptor and the donor.
Therefore, providing a pristine decay rate for the donor equals to [6]:
being the decay rate of the pristine donor, the complete FRET system (donor + acceptor) will be characterized by a new decay rate [6]:
resulting from the summation of two contributions: (i) , the decay rate of the pristine donor as defined before, and being the FRET rate. Here, is the decay rate of the donor measured at the energy of its active optical transition (fluorescence peak) in the presence of the acceptor. The FRET rate can be directly calculated from experimental measurements carried out to retrieve the values of and and by using Equation (3) [1,6]:
A parameter called “FRET efficiency” (EFRET) can also be defined as the ratio between the FRET rate over that of all other possible relaxation channels for the donor so that [1,6,7]:
All the quantities appearing in Equation (4) depend on the LDOS of the electromagnetic environment in which the fluorophores are immersed, and a still ongoing long-standing debate arises on whether the FRET rate and the FRET efficiency can be modified by structuring the LDOS distribution [8,9,10,11]. A noticeable theoretical contribution was recently given by Cortes and Jacob, which unraveled the skein [12].
2. FRET Enhancement Sensing Techniques
Dipole–Dipole interaction can be enhanced through several mechanisms. In principle, all the pathways that allow to increase the photophysical properties of a fluorophore can also determine an increase in its dipole–dipole interaction.
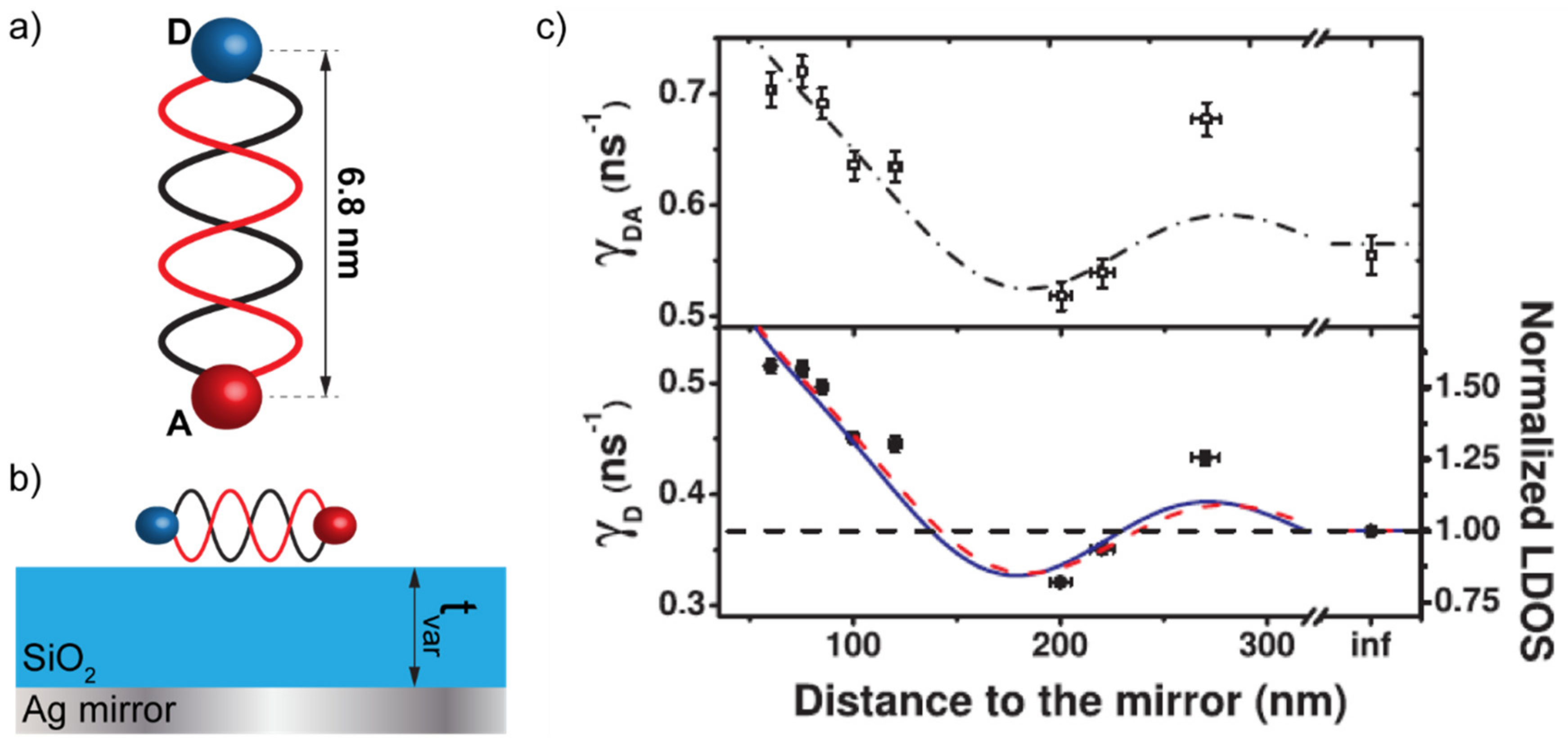
The LDOS appearing in the formulation of the Fermi golden rule can be tailored by structuring the electromagnetic environment in which the fluorophore is immerged. The existence of cavity resonances or plasmonic modes in the surrounding can, therefore, play a substantial role in the modification of the spontaneous emission decay rate and, as a consequence, in the possible dipole–dipole interaction with neighboring molecules [13,14,15]. A seminal work by Chance et al., investigated the dependence of the FRET rate and efficiency on the distance between a donor/acceptor pair (DAP) and a metallic mirror [6]. The metallic mirror is, perhaps, the simplest way to structure the LDOS around a DAP by changing the distance between them. In such a configuration, an oscillation of the emission rate of the DAP was reported as a function of the distance with the mirror, in accordance with the oscillations of the LDOS. Chance et al. concluded that, while the FRET efficiency is influenced by the LDOS configuration, the FRET rate is not [6]. Blum et al. confirmed these findings 34 years later by setting up an experimental procedure that became de facto a benchmark in these kinds of investigations [7]. In particular, they used a DAP constituted by two fluorophores, Atto488 (donor) and Atto565 (acceptor), separated by 15-base-pairs DNA strands, used as a 6.8 nm spacer between the two fluorophores (see Figure 1a). Then, they placed the DAP on top of a SiO2 film used as a variable-thickness spacer between the DAP and a thick Ag mirror (see Figure 1b). Their measurements confirmed the oscillations found by Chance et al. in the emission rate of the DAP as a function of the thickness of the SiO2 layer (see Figure 1c).
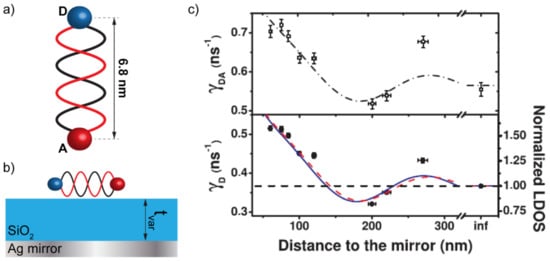
Figure 1.
(a) 15-base-pairs DNA strand used as a spacer between the donor (D) and the acceptor (A) molecules constituting the donor/acceptor pair (DAP). (b) Photonic framework consisting in a Ag mirror and a SiO2 layer used as a variable-thickness spacer between the DAP and the mirror. (c) Donor decay rate (bottom panel) compared to the donor/acceptor decay rate while increasing the distance from the Ag mirror by increasing the thickness of the SiO2 layer. Panel (c) is reproduced from reference [7]. Copyright (2022) by the American Physical Society—https://doi.org/10.1103/PhysRevLett.109.203601 (accessed on 24 March 2022).
It is interesting to mention that Patra et al. recently investigated the bare scattering response of a SiO2/Ag bilayer as a function of the thickness of the SiO2 layer, reporting the insurgence of the so-called “pseudo-cavity resonances” [16]. Even though Patra et al. also reported the strong interaction occurring between one single fluorophore and pseudo-cavity resonances, nothing has been reported so far on the capability of these resonances to modify the photodynamics of the FRET process. The pseudo-cavity resonances, to which an oscillation of reflectance and absorbance at the resonant wavelength is associated, could potentially explain the behavior reported by both Chance et al. and Blum et al.
After these two examples, many more contributions came, trying to enforce or, sometimes, contradict these conclusions that, however, still remain a compass in this field.
In the following, we examine some of the most important studies that explore the possibility of altering the FRET photodynamics through structuring the electromagnetic environment around the DAP. We distinguish two categories of interactions, the ones related to cavity modes and the ones related to plasmonic interactions with metallic nanostructures.
2.1. Cavity-Enhanced FRET
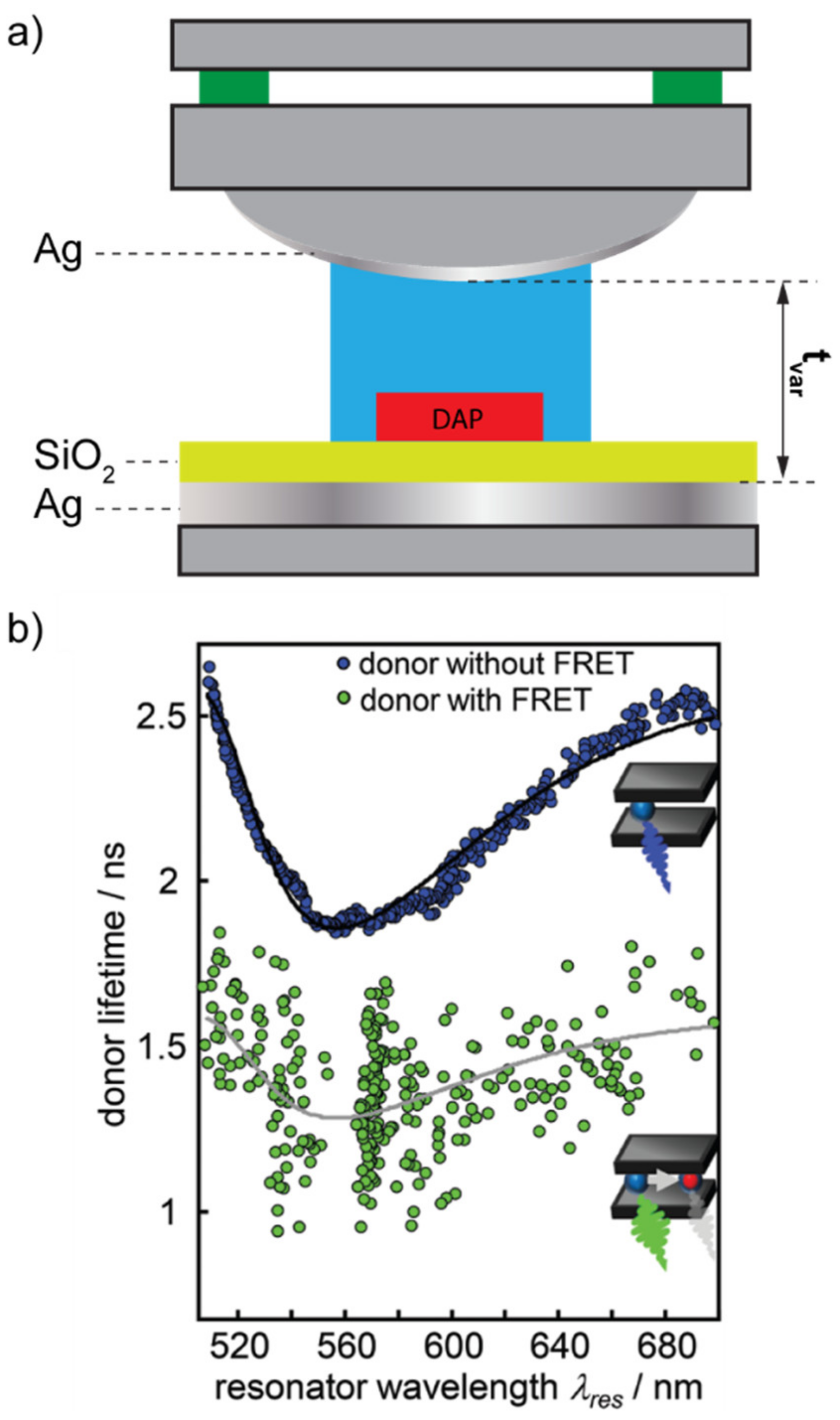
Fabry–Pérot cavities are exceptional for structuring the LDOS around a fluorophore. These resonators are, indeed, a standard in Cavity-Quantum Electro-Dynamics (C-QED) studies and, in particular, in those related to the so-called “Purcell effect”. The Purcell effect is a phenomenon according to which the decay rate of an emitter is enhanced by a resonant electromagnetic environment in tune with the radiative optical transitions of the emitting molecule [17]. Purcell effect originates from the increase in LDOS introduced in the proximity of the emitter by a resonant cavity and describes how the probability of such a fluorophore to spontaneously decay through radiative processes can be enhanced by a factor f = 3Qλ3/(4π2V), where Q is the quality factor of the resonant cavity, λ is the wavelength associated to the active optical transition and V is a quantity called “modal volume [18]. The capability of the Purcell effect to enhance the photoluminescence of a single fluorophore can do the same in the FRET mechanism, provided that a suitably high Q-factor resonance is used. Konrad et al. recently demonstrated this by means of a tunable Fabry–Pérot cavity [19]. In their work, the authors consider a FRET pair constituted by two dyes of the same family (Atto488 as a donor and Atto590 as an acceptor), linked to a DNA strand, similar to the case of Figure 1a. The decay lifetimes of the FRET pair have been measured both in the pristine configuration (stand-alone FRET pair) and by placing them inside a tunable Fabry–Pérot microcavity, directly built over a confocal microscope (Purcell-effect-assisted FRET) (Figure 2a). As expected, the static photoluminescence spectrum of the Purcell-effect-assisted FRET pair was substantially modified by the optical response of the cavity, as reported in other works [13]. However, no exceptional modification of the decay lifetimes of the FRET pair was detected in the presence of the Purcell effect (Figure 2b).
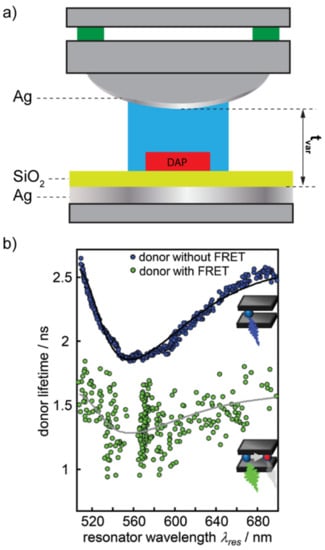
Figure 2.
(a) Sketch of the tunable Fabry–Pérot resonator built over a common microscope. (b) Donor lifetime with and without the presence of the acceptor. Panel (b) is reproduced from reference [19] with permission from the Royal Society of Chemistry.
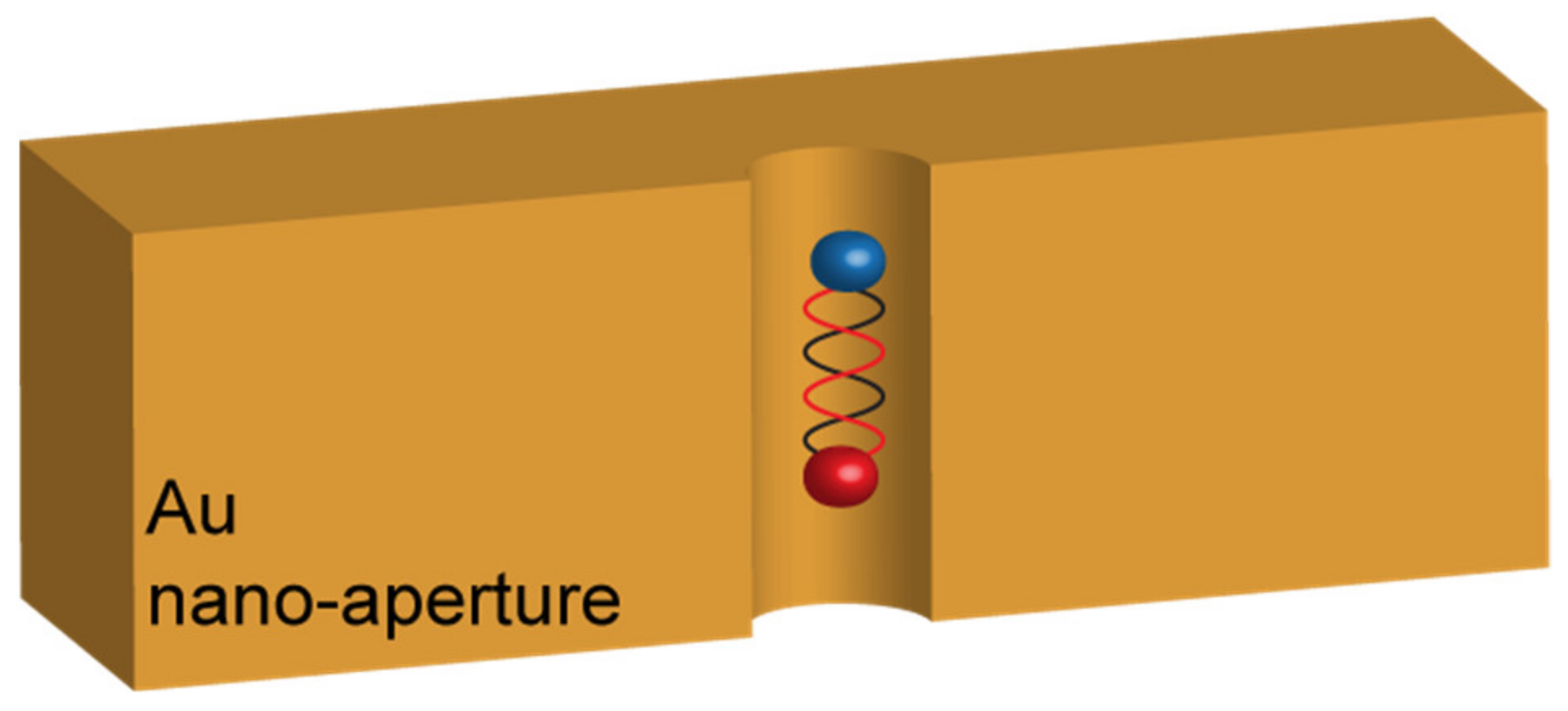
Such a result let the authors conclude that the low-Q-factor of their resonator was responsible for the insufficient FRET enhancement. Schleifenbaum et al. enforced these conclusions with similar experiments in Fabry–Pérot cavities but reported that a certain degree of FRET rate enhancement could be obtained due to the capability of the resonant cavities to modify the donor and acceptor populations [20]. A very different result was, on the contrary, obtained by Ghenuche et al. [21]. In their work, the authors investigated the capability of a nanostructured electromagnetic environment to enhance the FRET rate between a DAP very similar to the one used by Konrad et al. In particular, the DAP was composed of Atto550 and Atto647N as the donor and the acceptor, respectively. The inter-distance between these two dyes was finely controlled in a range spanning from 3.4 to 13.6 nm by linking them to DNA strands made of increasing numbers of base pairs. The resonant nanostructure designed to interact with the FRET pair was made of circular nano-apertures of different diameters ranging from 160 nm to 380 nm, milled in a 150 nm thick gold substrate (Figure 3). The variation of the nano-apertures diameters provided a different LDOS distribution around the DAP.
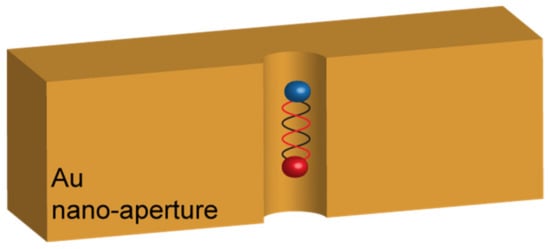
Figure 3.
Sketch of the architecture proposed by Grenuche et al. in reference [21], consisting in nano-apertures with different diameters milled in an Au film. The interaction occurs when the single DAP falls inside the hole.
The authors demonstrated not only that the FRET enhancement scales linearly with the LDOS but that this effect is larger the further apart the two molecules are, which is a conclusion that opens up an unexpected scenario for very-long-range dipole–dipole interaction. Wang et al. also demonstrated that a complete Fabry–Pérot cavity is not strictly necessary to enhance the FRET rate. In their work, the authors demonstrate that a simple Al/SiO2 bilayer can control both the FRET rate and efficiency between emitting nano-diamonds and graphene by tuning the thickness of the SiO2 layer [22].
Whispering gallery modes (WGM) have been demonstrated to be capable of enhancing the FRET rate of a DAP as well. Folan et al. demonstrated such a mechanism within a 10 μm diameter glycerol particle. The authors reported an enhancement of conventional FRET occurring in the adopted DAP by more than a factor of 100 [23]. Götzinger et al., reported WGM-mediated FRET over distances of tens of micrometers by using high-Q-factor silica microspheres [24]. They reported FRET efficiency enhancement of more than five orders of magnitude. Jana et al. demonstrated this phenomenon by adopting a particular configuration in which the donors (colloidal quantum dots) were placed within polymeric microspheres, manifesting high-Q-factor (>4000) WGM [25]. In this case, the acceptors (dye nanoparticles) were placed outside the microsphere. The authors demonstrated that energy transfer from WGM to acceptors placed in the vicinity of the cavity surface was largely enhanced by the high-Q-factor of the resonances and that FRET could take place even at distances of about 100 nm.
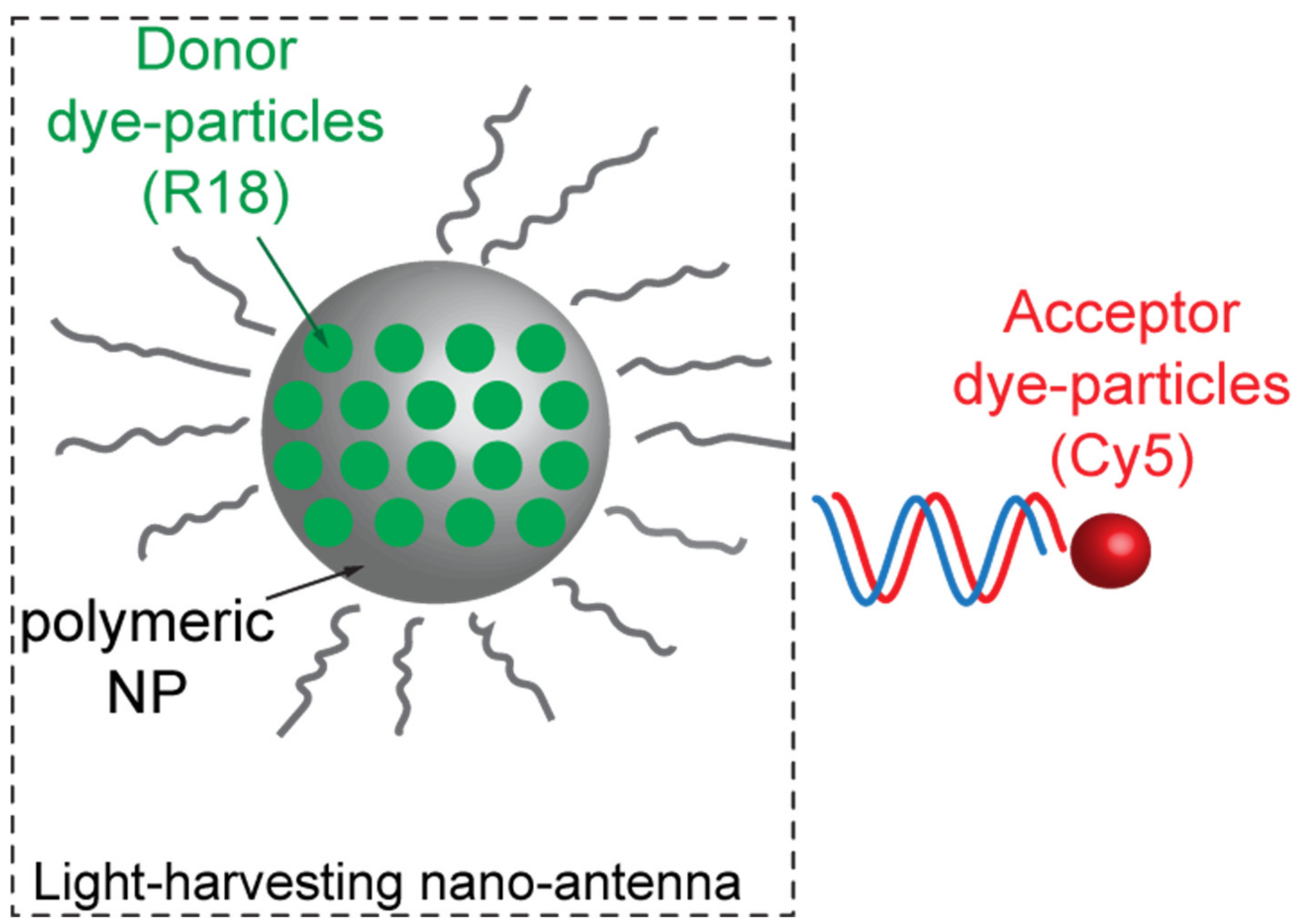
Loading polymeric NPs with a large number of donor molecules revealed such a proficient technique also for bio-sensing applications and with no strict necessity to leverage on high-Q-factor resonances. Melnychuk et al., for example, demonstrated a 2000-fold enhancement of FRET efficiency through a very peculiar technique based on the so-called light-harvesting nano-antennas [26]. These structures are constituted by 40 nm PMMA nanoparticles, functionalized on their surface with oligonucleotides. Each PMMA nanoparticle is loaded with about 3200 hydrophobic rhodamines (R18) molecules acting as a very bright donor (Figure 4). In order to prevent aggregation and quenching, rhodamine molecules were capped with a hydrophobic counterion (F5-TPB) (Figure 4). The PMMA nanoparticles are then capped with a capture oligonucleotide complementary to a nucleic acid fragment, encoding a marker of interest (SurC) for the specific sensing application. This marker is hybridized with the selected FRET acceptor Cy5. Once the oligonucleotide captures a functionalized marker, the FRET can occur, and thousands of rhodamine molecules can transfer energy to a few acceptors. Such a mechanism is responsible for an exceptional FRET efficiency enhancement.
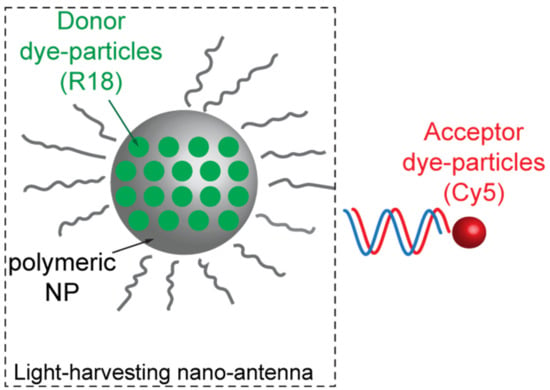
Figure 4.
Sketch of the light-harvesting nano-antenna system as envisioned by Melnychuk in reference [26].
The dye-loaded polymeric nanoparticles approach proved its validity also for the 3D structuring of the DAPs. Galisteo-Lopez et al., for example, demonstrated exceptional FRET enhancement achieved by geometrically structuring PMMA spheres in a 3D photonic crystal architecture [27]. The capability of photonic crystals to enhance the energy transfer dynamics has also been demonstrated by Yang et al., that demonstrated that FRET occurring between Fluorescein and Rhodamine B could be enhanced by inhibiting the radiative emission of the donor dye by appropriately designing the photonic bandgap of the periodic structure [28]. The same group demonstrated the capability of inverse opal photonic crystals to enhance the energy transfer occurring in a rare-earths-based DAP made of Tb3+ (donor) and Eu3+ (acceptor) [29].
2.2. Plasmon-Enhanced FRET
The capability of metals to improve the photophysical properties of a fluorophore are well-known. The mechanisms usually associated with such a process are two: the so-called (i) Metal-Enhanced Fluorescence (MEF), also sometimes called “Surface Plasmon Coupled Emission” (SPCE) [30], and the so-called “Surface Plasmon Coupled Absorption” (SPCA). The former phenomenon was thoroughly described by Lakowicz in 2005 [30]. One of the contradictions encountered when making fluorescence and metal deal together is that it is well-known that metals tend to quench fluorescence, offering very favorable non-radiative relaxation channels for the molecules. It is, however, true, as explained by Lakowicz, that if radiating plasmons are excited, metals can, instead, enhance the fluorescence. This is the case for metallic nanoparticles. However, the peculiar nature of localized plasmonic resonance, which implies both a scattering and absorptive nature, means that small colloidal nanoparticles tend to quench fluorescence while big ones enhance it. This is mainly due to the fact that the scattering cross-section in the visible range of small nanoparticles is negligible with respect to the absorption cross-section. On the contrary, large nanoparticles, in which scattering processes are predominant over absorptive ones, tend to increase the fluorescence of interacting emitters, behaving such as small antennas. Such a phenomenon is sometimes called “Surface Plasmon Coupled Emission” and is extremely useful for high-resolution sensing applications based on fluorescence [31,32,33,34,35,36,37].
The second mechanism that allows enhancing the spontaneous emission of a fluorophore is called “Surface Plasmon Enhanced Absorption”. As said before, plasmonic resonances share with antennas a scattering and dual absorptive nature. This means that nanoparticles and/or nanostructures can absorb light and make it available for the excitation of a fluorophore under the shape of evanescent waves. Such a mechanism can enhance the emission processes [38,39]. A combination of SPEA and SPCE has been recently used to tailor the Purcell effect in a system made of a metal/dielectric double cavity on top of which a CsPbBr3 perovskite was deposited, even though the plasmonic contribution to this phenomenon is still under investigation [40].
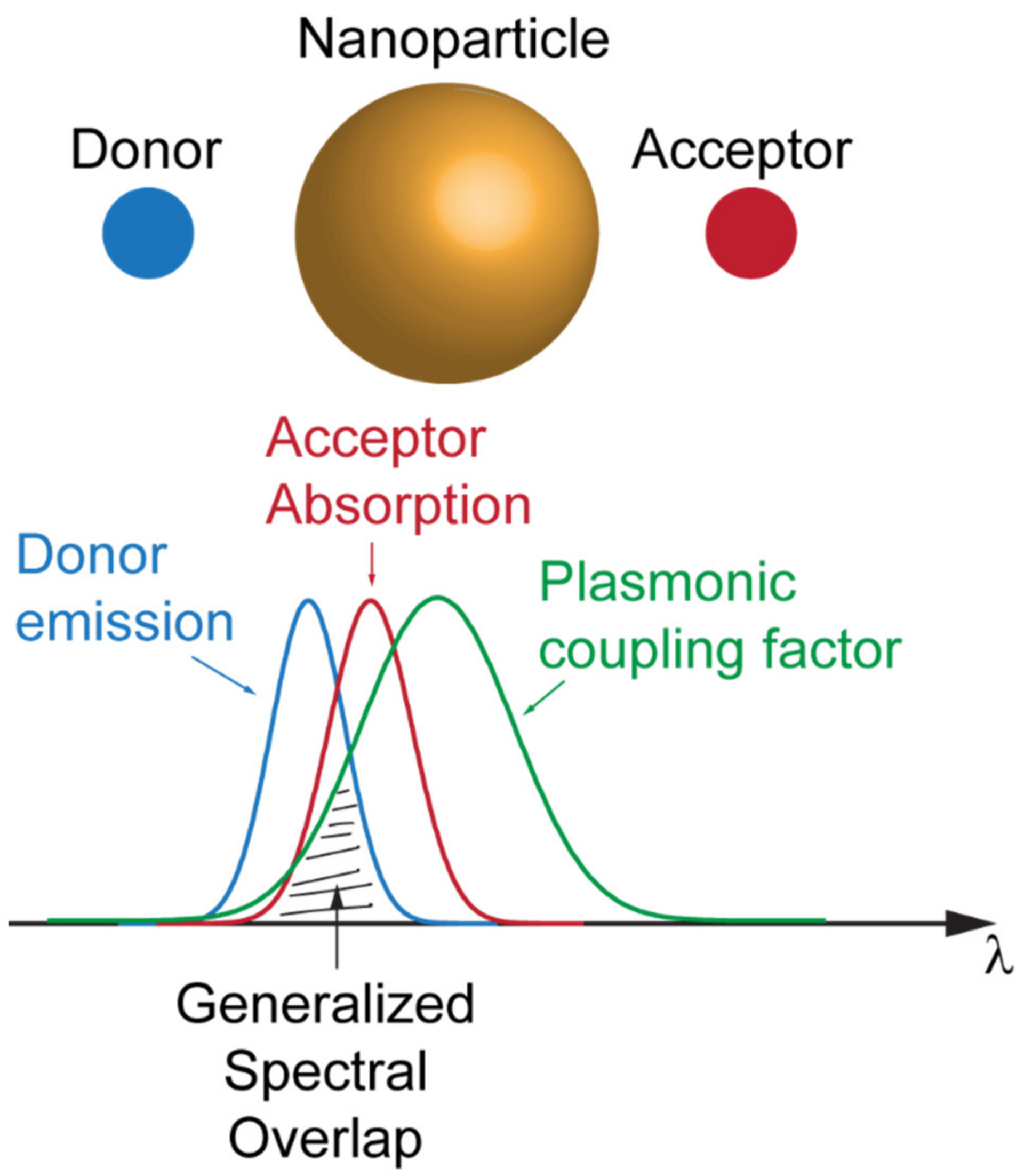
The importance of framing the interplay between plasmonic resonances and FRET has been recently summarized in the work of Hsu et al., where a theoretical ruler for the plasmon-coupled resonant energy transfer (PC-RET) has been achieved by the definition of a “generalized spectral overlap” concept. Hsu et al. demonstrated that the enhancement of the FRET dynamics could be achieved by finely designing the spectral overlap of three quantities: the donor emission spectrum, the acceptor absorption spectrum, and a coupling factor (the relative orientation of the acceptor and the electric field generated by the donor) that is determined by the spectral response of the plasmonic resonator mediating the coupling phenomenon (see Figure 5) [41]. The case for an anisotropic plasmonic nanoparticle has been investigated by Vincent and Carminiati under the definition of “magneto-optical FRET” [42].
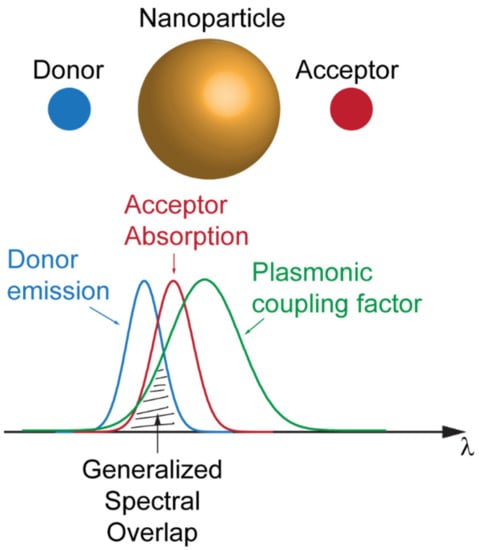
Figure 5.
Schematic of the coupling factor given by the spectral overlap of the emission of the donor, absorption of the acceptor and plasmonic resonance of the nanoparticle.
Stemming from this broad theoretical generalization, the capability of plasmonic resonators to structure the LDOS in the proximity of the DAP, and produce significant modification of both the rate and efficiency of the dipole–dipole resonant interaction between molecules, has been broadly experimentally documented, and all the associated phenomena usually go under the label of Plasmon-enhanced FRET (PE-FRET) [43]. Plasmonic nano-antennas have been among the first plasmonic structures investigated for PE-FRET applications. De Torres et al. demonstrated that the mediation of the components of the plasmonic field radiated by nano-antennas could also induce FRET and even in a configuration in which this phenomenon would be forbidden, as in the case of perpendicularly oriented molecules [44,45]. Plasmonic nanoparticles, such as core shells, have been broadly reported as efficient FRET enhancers [46,47]. Among the great advantages carried out by these architectures, there is the possibility to finely tune the inter-distance between the metallic core and the DAP, as demonstrated by Lessard-Viger et al. [48,49]. Nano pop-corns are among these. Asgar et al. reported a FRET rate increase from 4 to 20 ns−1 between organic cyanine3 carboxylic acid (Cy3, donor) molecules and inorganic CdSe colloidal quantum dots (QDs, acceptor) under the influence of a plasmonic field structured by the presence of Au nano-popcorns [50]. Plasmonic nano-gratings have been used by Steele et al. to increase the FRET efficiency of a DAP constituted by Atto-532 and Atto-633 molecules [51]. The largest efficiency enhancement has been found when the plasmonic response of the grating was in tune with the active optical transition of the acceptor.
3. FRET Inhibition Sensing Techniques
Sensing applications relying on FRET can as well leverage its suppression.
If plasmonics lies at the basis of the enhancement/suppression mechanism, the suppression of FRET is, interestingly, the other face of the medal of a phenomenon described before and called PC-RET. Jeong et al. demonstrated, indeed, that if a quantum-confined emitter (a quantum dot) is linked to a plasmonic nanoparticle, its spontaneous emission can be either enhanced or suppressed. In this latter case, the suppression occurs due to the effective cancellation of the electromagnetic radiation through destructive interference [52].
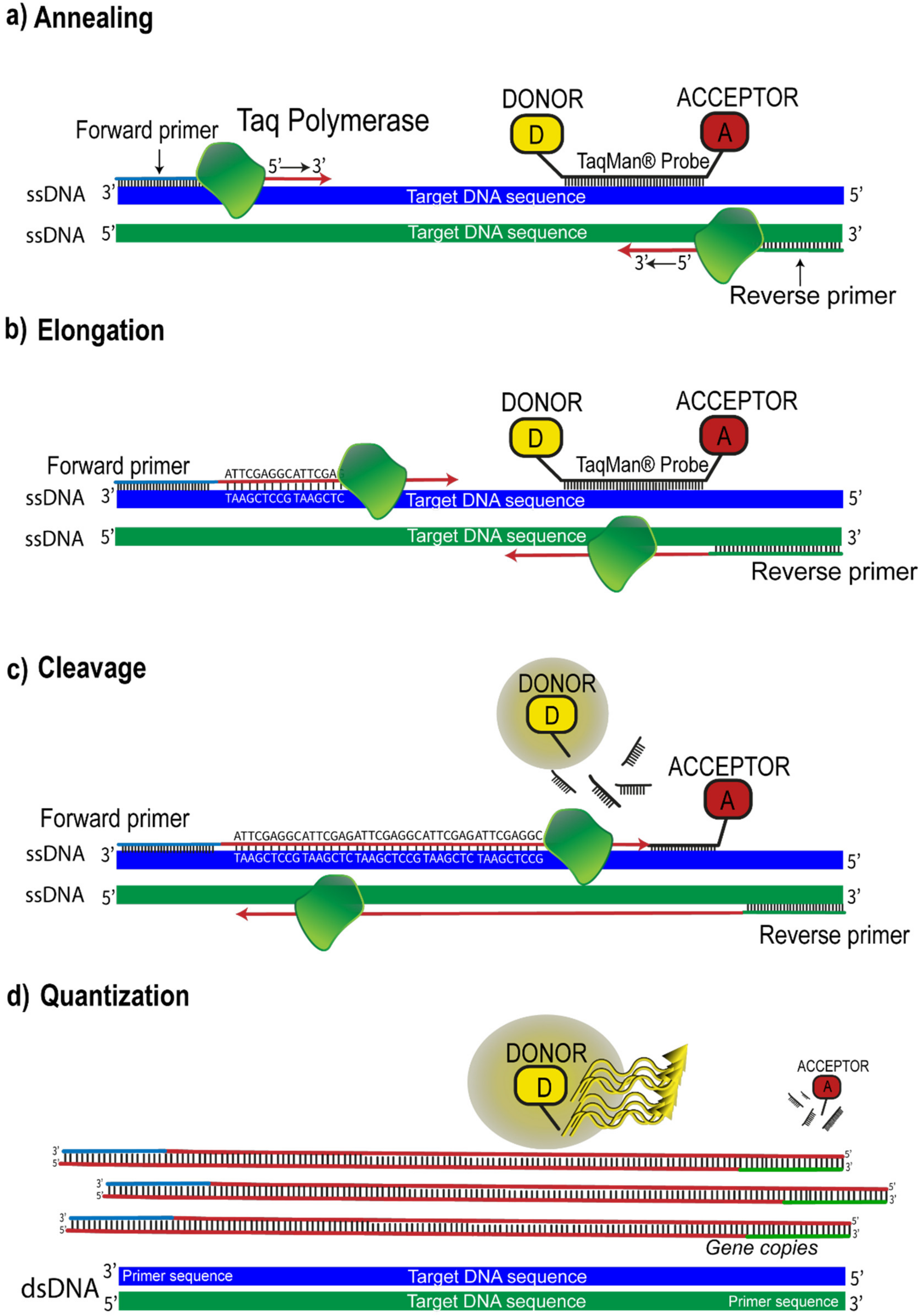
However, the most widely employed FRET suppression method remains to increase the separation between DAPs. This mechanism is the basis of well-known techniques such as the famous “Real Time Quantitative Polymerase Chain Reaction” (qRT-PCR), which allows the simultaneous amplification and quantification of specific DNA molecules. In particular, qRT-PCR is the method used in molecular biology for gene expression analysis, genotyping, and routine DNA quantification. Classical PCR allows amplifying in vitro specific sequence DNA by the following steps: (i) denaturation of double-strand DNA (dsDNA) template in single-strands DNA (ssDNA), (ii) annealing, in which two short single-stranded DNA (primers) hybridize by complementary DNA template 5′-P and 3′-OH ends (see Figure 6a), defining the target that will be amplified and, lastly, (iii) elongation, that consists in the polymerization of the new strand carried out by the Taq DNA polymerase enzyme from 5′-P end to 3′-OH end (see Figure 6b). These steps represent a reaction cycle that is repeated until a plateau phase is reached, in which the reaction reagents are exhausted. The qRT-PCR technique combines the classical PCR with FRET, using fluorogenic probes. Thus, the employment of probes allows the correlation of PCR products to changes in fluorescence intensity during each reaction cycle. Although several types of fluorogenic probes have been developed, the most widely used is the so-called “TaqMan”® probe (Thermo Fisher Scientific, Waltham, MA, USA). The TaqMan® probe is an oligonucleotide composed of 20–30 nucleotides, respectively labeled at the 5′-P end with a donor species, the reporter capable of emitting a fluorescent signal, and at the 3′-OH end with an acceptor species, which quenches fluorescence through non-radioactive channels. In this conformation, the two species, which constitute the DAP, are in such close proximity that they can interact and produce FRET. The region of DNA to which the oligonucleotide binds by complementarity is included between PCR primers (forward and reverse in Figure 6). When a new strand polymerization begins, the 5′-3′ exonuclease enzyme activity hydrolyzes the TaqMan® probe, bound to the templates, releasing the fluorophore (donor). This causes the physical separation of the two species (cleavage step in Figure 6c), resulting in the suppression of the FRET mechanism. Meanwhile, as the reaction proceeds, the produced fluorescence is captured and correlated to the amount of target gene copies (see Figure 6d) [45,46,47].
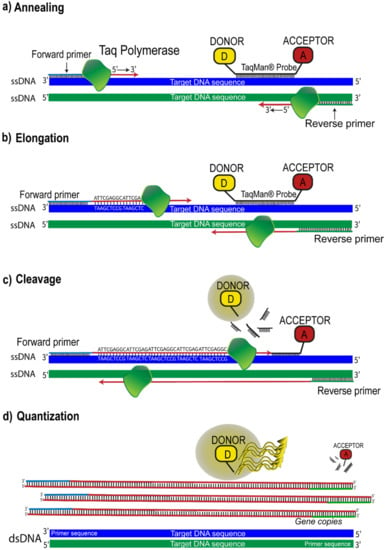
Figure 6.
qRT-PCR model using TaqMan® chemistry: (a) The enzyme Taq DNA Polymerase binds to the primer and starts to synthesize a new strand from the ssDNA templates; (b) elongation of new DNA strands; (c) the 5′-3′ exonuclease enzyme activity hydrolyzes the TaqMan® probe bound to the templates, releasing the fluorophore (donor); (d) the fluorescent signal detected is proportional to the amount of amplified gene produced in each cycle.
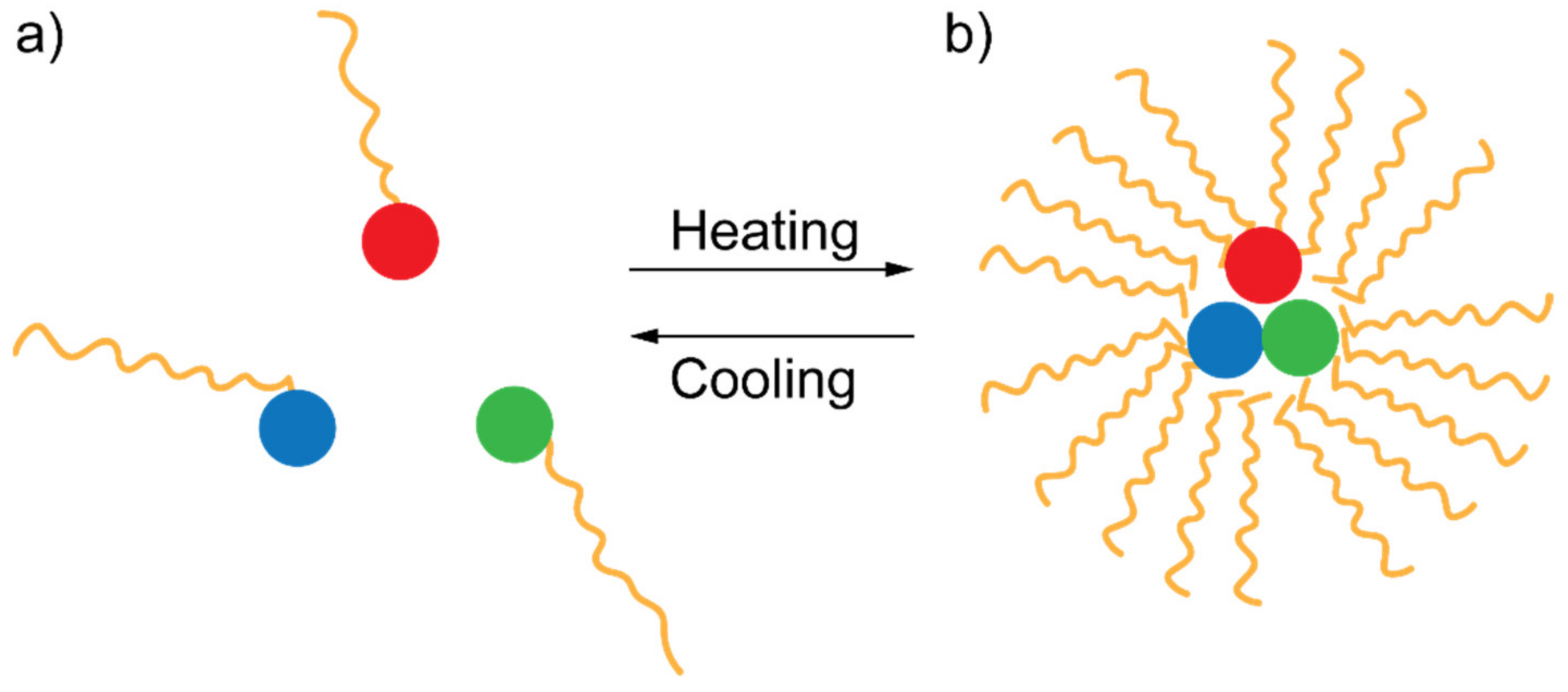
FRET inhibition based on the physical separation between DAP has been recently used as an effective technique for detecting the presence of glucose in blood [53]. The system proposed by Meledeo et al. consisted of a DAP relying on two fluorophores, namely tetramethylrhodamine isothiocyanate (TRITC) and fluorescein isothiocyanate (FITC), linked to concanavalin A and dextran, respectively. In the absence of glucose, concanavalin A and dextran are very close in solution, and FRET can occur. However, once glucose is added, due to the higher affinity of concanavalin to glucose, a significant displacement between concanavalin and dextran (and between the two linked fluorophores, as a consequence) happens, preventing the FRET from occurring [53]. FRET inhibition via DAP separation can be induced by increasing the temperature of the environment surrounding the DAP as well. Such a technique has been recently used to detect the temperature of a cell hosting a peculiar “FRET thermometer” [54]. This system is constituted by a FRET triplet rather than a FRET pair. Such a configuration allows dipole–dipole interactions to occur between three cascaded dyes: (i) a blue-emitting donor (BED), (ii) a green-emitting donor/acceptor (GEDA) acting as a FRET bridge, and a red-emitting acceptor (REA).
These fluorophores are immersed in a thermo-responsive polymeric matrix that reversibly shrinks upon heating. While heating, the thermo-responsive polymer brings the fluorophores close to each other, thus allowing the cascade-FRET to occur (see Figure 7). The authors also demonstrated that if this system is embedded in a biological cell, it can report the cellular temperature with exceptional precision [54].
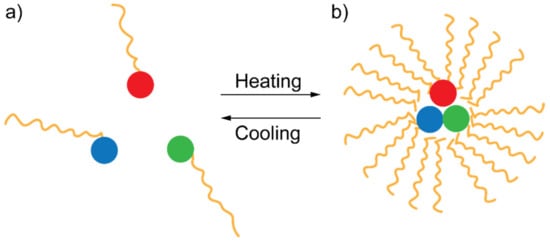
Figure 7.
Sketch of the heating/cooling mechanism allowing or hindering cascade FRET according to which the FRET triplet switches between two configurations: (a) one in which the components of the FRET triplet are far apart and (b) another in which they are close each other.
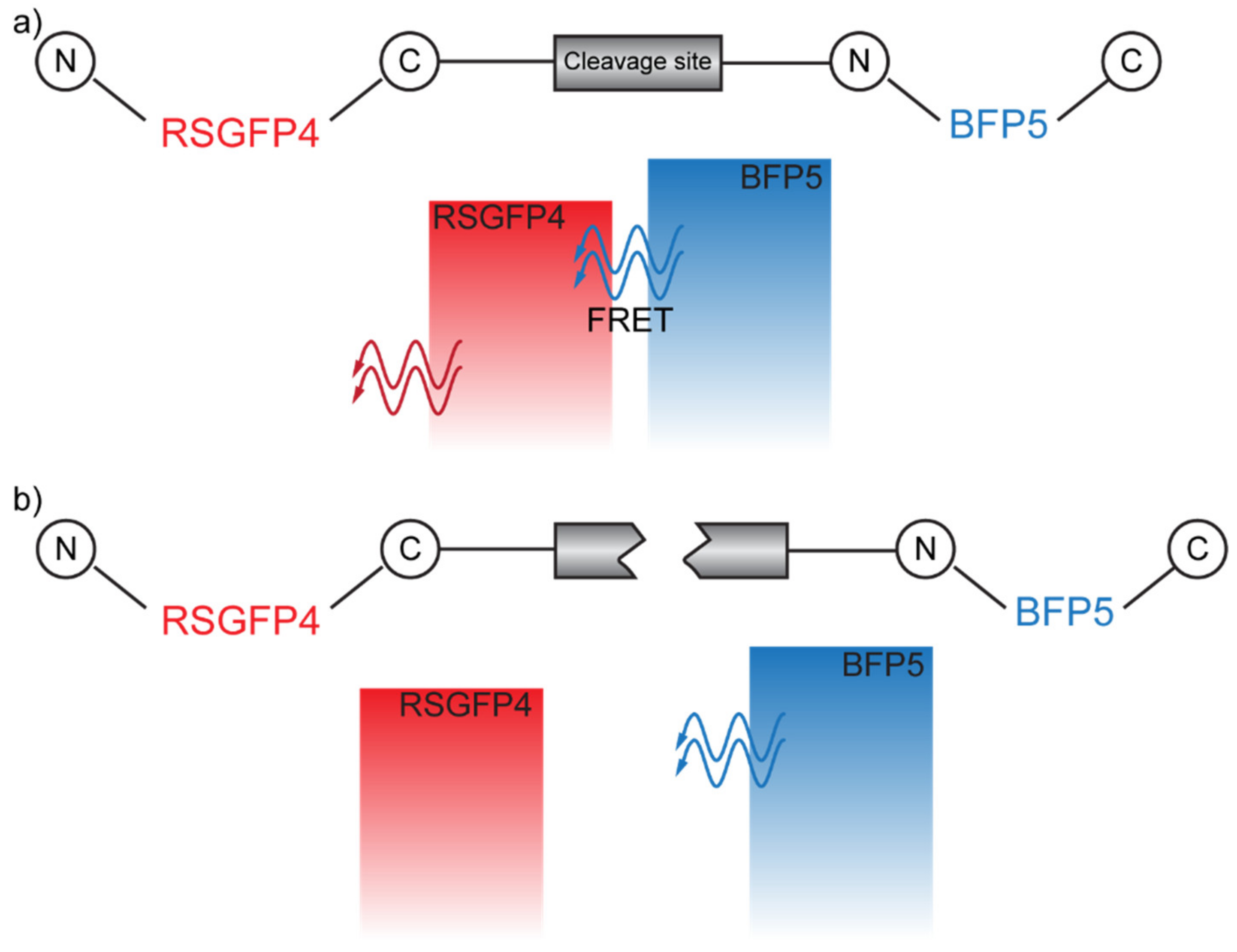
The displacement between the donor and the acceptor pair can also be achieved via enzymatic reactions. This is the working principle that allowed Mitra et al. to detect a Factor Xa, the active form of an enzyme involved in several steps in the blood coagulative cascade, through FRET [43]. In particular, here, the DAP is constituted by two variants of the green fluorescent protein (GFP): (i) RSGFP4, acting as an acceptor, and (ii) BFP5, acting as a donor. The two fluorophores are connected to both sides of a polypeptide containing the cleavage site of Factor Xa, in the absence of which FRET can occur (Figure 8a). When Factor Xa is introduced, it cleaves the site and prevents FRET between the DAP (Figure 8b).
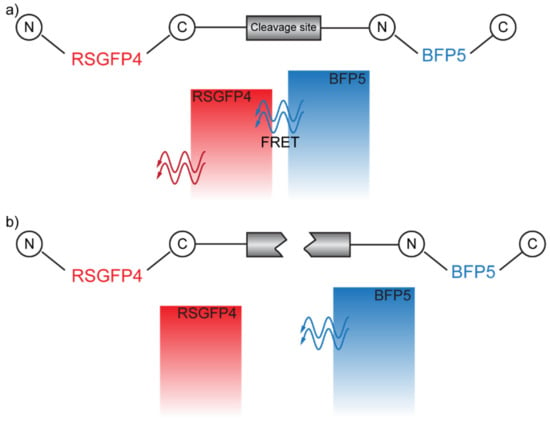
Figure 8.
(a) FRET-allowed configuration. (b) FRET-inhibited configuration in which the cleavage site has been cut by the Factor Xa.
Reducing FRET by separating fluorophores has been successfully demonstrated for QDs-based DAPs as well. Zhang et al., for example, achieved an effective suppression of FRET between red-emitting II-VI quantum dots (QDs) by separating them with a wider band of blue-emitting QDs. By this technique, they were able to enhance the photoluminescence quantum yield (PLQY) of the red-emitting QDs by 1.5 times [55]. A similar technique was employed by Mikhailov et al., that exploited ZnSe shells to separate epitaxially-grown Cd(Zn)Se QDs layers [56].
In some particular cases, the DAP is not strictly composed of two fluorophores. This is the case for Metal-Organic Frameworks (MOFs) that can be used as alternative acceptors. Xu et al. demonstrated a Bisphenol A sensor based on this mechanism [57]. In particular, the DAP is composed of a metal-organic framework (MOF), used as the acceptor, and an up-conversion nanoparticle (UCNPS) as the donor. The UCNPS donor is linked to an aptamer (DNA1) with a high affinity with Bisphenol A. The MOF acceptor is linked to a DNA complementary to the aptamer (DNA2). In the absence of Bisphenol A, FRET can occur between UCNPS and MOF since DNA1 and DNA2 are free to pair up. When Bisphenol A is present, it bounds to the aptamer (UCNPS), making it unavailable to be linked to the MOF-functionalized DNA2 and, therefore, the FRET mechanism cannot occur. Au nanoparticles (AuNPs) can constitute a valid alternative to fluorophores as acceptors too. Wang and Guo demonstrated the possibility of using a Quantum-Dots (QDs)/AuNPs based DAP to detect the presence of Pb2+. In particular, their FRET system is constituted by positively-charged cysteamine-capped CdTe-QDs (CA-CdTe-QDs) acting as the donor and negatively-charged 11-mercaptoundecanoic-acid-capped AuNPs (MUA-AuNPs) acting as acceptor. The plasmonic resonance of these particular NPs is tailored to match the emission of QDs, and, in these conditions, they constitute an efficient FRET pair. The authors demonstrated that, in the presence of Pb+2, chelation occurring in MUA-AuNPs induces the aggregation of the NPs, thus shifting their plasmonic resonance. As a consequence, the donor emission is no longer tuned to the acceptor absorbance, and FRET is prevented [58].
4. Conclusions
To conclude, we browsed in this review some of the most remarkable examples of FRET-based sensing, founded on either enhancing or suppressing the resonant energy transfer between fluorophores. We began clarifying the very nature of FRET and elucidating the physics behind this fascinating phenomenon. We went through the long-debated question of whether FRET rate and/or FRET efficiency depend or not on how the electromagnetic environment interacts with the DAP and show some of the most important experimental contributions in this direction, also providing an innovative key to reading some of the experimental evidence in terms of the recently described “pseudo-cavity modes”. We then moved on to describe the two main ways to enhance the FRET performances, both related to the local density of states of the electromagnetic environment surrounding the FRET pair, that consist of (i) the interaction with cavities and (ii) with metals through plasmonic processes. Regarding the cavities, we have shown the important case of the enhancement of FRET via the Purcell effect together with some of the most peculiar cavities such as nano-apertures and whispering gallery modes. Regarding plasmonics, we described the two important mechanisms going under the name of “Surface Plasmon Coupled Emission (SPCE)” and “Surface Plasmon Enhanced Absorption (SPEA)”, demonstrating their validity in enhancing FRET performances. We then mention the new important concept of a “generalized spectral overlap”, used as a ruler for plasmon-enhanced FRET.
The second part of the review is dedicated to all those sensing techniques based on FRET inhibition. We show that the main path to inhibit FRET remains to provide a physical separation between the donor and acceptor. The reported examples show that, basically, every mechanism capable of introducing a dislocation between the donor and the acceptor can be sensed via FRET. We brought the cases of chemical reactions, including glucose, and even the capability of enzymatic reactions to hinder the FRET efficiency. In this very last example, two biological dyes have been used as a DAP, demonstrating that, potentially, FRET pairs can be directly engineered in biological systems such as bacteria, whose replication could potentially open to serial production of FRET sensors, especially envisioning sustainable sensing techniques.
Our review could be seen as a hand guide for the design of innovative FRET DAP, especially in a biological context where bio-inspired DAP can be replicated countless times via expression in prokaryotic cells. Moreover, our review fosters a multidisciplinary look towards innovative FRET-based sensing techniques involving emerging plasmonic/photonic nanostructures such as the so-called meta-materials and meta-surfaces to access still unreachable FRET-based sensing performances.
Author Contributions
Conceptualization, V.C., F.A. and A.D.L.; Data Curation, All authors; FRET inhibition techniques, A.P., O.F., F.L. and F.A.; FRET Enhancement Techniques, V.C., A.D.L.; Writing, V.C.; Supervision, V.C., F.A. and A.D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This work has been entirely carried out in the framework of the joint lab “ELIPHOMOS” between CNR Nanotec and the Department of Physics of University of Calabria.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Novotny, L.; Hecht, B. Principles of Nano-Optics, 2nd ed.; Cambridge University Press: Cambridge, UK, 2012; ISBN 978-1-107-00546-4. [Google Scholar]
- Atkins, P.; de Paula, J.; Keeler, J. Atkins’ Physical Chemistry, 7th ed.; Oxford University Press: Oxford, UK, 2017; ISBN 978-0-19-876986-6. [Google Scholar]
- Griffiths, D.J. Introduction to Quantum Mechanics, 2nd ed.; Cambridge University Press: Cambridge, UK, 2005; p. 468. ISBN 978-1-107-17986-8. [Google Scholar]
- Kittel, C. Introduction to Solid State Physics, 7th ed.; Wiley, J., Ed.; Wiley: Hoboken, NJ, USA, 1996; ISBN 978-0-471-41526-8. [Google Scholar]
- Chance, R.R.; Prock, A.; Silbey, R. Molecular Fluorescence and Energy Transfer Near Interfaces. In Advances in Chemical Physics; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1978; pp. 1–65. ISBN 978-0-470-14256-1. [Google Scholar]
- Blum, C.; Zijlstra, N.; Lagendijk, A.; Wubs, M.; Mosk, A.P.; Subramaniam, V.; Vos, W.L. Nanophotonic Control of the Forster Resonance Energy Transfer Efficiency. Phys. Rev. Lett. 2012, 109, 203601. [Google Scholar] [CrossRef] [PubMed]
- Hopmeier, M.; Guss, W.; Deussen, M.; Göbel, E.O.; Mahrt, R.F. Enhanced Dipole-Dipole Interaction in a Polymer Microcavity. Phys. Rev. Lett. 1999, 82, 4118–4121. [Google Scholar] [CrossRef]
- Andrew, P.; Barnes, W.L. Förster Energy Transfer in an Optical Microcavity. Science 2000, 290, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, C.E.; Ginger, D.S.; Greenham, N.C. Enhanced Förster energy transfer in organic/inorganic bilayer optical microcavities. Chem. Phys. Lett. 2001, 338, 83–87. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Shen, Y.; D’Auria, S.; Malicka, J.; Fang, J.; Gryczynski, Z.; Gryczynski, I. Radiative Decay Engineering: 2. Effects of Silver Island Films on Fluorescence Intensity, Lifetimes, and Resonance Energy Transfer. Anal. Biochem. 2002, 301, 261–277. [Google Scholar] [CrossRef]
- Cortes, C.L.; Jacob, Z. Fundamental figures of merit for engineering Förster resonance energy transfer. Opt. Express 2018, 26, 19371–19387. [Google Scholar] [CrossRef]
- Caligiuri, V.; Biffi, G.; Palei, M.; Martín-García, B.; Pothuraju, R.D.; Bretonnière, Y.; Krahne, R. Angle and Polarization Selective Spontaneous Emission in Dye-Doped Metal/Insulator/Metal Nanocavities. Adv. Opt. Mater. 2019, 8, 1901215. [Google Scholar] [CrossRef]
- Caligiuri, V.; Palei, M.; Biffi, G.; Krahne, R. Hybridization of epsilon-near-zero modes via resonant tunneling in layered metal-insulator double nanocavities. Nanophotonics 2019, 8, 1505–1512. [Google Scholar] [CrossRef]
- Dionne, J.A.; Lezec, H.J.; Atwater, H.A. Highly confined photon transport in subwavelength metallic slot waveguides. Nano Lett. 2006, 6, 1928–1932. [Google Scholar] [CrossRef]
- Patra, A.; Caligiuri, V.; Krahne, R.; Luca, A.D. Strong Light–Matter Interaction and Spontaneous Emission Reshaping via Pseudo-Cavity Modes. Adv. Opt. Mater. 2021, 9, 2101076. [Google Scholar] [CrossRef]
- Purcell, E.M. Spontaneous Emission Probabilities at Radio Frequencies. Proc. Am. Phys. Soc. 1946, 69, 839. [Google Scholar]
- Caligiuri, V.; Leone, F.; Annesi, F.; Pane, A.; Bartolino, R.; De Luca, A. Envisioning Quantum Electrodynamic Frameworks Based on Bio-Photonic Cavities. Photonics 2021, 8, 470. [Google Scholar] [CrossRef]
- Konrad, A.; Metzger, M.; Kern, A.M.; Brecht, M.; Meixner, A.J. Controlling the dynamics of Förster resonance energy transfer inside a tunable sub-wavelength Fabry–Pérot-resonator. Nanoscale 2015, 7, 10204–10209. [Google Scholar] [CrossRef]
- Schleifenbaum, F.; Kern, A.M.; Konrad, A.; Meixner, A.J. Dynamic control of Förster energy transfer in a photonic environment. Phys. Chem. Chem. Phys. 2014, 16, 12812–12817. [Google Scholar] [CrossRef]
- Ghenuche, P.; de Torres, J.; Moparthi, S.B.; Grigoriev, V.; Wenger, J. Nanophotonic Enhancement of the Förster Resonance Energy-Transfer Rate with Single Nanoapertures. Nano Lett. 2014, 14, 4707–4714. [Google Scholar] [CrossRef]
- Wang, J.-F.; Zhou, H.-L.; Xiong, X.; Li, Q.; Cheng, Z.-D.; Liu, Z.-H.; Yan, F.-F.; Lin, S.-R.; Xu, J.-S.; Wang, G.-Z.; et al. Cavity-enhanced energy transfer between nano-emitters and monolayer graphene. Carbon 2020, 161, 794–799. [Google Scholar] [CrossRef]
- Folan, L.M.; Arnold, S.; Druger, S.D. Enhanced energy transfer within a microparticle. Chem. Phys. Lett. 1985, 118, 322–327. [Google Scholar] [CrossRef]
- Götzinger, S.; de S. Menezes, L.; Mazzei, A.; Kühn, S.; Sandoghdar, V.; Benson, O. Controlled Photon Transfer between Two Individual Nanoemitters via Shared High-Q Modes of a Microsphere Resonator. Nano Lett. 2006, 6, 1151–1154. [Google Scholar] [CrossRef][Green Version]
- Jana, S.; Xu, X.; Klymchenko, A.; Reisch, A.; Pons, T. Microcavity-Enhanced Fluorescence Energy Transfer from Quantum Dot Excited Whispering Gallery Modes to Acceptor Dye Nanoparticles. ACS Nano 2021, 15, 1445–1453. [Google Scholar] [CrossRef]
- Melnychuk, N.; Klymchenko, A.S. DNA-Functionalized Dye-Loaded Polymeric Nanoparticles: Ultrabright FRET Platform for Amplified Detection of Nucleic Acids. J. Am. Chem. Soc. 2018, 140, 10856–10865. [Google Scholar] [CrossRef]
- Galisteo-López, J.F.; Ibisate, M.; Muñoz, A.; López, C. 3D photonic crystals from highly monodisperse FRET-based red luminescent PMMA spheres. J. Mater. Chem. C 2015, 3, 3999–4006. [Google Scholar] [CrossRef][Green Version]
- Yang, Z.; Zhou, X.; Huang, X.; Zhou, J.; Yang, G.; Xie, Q.; Sun, L.; Li, B. Energy transfer between fluorescent dyes in photonic crystals. Opt. Lett. 2008, 33, 1963–1965. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huang, X.; Sun, L.; Zhou, J.; Yang, G.; Li, B.; Yu, C. Energy transfer enhancement in Eu3+ doped TbPO4 inverse opal photonic crystals. J. Appl. Phys. 2009, 105, 083523. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Radiative decay engineering 5: Metal-enhanced fluorescence and plasmon emission. Anal. Biochem. 2005, 337, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.G.; Moh, K.J.; Yuan, X.-C. Surface plasmon-coupled emission from shaped PMMA films doped with fluorescence molecules. Opt. Express 2010, 18, 12185–12190. [Google Scholar] [CrossRef]
- Gryczynski, I.; Malicka, J.; Gryczynski, Z.; Lakowicz, J.R. Surface plasmon-coupled emission with gold films. J. Phys. Chem. B 2004, 108, 12568–12574. [Google Scholar] [CrossRef]
- Cao, S.-H.; Zhai, Y.-Y.; Xie, K.-X.; Li, Y.-Q. Surface Plasmon-Coupled Emission. In Surface Plasmon Enhanced, Coupled and Controlled Fluorescence; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 241–256. ISBN 978-1-119-32516-1. [Google Scholar]
- Gryczynski, Z.; Gryczynski, I.; Matveeva, E.; Malicka, J.; Nowaczyk, K.; Lakowicz, J.R. Surface-Plasmon–Coupled Emission: New Technology for Studying Molecular Processes. In Methods in Cell Biology, 4th ed.; New Developments; Academic Press: Cambridge, MA, USA, 2004; Volume 75, pp. 73–104. [Google Scholar]
- Chen, M.; Cao, S.-H.; Li, Y.-Q. Surface plasmon–coupled emission imaging for biological applications. Anal. Bioanal. Chem. 2020, 412, 6085–6100. [Google Scholar] [CrossRef]
- Badiya, P.K.; Patnaik, S.G.; Srinivasan, V.; Reddy, N.; Manohar, C.S.; Vedarajan, R.; Mastumi, N.; Belliraj, S.K.; Ramamurthy, S.S. Ag-protein plasmonic architectures for surface plasmon-coupled emission enhancements and Fabry-Perot mode-coupled directional fluorescence emission. Chem. Phys. Lett. 2017, 685, 139–145. [Google Scholar] [CrossRef]
- Badiya, P.K.; Srinivasan, V.; Ramamurthy, S.S. Silver–Graphene oxide based plasmonic spacer for surface plasmon-coupled fluorescence emission enhancements. Mater. Res. Express 2017, 4, 065002. [Google Scholar] [CrossRef]
- Murai, S.; Oka, S.; Azzam, S.I.; Kildishev, A.V.; Ishii, S.; Tanaka, K. Enhanced absorption and photoluminescence from dye-containing thin polymer film on plasmonic array. Opt. Express 2019, 27, 5083–5096. [Google Scholar] [CrossRef]
- Ju, L.; Geng, B.; Horng, J.; Girit, C.; Martin, M.; Hao, Z.; Bechtel, H.A.; Liang, X.; Zettl, A.; Shen, Y.R.; et al. Graphene plasmonics for tunable terahertz metamaterials. Nat. Nanotechnol. 2011, 6, 630–634. [Google Scholar] [CrossRef]
- Caligiuri, V.; Palei, M.; Imran, M.; Manna, L.; Krahne, R. Planar Double-Epsilon-Near-Zero Cavities for Spontaneous Emission and Purcell Effect Enhancement. ACS Photonics 2018, 5, 2287–2294. [Google Scholar] [CrossRef]
- Hsu, L.Y.; Ding, W.; Schatz, G.C. Plasmon-Coupled Resonance Energy Transfer. J. Phys. Chem. Lett. 2017, 8, 2357–2367. [Google Scholar] [CrossRef]
- Vincent, R.; Carminati, R. Magneto-optical control of Forster energy transfer. Phys. Rev. B 2011, 83, 165426. [Google Scholar] [CrossRef]
- Zong, H.; Wang, X.; Mu, X.; Wang, J.; Sun, M. Plasmon-Enhanced Fluorescence Resonance Energy Transfer. Chem. Rec. 2019, 19, 818–842. [Google Scholar] [CrossRef]
- De Torres, J.; Mivelle, M.; Moparthi, S.B.; Rigneault, H.; Van Hulst, N.F.; García-Parajó, M.F.; Margeat, E.; Wenger, J. Plasmonic Nanoantennas Enable Forbidden Förster Dipole–Dipole Energy Transfer and Enhance the FRET Efficiency. Nano Lett. 2016, 16, 6222–6230. [Google Scholar] [CrossRef]
- Ghenuche, P.; Mivelle, M.; de Torres, J.; Moparthi, S.B.; Rigneault, H.; Van Hulst, N.F.; García-Parajó, M.F.; Wenger, J. Matching Nanoantenna Field Confinement to FRET Distances Enhances Förster Energy Transfer Rates. Nano Lett. 2015, 15, 6193–6201. [Google Scholar] [CrossRef]
- Asselin, J.; Viger, M.L.; Boudreau, D. Metal-Enhanced Fluorescence and FRET in Multilayer Core-Shell Nanoparticles. Adv. Chem. 2014, 2014, e812313. [Google Scholar] [CrossRef]
- Kochuveedu, S.T.; Son, T.; Lee, Y.; Lee, M.; Kim, D.; Kim, D.H. Revolutionizing the FRET-based light emission in core-shell nanostructures via comprehensive activity of surface plasmons. Sci. Rep. 2014, 4, 4735. [Google Scholar] [CrossRef]
- Lessard-Viger, M.; Rioux, M.; Rainville, L.; Boudreau, D. FRET Enhancement in Multilayer Core−Shell Nanoparticles. Nano Lett. 2009, 9, 3066–3071. [Google Scholar] [CrossRef]
- Viger, M.L.-; Brouard, D.; Boudreau, D. Plasmon-Enhanced Resonance Energy Transfer from a Conjugated Polymer to Fluorescent Multilayer Core−Shell Nanoparticles: A Photophysical Study. J. Phys. Chem. C 2011, 115, 2974–2981. [Google Scholar] [CrossRef]
- Asgar, H.; Jacob, L.; Hoang, T.B. Fast spontaneous emission and high Förster resonance energy transfer rate in hybrid organic/inorganic plasmonic nanostructures. J. Appl. Phys. 2018, 124, 103105. [Google Scholar] [CrossRef]
- Steele, J.M.; Ramnarace, C.M.; Farner, W.R. Controlling FRET Enhancement Using Plasmon Modes on Gold Nanogratings. J. Phys. Chem. C 2017, 121, 22353–22360. [Google Scholar] [CrossRef]
- Jeong, Y.; Schatz, G.C. Enhancement and Suppression of Resonance Energy Transfer Near Metal Nanoparticles. J. Phys. Chem. C 2020, 124, 20589–20597. [Google Scholar] [CrossRef]
- Meledeo, M.A.; Ibey, B.L.; O’Neal, D.P.; Pishko, M.V.; Cote, G.L. Investigation of pH and temperature effects on FRET systems for glucose sensing. In Proceedings of the Optical Diagnostics and Sensing of Biological Fluids and Glucose and Cholesterol Monitoring II, SPIE, San Jose, CA, USA, 19–25 January 2002; Volume 4624, pp. 55–65. [Google Scholar]
- Hu, X.; Li, Y.; Liu, T.; Zhang, G.; Liu, S. Intracellular Cascade FRET for Temperature Imaging of Living Cells with Polymeric Ratiometric Fluorescent Thermometers. ACS Appl. Mater. Interfaces 2015, 7, 15551–15560. [Google Scholar] [CrossRef]
- Zhang, H.; Su, Q.; Chen, S. Suppressing Förster Resonance Energy Transfer in Close-Packed Quantum-Dot Thin Film: Toward Efficient Quantum-Dot Light-Emitting Diodes with External Quantum Efficiency over 21.6%. Adv. Opt. Mater. 2020, 8, 1902092. [Google Scholar] [CrossRef]
- Mikhailov, T.N.; Evropeytsev, E.A.; Belyaev, K.G.; Kaibyshev, V.H.; Toropov, A.A.; Rodina, A.V.; Ivanov, S.V.; Pozina, G.; Shubina, T.V. Suppression of slow decaying emission in II-VI quantum dots with Förster resonance energy transfer. J. Phys. Conf. Ser. 2017, 917, 062048. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, L.; Long, L.; Zhu, S.; Chen, M.; Ding, L.; Cheng, Y. Metal Organic Frame-Upconverting Nanoparticle Assemblies for the FRET Based Sensor Detection of Bisphenol A in High-Salt Foods. Front. Bioeng. Biotechnol. 2020, 8, 626269. [Google Scholar] [CrossRef]
- Wang, X.; Guo, X. Ultrasensitive Pb2+ detection based on fluorescence resonance energy transfer (FRET) between quantum dots and gold nanoparticles. Analyst 2009, 134, 1348–1354. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).