Effect of Acidification on Clay Minerals and Surface Properties of Brown Soil
Abstract
:1. Introduction
2. Materials and Methods
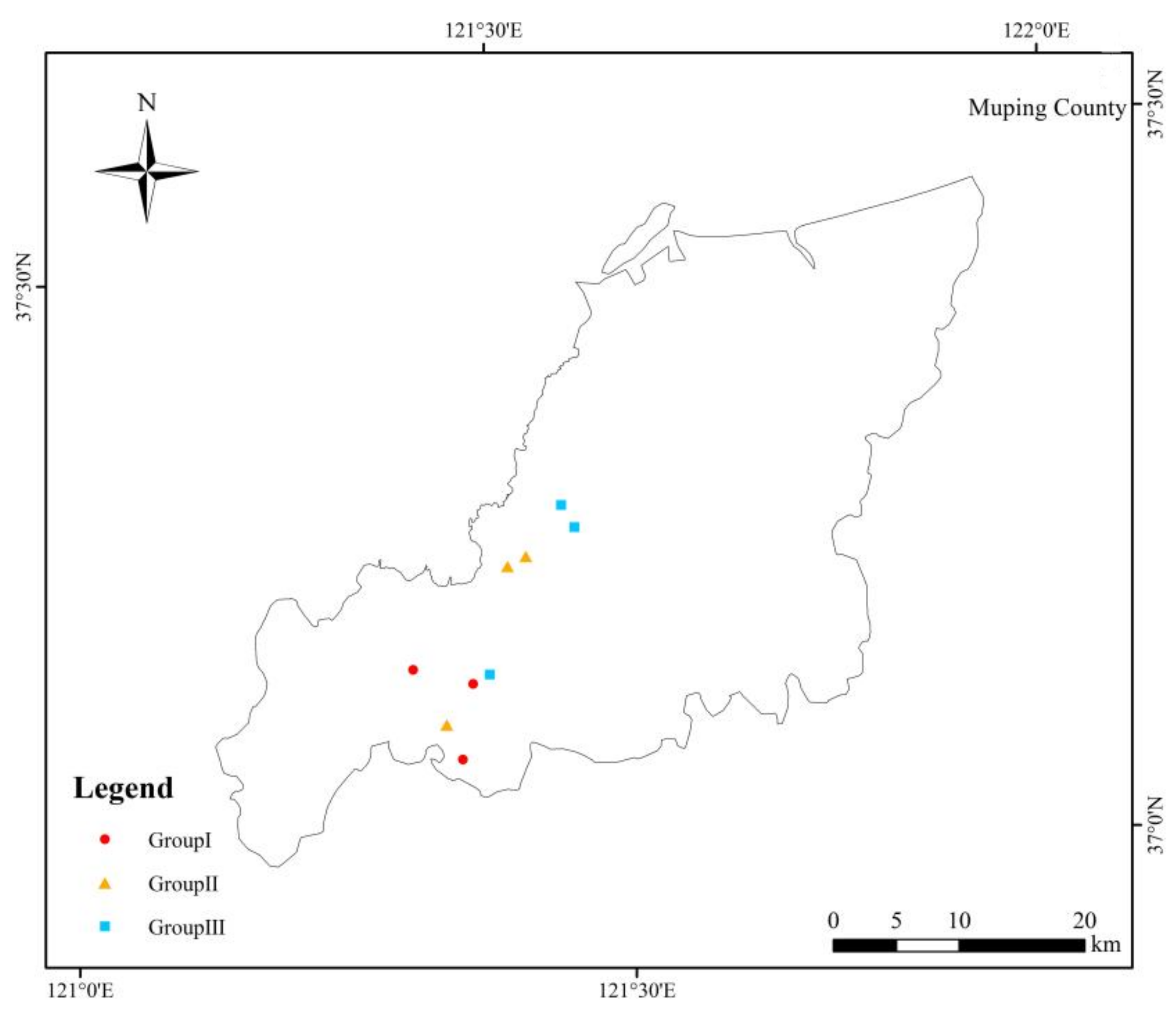
2.1. Soil Samples
2.2. Sample Measurements
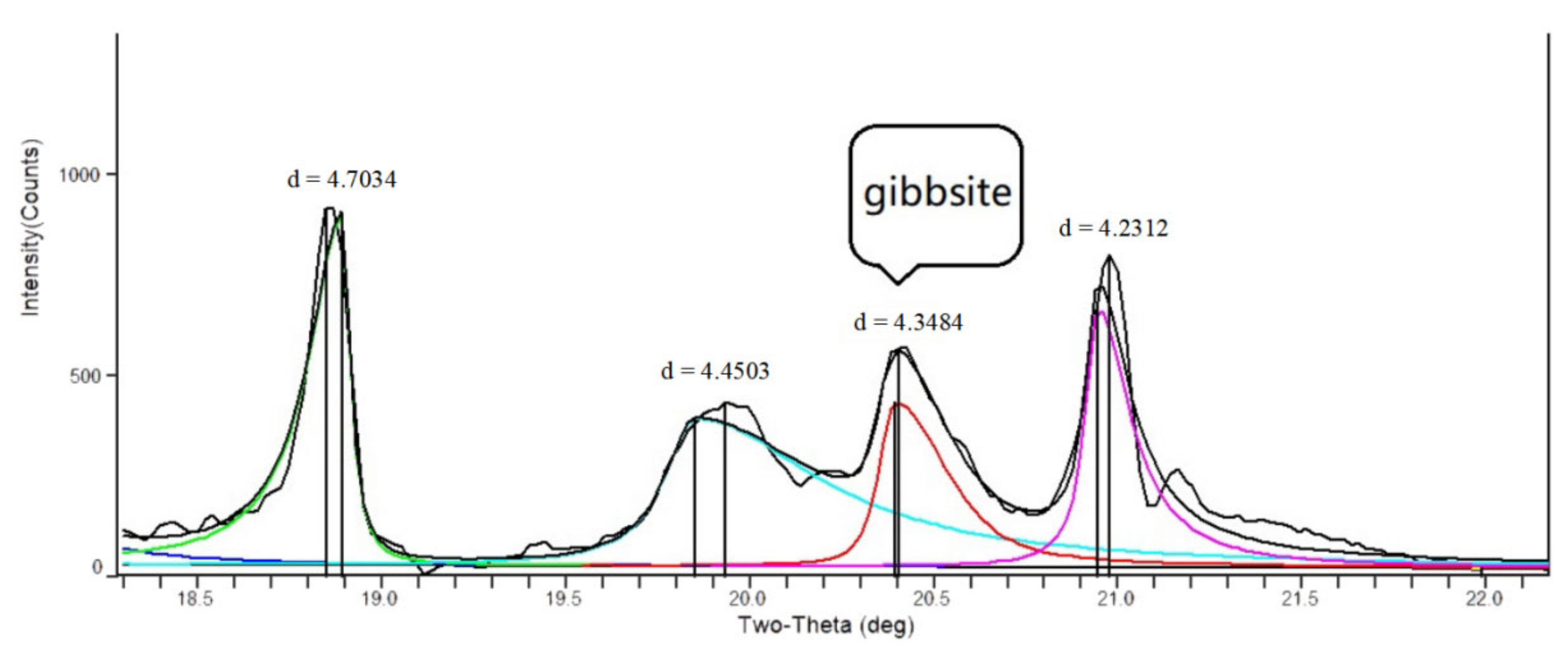
2.3. Clay Minerals Extraction and Determination
2.4. Statistical Analysis
3. Results
3.1. Soil Properties
3.2. Species and Content of Clay Minerals
3.3. Surface Properties
3.4. Correlation of Basic Soil Properties, Clay Minerals, and Surface Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raza, S.; Zamanian, K.; Ullah, S.; Kuzyakov, Y.; Virto, I.; Zhou, J. Inorganic carbon losses by soil acidification jeopardize global efforts on carbon sequestration and climate change mitigation. J. Clean. Prod. 2021, 315, 128036. [Google Scholar] [CrossRef]
- Tafesse, S.; Braam, C.; Mierlo, B.; Lemaga, B.; Struik, P.C. Association between soil acidity and bacterial wilt occurrence in potato production in Ethiopia. Agronomy 2021, 11, 1541. [Google Scholar] [CrossRef]
- Yan, P.; Wu, L.Q.; Wang, D.; Fu, J.; Shen, C.; Li, X.; Zhang, L.P.; Zhang, L.; Fan, L.C.; Han, W.Y. Soil acidification in Chinese tea plantations. Sci. Total. Environ. 2020, 715, 136963. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.P.; Chen, H.Y.; Searle, E.B.; Sardans, J.; Ciais, P.; Peñuelas, J.; Huang, Z.Q. Whole soil acidification and base cation reduction across subtropical China. Geoderma 2020, 361, 114107. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, J.L.; Zhao, X.R.; Yang, S.H.; Zhang, G.L. Contribution of different proton sources to the acidification of red soil with maize cropping in subtropical China. Geoderma 2021, 392, 114995. [Google Scholar] [CrossRef]
- Hamidi, N.H.; Ahmed, O.H.; Omar, L.; Ch’ng., H.Y. Combined Use of Charcoal, Sago Bark Ash, and Urea Mitigate Soil Acidity and Aluminium Toxicity. Agronomy 2021, 11, 1799. [Google Scholar] [CrossRef]
- Mokolobate, M.S. An Evaluation of the Use of Organic Amendments to Ameliorate Aluminum Toxicity and Phosphorus Deficiency in an Acid Soil. Master’s Thesis, University of Natal, Pietermaritzburg, South Africa, 2001. [Google Scholar]
- Matocha, C.J.; Grove, J.H.; Karathanasis, T.D.; Vandiviere, M. Changes in soil mineralogy due to nitrogen fertilization in an agroecosystem. Geoderma 2016, 263, 176–184. [Google Scholar] [CrossRef]
- Xu, R.K.; Zhao, A.Z.; Jiang, J. Effect of acidification on CEC and mineral compositions of yellow brown soils in tea gardens. Ecol. Environ. Sci. 2011, 20, 1395–1398. [Google Scholar] [CrossRef]
- Tao, L.; Wen, X.C.; Li, H.; Huang, C.; Jiang, Y.; Liu, D.; Sun, B. Influence of manure fertilization on soil phosphorous retention and clay mineral transformation: Evidence from a 16-year long-term fertilization experiment. Appl. Clay. Sci. 2021, 204, 106021. [Google Scholar] [CrossRef]
- Leng, S.Y.; Song, C.Q. Relationship between soil acidity and surface electrochemical properties. Adv. Earth. Sci. 2013, 28, 1170–1172. [Google Scholar] [CrossRef]
- Khawmee, K.; Suddhiprakarn, A.; Kheoruenromne, I.; Singh, B. Surface charge properties of kaolinite from Thai soils. Geoderma 2013, 192, 120–131. [Google Scholar] [CrossRef]
- Li, J.Y.; Xu, R.K.; Zhang, H. Iron oxides serve as natural anti-acidification agents in highly weathered soils. J. Soil. Sediment. 2012, 12, 876–887. [Google Scholar] [CrossRef]
- Liao, L.X.; Hu, H.G.; He, J.Z. Relationship between surface charge properties of several kinds of zonal soils in central Southern China and pH. Hua Zhong Nongye Xue Xuebao 2005, 24, 29–32. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation-a review. Earth-Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Yang, W.; Chen, Y.; Yang, L.; Xu, M.Q.; Jing, H.N.; Wu, P.; Wang, P. Spatial distribution, food chain translocation, human health risks, and environmental thresholds of heavy metals in a maize cultivation field in the heart of China’s karst region. J. Soil. Sediment. 2022, 22, 2654–2670. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Ayub, A.; Hussain, S.; Waraich, E.A.; Esawi, M.A.; Ishfaq, M.; Ahmad, M.; Ali, N.; Maqsood, M.F. Cadmium Toxicity in Plants: Recent Progress on Morpho-physiological Effects and Remediation Strategies. J. Soil. Sci. Plant. Nutr. 2022, 22, 212–269. [Google Scholar] [CrossRef]
- Hao, W.D.; Pudasainee, D.; Gupta, R.; Kashiwabara, T.; Alessi, D.S.; Konhauser, K.O. Effect of acidic conditions on surface properties and metal binding capacity of clay minerals. ACS. Earth. Space. Chem. 2019, 3, 2421–2429. [Google Scholar] [CrossRef]
- Wei, H.; Liu, Y.L.; Xiang, H.M.; Zhang, J.E.; Li, S.; Yang, J. Soil pH responses to simulated acid rain leaching in three agricultural soils. Sustainability 2020, 12, 280. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Xia, F.; Liu, X.; He, Y.; Xu, J.; Brookes, P.C. Effects of nitrogen fertilizer on the acidification of two typical acid soils in South China. J. Soil. Sediment. 2014, 14, 415–422. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Liang, H.; Zhao, J.; Zhang, Y.; Xu, C.; Shi, X.J. Long-term tobacco plantation induces soil acidification and soil base cation loss. Environ. Sci. Pollut. Res. Int. 2016, 23, 5442–5450. [Google Scholar] [CrossRef]
- Fu, C.C.; Zhang, H.B.; Chen, T.; Li, L.Z.; Liu, X.H.; Luo, Y.M. Spatial interpolation of orchard soil pH using soil type and planting duration as auxiliary information. Pedosphere 2020, 30, 628–637. [Google Scholar] [CrossRef]
- Shen, Y.Y.; Zhang, Z.Q.; Xue, Y. Study on the new dynamics and driving factors of soil pH in the red soil, hilly region of South China. Environ. Monit. Assess. 2021, 193, 304. [Google Scholar] [CrossRef] [PubMed]
- Peech, H.M. Hydrogen-Ion Activity, in Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; American Society of Agronomy and Soil Science Society of America: New York, NY, USA, 1965; pp. 914–926. [Google Scholar]
- Di, Z.Z.; Li, X.L. Effect of Phosphorus Applications on Soil charge. Liaoning Agric. Sci. 2014, 5, 12–15. Available online: https://kns.cnki.net/kcms/detail/21.1111.s.20141103.1536.001.html (accessed on 18 December 2022).
- Yukselen, Y.; Kaya, A. Comparison of methods for determining specific surface area of soils. J. Geotech. Geoenviron. Eng. 2006, 132, 931–936. [Google Scholar] [CrossRef]
- Ndzana, G.M.; Huang, L.; Wang, J.B.; Zhang, Z.Y. Characteristics of clay minerals in soil particles from an argillic horizon of alfisol in central China. Appl. Clay. Sci. 2018, 151, 148–156. [Google Scholar] [CrossRef]
- Das, R.; Purakayastha, T.J.; Das, D.; Ahmed, N.; Kumar, R.; Walia, S.S.; Singh, R.; Shukla, V.K.; Yadava, M.S.; Ravisankar, N. Effect of chemical pre-treatment for identifification of clay minerals in four soil orders by X-ray diffraction technique. Natl. Acad. Sci. Lett. 2022, 45, 39–44. [Google Scholar] [CrossRef]
- Moore, D.E.; Reynolds, R.C. X-ray Diffraction and the Identification of Clay Minerals, 2nd ed.; Oxford University Press: New York, NY, USA, 1997; p. 378. [Google Scholar]
- Środoń, J. Identifification and quantitative analysis of clay minerals. Dev. Clay. Sci. 2006, 1, 765–787. [Google Scholar] [CrossRef]
- Ou, J.Q.; Huang, W.H.; Lu, Y.; Li, B.; Yang, Y.; Tang, X.; Jia, C.J.; Qin, H.L. Clay mineral composition and spatial distribution of stagnic anthosols in Guangxi. Soils 2020, 52, 1290–1297. [Google Scholar] [CrossRef]
- Barré, P.; Montagnier, C.; Chenu, V.; Abbadie, L.; Velde, B. Clay minerals as a soil potassium reservoir: Observation and quantification through X-ray diffraction. Plant and Soil. 2008, 302, 213–220. [Google Scholar] [CrossRef]
- Xu, F.L.; Li, X.Y.; Huang, Q.Y.; Qu, S.X.; Zhou, J.W. Studies on the clay minerals of mountain soils in Hubeil and Hunan Province. I. The clay minerals of soils on the south slop of the Dabie Montains. J. Acta. Pedologica. Sinica. 1990, 27, 293–300. [Google Scholar]
- Hao, T.X.; Zhu, Q.C.; Zeng, M.F.; Shen, J.; Shi, X.; Liu, X.j.; Zhang, F.S.; Veries, W. Impacts of nitrogen fertilizer type and application rate on soil acidification rate under a wheat-maize double cropping system. J. Environ. Manag. 2020, 270, 110888. [Google Scholar] [CrossRef] [PubMed]
- Sposito, G. The Chemistry of Soils; Oxford University Press: New York, NY, USA, 1989; pp. 209–222. [Google Scholar]
- Nkoh, J.N.; Yan, J.; Xu, R.K.; Shi, R.Y.; Hong, Z.N. The mechanism for inhibiting acidification of variable charge soils by adhered Pseudomonas fluorescens. Environ. Pollut. 2020, 260, 114049. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ren, H.Y.; Liu, J.J.; Tian, Y.; Lu, S.G. Soil acidification induced decline disease of Myrica rubra: Aluminum toxicity and bacterial community response analyses. Environ. Sci. Pollut. Res. Int. 2022, 29, 45435–45448. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.Y.; Baquy, M.A.; Guan, P.; Yan, J.; Wang, R.; Xu, R.K.; Xie, L. Effect of soil acidification on the growth and nitrogen use efficiency of maize in ultisols. J. Soil. Sediment. 2020, 20, 1435–1445. [Google Scholar] [CrossRef]
- Liang, W.J.; Cai, Z.J.; Song, F.F.; Zhou, S.W.; Ai, T.C.; Xu, M.G. Relationships between maize growth and the pH, exchangeable aluminum and calcium of red soils derived from different parent materials. J. Agro. Environ. Sci. 2017, 36, 1544–1550. [Google Scholar] [CrossRef]
- Kämpf, N.; Scheinost, A.C.; Schulze, D.G. Oxide Minerals in Soils, in Handbook of Soil Sciences: Properties and Processes, 2nd ed.; Taylor & Francis: CRC Press: Boca Raton, FL, USA, 2012; pp. 16–22. [Google Scholar]
- Zhang, Z.Y.; Huang, L.; Liu, F.; Wang, M.K.; Ndzana, G.M.; Liu, Z.J. Transformation of clay minerals in nanoparticles of several zonal soils in China. J. Soil. Sediment. 2019, 19, 211–220. [Google Scholar] [CrossRef]
- Wilson, M.J. Weathering of the primary rock-forming minerals: Processes, products and rates. Clay. Miner. 2004, 39, 233–266. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, W.L. Chemical Equilibria in Soils; John Wiley and Sons Ltd.: New York, NY, USA, 1979; pp. 1–319. [Google Scholar]
- Wisawapipat, W.; Kheoruenromne, I.; Suddhiprakarn, A.; Gilkes, R.J. Surface charge characteristics of variable charge soils in Thailand. Soil. Res. 2010, 48, 337. [Google Scholar] [CrossRef]
- Zhao, Z.J. Evolution of surface electrochemical properties of typical paddy soils in two climatic zones and their effects on environmental behavior of phosphorus. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, June 2018. [Google Scholar]

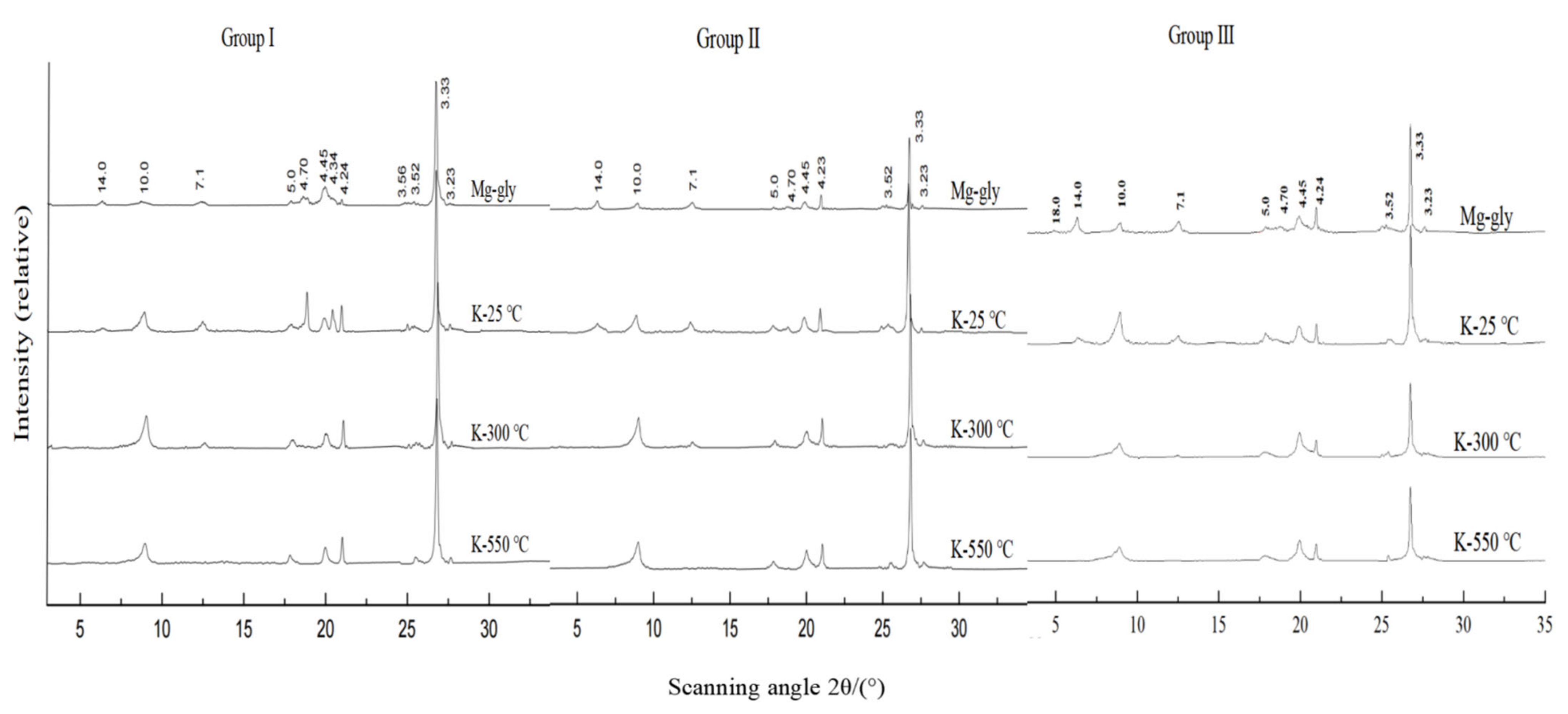
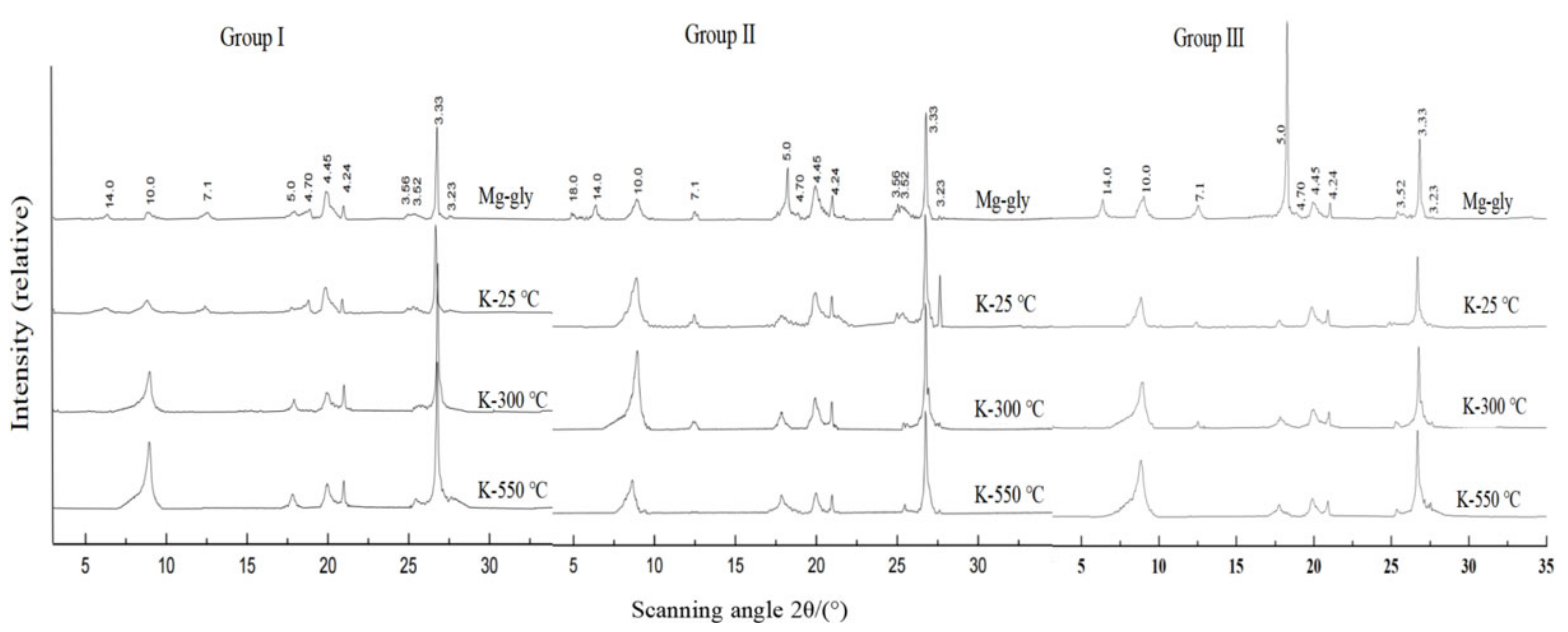





| Treatment | Montmorillonite | Vermiculite | Illite | Kaolinite | Chlorite | Gibbsite |
|---|---|---|---|---|---|---|
| Mg-gly | 18 | 14.2 | 10 | 7.1 | 14.2 | 4.34 |
| K-25 °C | 10–12 | 10 | 10 | 7.1 | 14.2 | 4.34 |
| K-300 °C | 10–12 | 10 | 10 | 7.1 | 14.2 | disappear |
| K-550 °C | 10 | 10 | 10 | disappear | 13.8 | disappear |
| Layer (cm) | Group | pH Value | EH+ (cmol∙kg−1) | EAl3+ (cmol∙kg−1) | SOM (g∙kg−1) | Clay (%) |
|---|---|---|---|---|---|---|
| 0–20 | Group I | 3.98 ± 0.20 Ba | 0.55 ± 0.03 Aa | 1.86 ± 0.03 Aa | 10.48 ± 1.25 Ab | 4.61 ± 0.80 Bb |
| Group II | 4.78 ± 0.08 Bb | 0.30 ± 0.04 Ab | 0.74 ± 0.06 Aab | 11.93 ± 0.76 Ab | 7.51 ± 1.13 Aa | |
| Group III | 5.76 ± 0.24 Bc | 0.13 ± 0.03 Ac | 0.54 ± 0.13 Aab | 15.83 ± 0.85 Aa | 8.07 ± 0.57 Aa | |
| 20–40 | Group I | 4.21 ± 0.15 Ba | 0.37 ± 0.04 ABa | 2.07 ± 0.18 Aa | 4.25 ± 0.33 Bb | 6.08 ± 0.90 ABb |
| Group II | 5.95 ± 0.22 Ab | 0.28 ± 0.01 Aa | 0.58 ± 0.03 Aab | 4.97 ± 1.07 Bb | 7.68 ± 0.82 Aab | |
| Group III | 6.79 ± 0.02 Ac | 0.12 ± 0.01 Ab | 0.27 ± 0.05 Bb | 7.66 ± 2.24 Ba | 8.41 ± 0.66 Ba | |
| 40–60 | Group I | 5.34 ± 0.08 Aa | 0.28 ± 0.04 Ba | 2.29 ± 0.47 Aa | 6.82 ± 0.69 Ba | 7.52 ± 1.17 Aa |
| Group II | 6.23 ± 0.23 Ab | 0.21 ± 0.02 Ba | 0.30 ± 0.05 Bb | 6.13 ± 0.42 Ba | 8.29 ± 1.91 Ba | |
| Group III | 7.08 ± 0.02 Ac | 0.06 ± 0.01 Bb | 0.15 ± 0.02 Bb | 5.02 ± 0.22 Bab | 8.79 ± 0.15 Ba |
| Layer (cm) | Group | Montmorillonite | Vermiculite | Illite | Kaolinite | Chlorite | Gibbsite |
|---|---|---|---|---|---|---|---|
| 0–20 | Group I | - | + | + | + | + | + |
| Group II | - | + | + | + | + | - | |
| Group III | + | + | + | + | + | - | |
| 20–40 | Group I | - | + | + | + | + | + |
| Group II | - | + | + | + | + | - | |
| Group III | + | + | + | + | + | - | |
| 40–60 | Group I | - | + | + | + | + | - |
| Group II | + | + | + | + | + | - | |
| Group III | - | + | + | + | + | - |
| Name | Importance Ranking | Explanatory Quantity (%) | Pseudo-F | p |
|---|---|---|---|---|
| pH | 1 | 50.2 | 25.2 | 0.002 |
| Clay | 2 | 5.8 | 3.2 | 0.088 |
| EAl | 3 | 3.8 | 2.2 | 0.128 |
| EH | 4 | 2.0 | 1.1 | 0.302 |
| SOM | 5 | 0.7 | 0.4 | 0.56 |
| Name | Importance Ranking | Explanatory Quantity (%) | Pseudo-F | p |
|---|---|---|---|---|
| I | 1 | 24.0 | 7.9 | 0.018 |
| Ch | 2 | 6.7 | 2.3 | 0.158 |
| V | 3 | 2.9 | 1.0 | 0.368 |
| K | 4 | 1.0 | 0.3 | 0.586 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, N.; Geng, N.; Wang, T.; Wang, H.; Pan, H.; Yang, Q.; Lou, Y.; Zhuge, Y. Effect of Acidification on Clay Minerals and Surface Properties of Brown Soil. Sustainability 2023, 15, 179. https://doi.org/10.3390/su15010179
Yin N, Geng N, Wang T, Wang H, Pan H, Yang Q, Lou Y, Zhuge Y. Effect of Acidification on Clay Minerals and Surface Properties of Brown Soil. Sustainability. 2023; 15(1):179. https://doi.org/10.3390/su15010179
Chicago/Turabian StyleYin, Na, Na Geng, Tingting Wang, Hui Wang, Hong Pan, Quangang Yang, Yanhong Lou, and Yuping Zhuge. 2023. "Effect of Acidification on Clay Minerals and Surface Properties of Brown Soil" Sustainability 15, no. 1: 179. https://doi.org/10.3390/su15010179





