Differential Impacts of Cropland Expansion on Soil Biological Indicators in Two Ecological Zones
Abstract
:1. Introduction
2. Materials and Methods
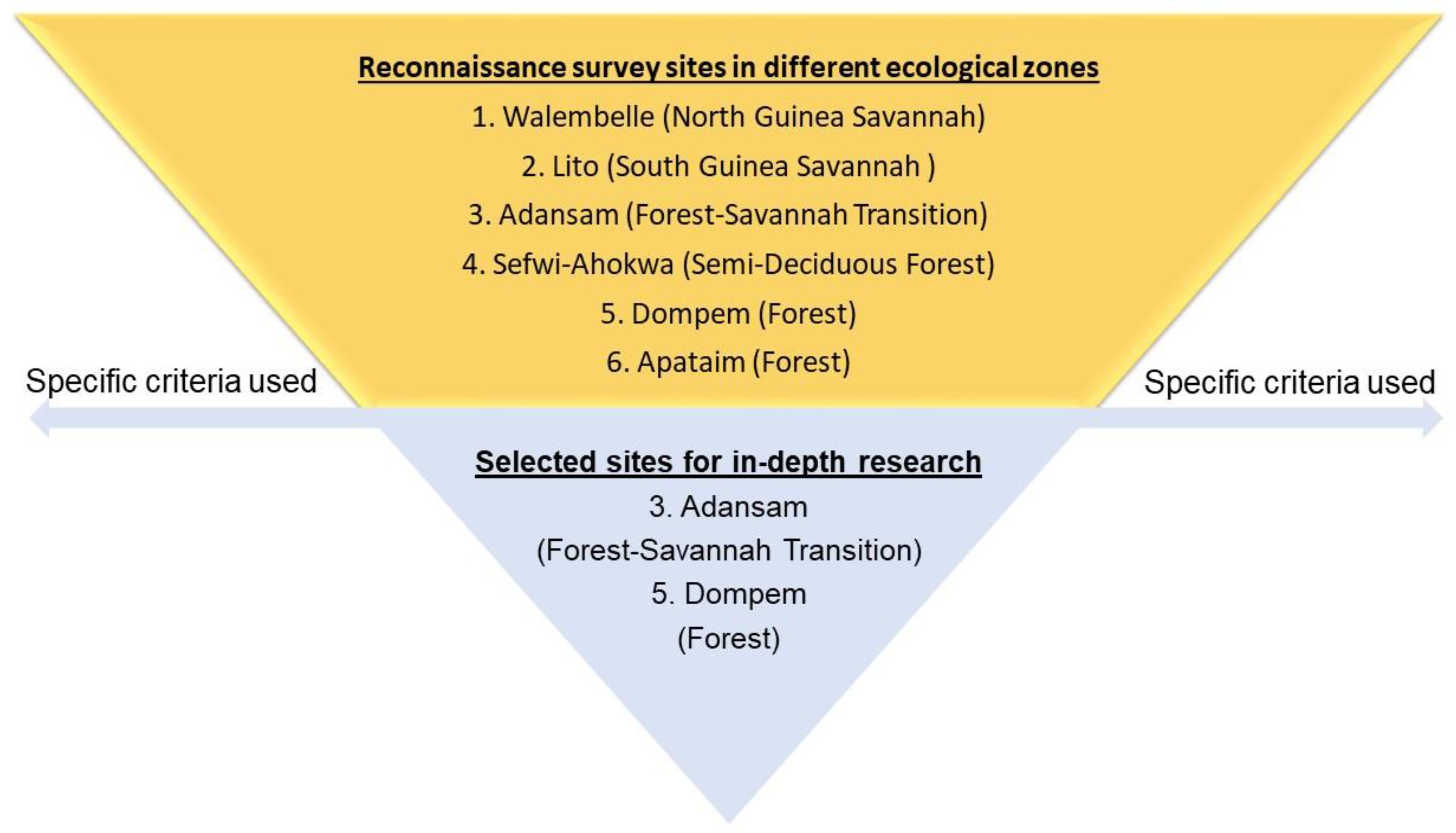
2.1. Study Sites and Sampling
2.2. Laboratory Procedures
2.2.1. Analysis of Basic Soil Properties
2.2.2. Labile C, Basal Respiration, and Microbial Biomass
2.3. Statistical Analysis
3. Results
3.1. Basic Soil Properties
3.2. Labile C, Basal Respiration, and Microbial Biomass
3.3. Effect of the Basic Soil Properties on Microbial Indices
4. Discussion
- Forest zone—clay loams/sandy clay loam textures with very strong acidity;
- Deciduous forest—sandy clay loams with slight acidity;
- Forest–savanna transition—sands with slight acidity;
- Southern Guinea savanna—sandy loams with strong acidity;
- Northern Guinea savanna zones—sandy loam with slight acidity; and
- Finally, the Adansam soils were arenic/sandy with slight acidity, while the Dompem soils were loams with very strong acidity [40].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jellason, N.P.; Robinson, E.J.Z.; Chapman, A.S.A.; Neina, D.; Devenish, A.J.M.; Po, J.Y.T.; Adolph, B. A systematic review of drivers and constraints on agricultural expansion in Sub-Saharan Africa. Land 2021, 10, 332. [Google Scholar] [CrossRef]
- de Valença, A.W.; Vanek, S.J.; Meza, K.; Ccanto, R.; Olivera, E.; Scurrah, M.; Lantinga, E.A.; Fonte, S.J. Land use as a driver of soil fertility and biodiversity across an agricultural landscape in the Central Peruvian Andes. Ecol. Appl. 2017, 27, 1138–1154. [Google Scholar] [CrossRef]
- Tilman, D. Global environmental impacts of agricultural expansion: The need for sustainable and efficient practices. Proc. Natl. Acad. Sci. USA 1999, 96, 5995–6000. [Google Scholar] [CrossRef]
- Laurance, W.F.; Sayer, J.; Cassman, K.G. Agricultural expansion and its impacts on tropical nature. Trends Ecol. Evol. 2014, 29, 107–116. [Google Scholar] [CrossRef]
- Barbier, E.B. Long run agricultural land expansion, booms and busts. Land Use Policy 2020, 93, 103808. [Google Scholar] [CrossRef]
- Delzeit, R.; Zabel, F.; Meyer, C.; Václavík, T. Addressing future trade-offs between biodiversity and cropland expansion to improve food security. Reg. Env. Chang. 2017, 17, 1429–1441. [Google Scholar] [CrossRef]
- Paula, F.S.; Rodrigues, J.L.M.; Zhou, J.; Wu, L.; Mueller, R.C.; Mirza, B.S.; Bohannan, B.J.M.; Nüsslein, K.; Deng, Y.; Tiedje, J.M.; et al. Land use change alters functional gene diversity, composition and abundance in Amazon forest soil microbial communities. Mol. Ecol. 2014, 23, 2988–2999. [Google Scholar] [CrossRef] [PubMed]
- West, P.C.; Gibbs, H.K.; Monfreda, C.; Wagner, J.; Barford, C.C.; Carpenter, S.R.; Foley, J.A. Trading carbon for food: Global comparison of carbon stocks vs. crop yields on agricultural land. Proc. Natl. Acad. Sci. USA 2010, 107, 19645–19648. [Google Scholar] [CrossRef]
- Felix, L.; Houet, T.; Verburg, P.H. Mapping biodiversity and ecosystem service trade-offs and synergies of agricultural change trajectories in Europe. Env. Sci. Policy 2022, 136, 387–399. [Google Scholar] [CrossRef]
- Jellason, N.P.; Robinson, E.J.; Katic, P.; Davies, J.E.; Devenish, A.J.; Po, J.Y.; Martin, A.; Adanu, S.K.; Gebrehiwot, T.; Teklewold, H.; et al. Winners and losers: Exploring the differential impacts of agricultural expansion in Ethiopia and Ghana. Curr. Res. Env. Sustain. 2022, 4, 100176. [Google Scholar] [CrossRef]
- Akinyemi, F.O.; Speranza, C.I. Agricultural landscape change impact on the quality of land: An African continent-wide assessment in gained and displaced agricultural lands. Int. J. Appl. Earth Obs. Geoinf. 2022, 106, 102644. [Google Scholar] [CrossRef]
- Gomes, L.; Simões, S.J.; Dalla Nora, E.L.; de Sousa-Neto, E.R.; Forti, M.C.; Ometto, J.P.H. Agricultural expansion in the Brazilian Cerrado: Increased soil and nutrient losses and decreased agricultural productivity. Land 2019, 8, 12. [Google Scholar] [CrossRef]
- Rukundo, E.; Liu, S.; Dong, Y.; Rutebuka, E.; Asamoah, E.F.; Xu, J.; Wu, X. Spatio-temporal dynamics of critical ecosystem services in response to agricultural expansion in Rwanda, East Africa. Ecol. Indic. 2018, 89, 696–705. [Google Scholar] [CrossRef]
- Locatelli, J.L.; Santos, R.S.; Cherubin, M.R.; Cerri, C.E. Changes in soil organic matter fractions induced by cropland and pasture expansion in Brazil's new agricultural frontier. Geoderma Reg. 2022, 28, e00474. [Google Scholar] [CrossRef]
- Blécourt, M.D.; Röder, A.; Groengroeft, A.; Baumann, S.; Frantz, D.; Eschenbach, A. Deforestation for agricultural expansion in SW Zambia and NE Namibia and the impacts on soil fertility, soil organic carbon-and nutrient levels. Biodivers Ecol. 2018, 6, 242–250. [Google Scholar] [CrossRef]
- De Blécourt, M.; Gröngröft, A.; Baumann, S.; Eschenbach, A. Losses in soil organic carbon stocks and soil fertility due to deforestation for low-input agriculture in semi-arid southern Africa. J. Arid. Environ. 2019, 165, 88–96. [Google Scholar] [CrossRef]
- Janes-Bassett, V.; Bassett, R.; Yumashev, D.; Blair, G.; Davies, J. Mapping regional impacts of agricultural expansion on terrestrial carbon storage. Reg. Stud. Reg. Sci. 2021, 8, 336–340. [Google Scholar] [CrossRef]
- Oliveira, D.M.S.; Williams, S.; Cerri, C.E.P.; Paustian, K. Predicting soil C changes over sugarcane expansion in Brazil using the DayCent model. GCB Bioenergy 2017, 9, 1436–1446. [Google Scholar] [CrossRef]
- Chaplin-Kramer, R.; Sharp, R.P.; Mandle, L.; Sim, S.; Johnson, J.; Butnar, I.; Milài Canals, L.; Eichelberger, B.A.; Ramler, I.; Mueller, C.; et al. Spatial patterns of agricultural expansion determine impacts on biodiversity and carbon storage. Proc. Natl. Acad. Sci. USA 2015, 112, 7402–7407. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Wang, J. A global analysis of agricultural productivity and water resource consumption changes over cropland expansion regions. Agric Ecosyst. Env. 2021, 321, 107630. [Google Scholar] [CrossRef]
- Grecchi, R.C.; Gwyn, Q.H.J.; Bénié, G.B.; Formaggio, A.R.; Fahl, F.C. Land use and land cover changes in the Brazilian Cerrado: A multidisciplinary approach to assess the impacts of agricultural expansion. Appl. Geogr. 2014, 55, 300–312. [Google Scholar] [CrossRef]
- Park, E.; Loc Ho, H.; van Binh, D.; Kantoush, S.; Poh, D.; Alcantara, E.; Try, S.; Lin, Y.N. Impacts of agricultural expansion on floodplain water and sediment budgets in the Mekong River. J. Hydrol. 2022, 605, 127296. [Google Scholar] [CrossRef]
- Potapov, P.; Turubanova, S.; Hansen, M.C.; Tyukavina, A.; Zalles, V.; Khan, A.; Song, X.-P.; Pickens, A.; Shen, Q.; Cortez, J. Global maps of cropland extent and change show accelerated cropland expansion in the twenty-first century. Nat. Food 2022, 3, 19–28. [Google Scholar] [CrossRef]
- Viglizzo, E.F.; Frank, F.C.; Carreño, L.V.; Jobbagy, E.G.; Pereyra, H.; Clatt, J.; Pincén, D.; Ricard, M.F. Ecological and environmental footprint of 50 years of agricultural expansion in Argentina. Global Chang. Biol. 2011, 17, 959–973. [Google Scholar] [CrossRef]
- Kostin, J.E.; Cesarz, S.; Lochner, A.; Schädler, M.; Macdonald, C.A.; Eisenhauer, N. Land-use drives the temporal stability and magnitude of soil microbial functions and modulates climate effects. Ecol. Appl. 2021, 31, e02325. [Google Scholar] [CrossRef]
- Lal, R.; Cummings, D.J. Clearing a tropical forest I. Effects on soil and micro-climate. Field Crop Res. 1979, 2, 91–107. [Google Scholar] [CrossRef]
- Beza, E.; Silva, J.V.; Kooistra, L.; Reidsma, P. Review of yield gap explaining factors and opportunities for alternative data collection approaches. Eur. J. Agron. 2017, 82, 206–222. [Google Scholar] [CrossRef]
- Sileshi, G.; Akinnifesi, F.K.; Debusho, L.K.; Beedy, T.; Ajayi, O.C.; Mong’omba, S. Variation in maize yield gaps with plant nutrient inputs, soil type and climate across sub-Saharan Africa. Field Crop Res. 2010, 116, 1–13. [Google Scholar] [CrossRef]
- Boullouz, M.; Bindraban, P.S.; Kissiedu, I.N.; Kouame, A.K.K.; Devkota, K.P.; Atakora, W.K. An integrative approach based on crop modeling and geospatial and statistical analysis to quantify and explain the maize (Zea mays) yield gap in Ghana. Front. Soil Sci. 2022, 2, 68. [Google Scholar] [CrossRef]
- Piquer-Rodríguez, M.; Butsic, V.; Gärtner, P.; Macchi, L.; Baumann, M.; Gavier Pizarro, G.; Volante, J.N.; Gasparri, I.N.; Kuemmerle, T. Drivers of agricultural land-use change in the Argentine Pampas and Chaco regions. Appl. Geogr. 2018, 91, 111–122. [Google Scholar] [CrossRef]
- Franks, P.; Hou-Jones, X.; Fikreyesus, D.; Sintayehu, M.; Mamuye, S.; Danso, E.Y.; Meshack, C.K.; McNicol, I.; van Soesbergen, A. Reconciling Forest Conservation with Food Production in Sub-Saharan Africa: Case Studies from Ethiopia, Ghana and Tanzania; International Institute for Environment and Development: London, UK, 2017. [Google Scholar]
- Baćmaga, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J.; Paprocki, Ł. Role of forest site type in determining bacterial and biochemical properties of soil. Ecol. Indic. 2022, 135, 108557. [Google Scholar] [CrossRef]
- Cui, H.; Wagg, C.; Wang, X.; Liu, Z.; Liu, K.; Chen, S.; Chen, J.; Song, H.; Meng, L.; Wang, J.; et al. The loss of above- and belowground biodiversity in degraded grasslands drives the decline of ecosystem multifunctionality. Appl. Soil Ecol. 2022, 172, 104370. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; van der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Cappelli, S.L.; Domeignoz-Horta, L.A.; Loaiza, V.; Laine, A.-L. Plant biodiversity promotes sustainable agriculture directly and via belowground effects. Trends Plant Sci. 2022, 27, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Bardgett, R.D.; Vitousek, P.M.; Maestre, F.T.; Williams, M.A.; Eldridge, D.J.; Lambers, H.; Neuhauser, S.; Gallardo, A.; García-Velázquez, L.; et al. Changes in belowground biodiversity during ecosystem development. Proc. Natl. Acad. Sci. USA 2019, 116, 6891–6896. [Google Scholar] [CrossRef]
- Liu, L.; Huang, X.; Zhang, J.; Cai, Z.; Jiang, K.; Chang, Y. Deciphering the relative importance of soil and plant traits on the development of rhizosphere microbial communities. Soil Biol. Biochem. 2020, 148, 107909. [Google Scholar] [CrossRef]
- de Souza, G.P.; de Figueiredo, C.C.; de Sousa, D.M.G. Relationships between labile soil organic carbon fractions under different soil management systems. Sci. Agric. 2016, 73, 535–542. [Google Scholar] [CrossRef]
- Neina, D.; Agyarko-Mintah, E. Duration of cultivation has varied impacts on soil charge properties in different agro-ecological zones of Ghana. Land 2022, 11, 1633. [Google Scholar] [CrossRef]
- Tosi, M.; Correa, O.S.; Soria, M.A.; Vogrig, J.A.; Sydorenko, O.; Montecchia, M.S. Land-use change affects the functionality of soil microbial communities: A chronosequence approach in the Argentinian Yungas. Appl. Soil Ecol. 2016, 108, 118–127. [Google Scholar] [CrossRef]
- Winding, A.; Hund-Rinke, K.; Rutgers, M. The use of microorganisms in ecological soil classification and assessment concepts. Ecotoxicol. Env. Saf. 2005, 62, 230–248. [Google Scholar] [CrossRef] [PubMed]
- Wobeng, N.B.M.; Banfield, C.C.; Megueni, C.; Mapongmetsem, P.M.; Dippold, M.A. Impact of legumes on soil microbial activity and C cycle functions in two contrasting Cameroonian agro-ecological zones. Pedobiologia 2020, 81–82, 150662. [Google Scholar] [CrossRef]
- Wall, D.H.; Nielsen, U.N.; Six, J. Soil biodiversity and human health. Nature 2015, 528, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Neina, D.; Adolph, B. Sulphur contents in arable soils from four agro-ecological zones of Ghana. Land 2022, 11, 1866. [Google Scholar] [CrossRef]
- Weil, R.R.; Islam, K.R.; Stine, M.A.; Gruver, J.B.; Samson-Liebig, S.E. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 2003, 18, 3–17. [Google Scholar] [CrossRef]
- Blair, G.J.; Lefroy, R.D.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459. [Google Scholar] [CrossRef]
- Isermeyer, H. Eine einfache Methode zur Bestimmung der Bodenatmung und der Karbonate im Boden. Z. Pflanzenernaehr. Dueng. Bodenk. 1952, 56, 26–38. [Google Scholar] [CrossRef]
- Creamer, R.E.; Schulte, R.; Stone, D.; Gal, A.; Krogh, P.H.; Lo Papa, G.; Murray, P.J.; Pérès, G.; Foerster, B.; Rutgers, M.; et al. Measuring basal soil respiration across Europe: Do incubation temperature and incubation period matter? Ecol. Indic. 2014, 36, 409–418. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Haney, R.; Franzluebbers, A.; Hons, F.; Hossner, L.; Zuberer, D. Molar concentration of K2SO4 and soil pH affect estimation of extractable C with chloroform fumigation–extraction. Soil Biol. Biochem. 2001, 33, 1501–1507. [Google Scholar] [CrossRef]
- Jenkinson, D.S.; Powlson, D.S. The effects of biocidal treatments on metabolism in soil—V. Soil Biol. Biochem. 1976, 8, 209–213. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Errata: Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1953, 48, 907. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using SPSS, 3rd ed.; SAGE Publications Ltd.: London, UK, 2009; ISBN 978-1-84787-906-6. [Google Scholar]
- Cao, Y.; Chai, Y.; Jiao, S.; Li, X.; Wang, X.; Zhang, Y.; Yue, M. Bacterial and fungal community assembly in relation to soil nutrients and plant growth across different ecoregions of shrubland in Shaanxi, northwestern China. Appl. Soil. Ecol. 2022, 173, 104385. [Google Scholar] [CrossRef]
- Smith, L.C.; Orgiazzi, A.; Eisenhauer, N.; Cesarz, S.; Lochner, A.; Jones, A.; Bastida, F.; Patoine, G.; Reitz, T.; Buscot, F.; et al. Large-scale drivers of relationships between soil microbial properties and organic carbon across Europe. Global Ecol. Biogeogr. 2021, 30, 2070–2083. [Google Scholar] [CrossRef]
- Leng, X.; Feng, X.; Fu, B. Driving forces of agricultural expansion and land degradation indicated by Vegetation Continuous Fields (VCF) data in drylands from 2000 to 2015. Global Ecol. Conserv. 2020, 23, e01087. [Google Scholar] [CrossRef]
- Duval, M.E.; Galantini, J.A.; Martínez, J.M.; Limbozzi, F. Labile soil organic carbon for assessing soil quality: Influence of management practices and edaphic conditions. Catena 2018, 171, 316–326. [Google Scholar] [CrossRef]
- Culman, S.W.; Snapp, S.S.; Freeman, M.A.; Schipanski, M.E.; Beniston, J.; Lal, R.; Drinkwater, L.E.; Franzluebbers, A.J.; Glover, J.D.; Grandy, A.S.; et al. Permanganate oxidizable carbon reflects a processed soil fraction that is sensitive to management. Soil Sci. Soc. Am. J. 2012, 76, 494–504. [Google Scholar] [CrossRef]
- Bongiorno, G.; Bünemann, E.K.; Oguejiofor, C.U.; Meier, J.; Gort, G.; Comans, R.; Mäder, P.; Brussaard, L.; de Goede, R. Sensitivity of labile carbon fractions to tillage and organic matter management and their potential as comprehensive soil quality indicators across pedoclimatic conditions in Europe. Ecol. Indic. 2019, 99, 38–50. [Google Scholar] [CrossRef]
- Ramírez, P.B.; Fuentes-Alburquenque, S.; Díez, B.; Vargas, I.; Bonilla, C.A. Soil microbial community responses to labile organic carbon fractions in relation to soil type and land use along a climate gradient. Soil Biol. Biochem. 2020, 141, 107692. [Google Scholar] [CrossRef]
- Neina, D.; Buerkert, A.; Joergensen, R.G. Effects of land use on microbial indices in tantalite mine soils, Western Rwanda. Land Degrad. Develop. 2017, 28, 181–188. [Google Scholar] [CrossRef]
- Xu, X.; Schimel, J.P.; Janssens, I.A.; Song, X.; Song, C.; Yu, G.; Sinsabaugh, R.L.; Tang, D.; Zhang, X.; Thornton, P.E. Global pattern and controls of soil microbial metabolic quotient. Ecol. Monogr. 2017, 87, 429–441. [Google Scholar] [CrossRef]
- Ashraf, M.N.; Waqas, M.A.; Rahman, S. Microbial metabolic quotient is a dynamic indicator of soil health: Trends, implications and perspectives (Review). Eurasian Soil Sci. 2022, 55, 1794–1803. [Google Scholar] [CrossRef]
- Janssens, I.A.; Dieleman, W.; Luyssaert, S.; Subke, J.-A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G.; et al. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- da C. Jesus, E.; Marsh, T.L.; Tiedje, J.M.; Moreira, F.M.d.S. Changes in land use alter the structure of bacterial communities in Western Amazon soils. ISME J. 2009, 3, 1004–1011. [Google Scholar] [CrossRef]
- Li, T.; Zhang, J.; Wang, X.; Hartley, I.P.; Zhang, J.; Zhang, Y. Fungal necromass contributes more to soil organic carbon and more sensitive to land use intensity than bacterial necromass. Appl. Soil Ecol. 2022, 176, 104492. [Google Scholar] [CrossRef]
- He, L.; Mazza Rodrigues, J.L.; Soudzilovskaia, N.A.; Barceló, M.; Olsson, P.A.; Song, C.; Tedersoo, L.; Yuan, F.; Yuan, F.; Lipson, D.A.; et al. Global biogeography of fungal and bacterial biomass carbon in topsoil. Soil Biol. Biochem. 2020, 151, 108024. [Google Scholar] [CrossRef]
- Yang, X.; Ren, W.; Sun, B.; Zhang, S. Effects of contrasting soil management regimes on total and labile soil organic carbon fractions in a loess soil in China. Geoderma 2012, 177–178, 49–56. [Google Scholar] [CrossRef]
- Bruun, T.B.; Elberling, B.; Christensen, B.T. Lability of soil organic carbon in tropical soils with different clay minerals. Soil Biol. Biochem. 2010, 42, 888–895. [Google Scholar] [CrossRef]
- Koković, N.; Saljnikov, E.; Eulenstein, F.; Čakmak, D.; Buntić, A.; Sikirić, B.; Ugrenović, V. Changes in soil labile organic matter as affected by 50 years of fertilization with increasing amounts of nitrogen. Agronomy 2021, 11, 2026. [Google Scholar] [CrossRef]
- Filep, T.; Zacháry, D.; Jakab, G.; Szalai, Z. Chemical composition of labile carbon fractions in Hungarian forest soils: Insight into biogeochemical coupling between DOM and POM. Geoderma 2022, 419, 115867. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Garbeva, P.; van Elsas, J.D.; van Veen, J.A. Rhizosphere microbial community and its response to plant species and soil history. Plant Soil 2008, 302, 19–32. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Song, W.; Slik, J.W.F.; Sukri, R.S.; Jaafar, S.; Dong, K.; Adams, J.M. Distinctive Tropical Forest Variants Have Unique Soil Microbial Communities, But Not Always Low Microbial Diversity. Front. Microbiol. 2016, 7, 376. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Q.; Zhang, W.; Zhu, P.; Xiao, Q.; Wang, C.; Wu, L.; Tian, Y.; Xu, M.; Gunina, A. Stoichiometric imbalance of soil carbon and nutrients drives microbial community structure under long-term fertilization. Appl. Soil Ecol. 2021, 168, 104119. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Morreale, S.J.; Schneider, R.L.; Li, Z.; Wu, G.-L. Contributions of plant litter to soil microbial activity improvement and soil nutrient enhancement along with herb and shrub colonization expansions in an arid sandy land. Catena 2023, 227, 107098. [Google Scholar] [CrossRef]




| Ecological Zone | 1 SOC | POXC | POXC/SOC | CO2-C | Cmic | Cmic/SOC | qCO2 |
|---|---|---|---|---|---|---|---|
| mg kg−1 | % | µg g−1 | % | ||||
| Forest–savanna transition (Adansam) | 5.86 a | 46.71 a | 0.82 a | 25.60 a | 70.11 a | 1.31 | 0.40 a |
| Semi-deciduous forest (Sefwi-Ahokwa) | 17.03 b | 73.72 b | 0.44 b | 55.12 b | 52.65 a | 0.33 | 1.09 b |
| South Guinea savanna (Lito) | 8.90 a | 33.08 a | 0.38 b | 27.79 a | 145.25 b | 1.75 | 0.18 a |
| North Guinea savanna (Wallembelle) | 10.81 a | 27.96 a | 0.27 b | 28.81 a | 115.46 c | 1.24 | 0.25 a |
| CV (%) | 51 | 44 | 59 | 46 | 47 | 66 | 84 |
| p-Value | 0.014 | 0.001 | 0.020 | 0.017 | 0.007 | 0.126 | 0.002 |
| Farm Type | 1 pH | SOC | POXC | POXC/SOC |
|---|---|---|---|---|
| Water | g kg−1 | mg kg−1 | % | |
| Dompem | ||||
| Year one (forest) | 4.3 (0.16) | 28.49 (5.60) | 64.82 (6.90) | 0.26 (0.00) a |
| Year one (fallow) | 4.3 (0.15) | 21.81 (1.81) | 67.03 (4.02) | 0.32 (0.00) b |
| Three years | 4.4 (0.14) | 21.43 (3.70) | 53.91 (7.21) | 0.26 (0.00) a |
| Five years | 4.5 (0.13) | 16.82 (1.16) | 65.06 (5.22) | 0.39 (0.00) b |
| Ten years | 4.5 (0.13) | 16.89 (2.45) | 52.01 (3.82) | 0.33 (0.00) b |
| p-value | - | 0.086 | 0.175 | 0.001 |
| Adansam | ||||
| Year one (forest/fallow) | 6.3 (0.08) | 12.23 (0.63) a | 47.27 (2.85) | 0.39 (0.00) |
| Three years | 6.2 (0.15) | 10.29 (0.70) a | 38.03 (4.93) | 0.36 (0.00) |
| Five years | 6.4 (0.13) | 8.79 (0.69) b | 36.61 (2.36) | 0.43 (0.00) |
| Ten years | 6.3 (0.17) | 10.91 (0.73) a | 38.85 (2.55) | 0.37 (0.00) |
| p-value | - | 0.008 | 0.100 | 0.482 |
| POXC | %POXC/SOC | CO2-C | Cmic | qCO2 | %Cmic/SOC | |
|---|---|---|---|---|---|---|
| pH | 0.20 ns | - | - | −0.47 ns | - | −0.34 ns |
| SOC | 0.50 ns | −0.66 * | 0.76 ** | - | 0.39 ns | −0.66 * |
| EA | 0.46 ns | - | 0.57 * | 0.60 * | 0.61 * | −0.54 * |
| ECEC | 0.73 ** | - | 0.54 * | −0.63 * | 0.66 * | −0.71 ** |
| SEB | 0.73 ** | - | 0.68 ** | −0.64 * | 0.68 ** | −0.70 ** |
| Ald | −0.43 ns | −0.26 ns | - | - | - | - |
| Alox | −0.20 ns | −0.21 ns | - | - | - | - |
| Fed | −0.91 *** | −0.68 ** | −0.45 ns | 0.62 * | −0.59 * | 0.37 ns |
| Feox | −0.43 ns | −0.79 ** | - | 0.28 ns | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neina, D.; Agyarko-Mintah, E. Differential Impacts of Cropland Expansion on Soil Biological Indicators in Two Ecological Zones. Sustainability 2023, 15, 8138. https://doi.org/10.3390/su15108138
Neina D, Agyarko-Mintah E. Differential Impacts of Cropland Expansion on Soil Biological Indicators in Two Ecological Zones. Sustainability. 2023; 15(10):8138. https://doi.org/10.3390/su15108138
Chicago/Turabian StyleNeina, Dora, and Eunice Agyarko-Mintah. 2023. "Differential Impacts of Cropland Expansion on Soil Biological Indicators in Two Ecological Zones" Sustainability 15, no. 10: 8138. https://doi.org/10.3390/su15108138
APA StyleNeina, D., & Agyarko-Mintah, E. (2023). Differential Impacts of Cropland Expansion on Soil Biological Indicators in Two Ecological Zones. Sustainability, 15(10), 8138. https://doi.org/10.3390/su15108138







