Strength and Environmental Behaviours of Municipal Solid Waste Incineration Fly Ash for Cement-Stabilised Soil
Abstract
:1. Introduction
2. Material and MSWIFA Characteristics
2.1. Materials
2.2. MSWIFA Characteristics
2.2.1. Physical Characteristics
2.2.2. Chemical Characteristics
| Country/ Region | Remarks | CaO | Na2O | SiO2 | MgO | Fe2O3 | Al2O3 | SO3 | Cl− |
|---|---|---|---|---|---|---|---|---|---|
| FA, Hezhou | China | 35.7 | 6.5 | 2.5 | 1.6 | 0.8 | 0.6 | 4.0 | 31.5 |
| FA, Nanning | 35.0 | 10.7 | 2.2 | 1.8 | 0.6 | 0.5 | 6.0 | 23.8 | |
| FA, Dalian | 45.3 | 9.9 | 2.1 | 1.2 | 0.8 | 0.4 | - | 21.5 | |
| FA, Suzhou | 39.1 | 3.4 | 16.0 | 1.6 | 1.9 | 4.4 | 7.2 | 11.9 | |
| FA, Shanghai | 23.4 | 4.0 | 24.5 | 2.7 | 4.0 | 7.4 | 12.0 | 10.0 | |
| FA, Japan | - | 13.9 | 17.2 | 12.0 | 2.6 | 1.2 | 8.1 | - | 14.9 |
| FA, Denmark | - | 13–40 | 9–21 | 4–5 | 0.7–1.3 | 0.7–1.1 | 1–4 | 7–35 | 3–22 |
| FA, American | - | 2.2 | 2.9 | 6.5 | - | 3.3 | 0.4 | 2.9 | 33.2 |
| OPC, Hezhou | China | 51.9 | 0.3 | 18.2 | 1.3 | 3 | 4.6 | 3.1 | - |
2.2.3. Environmental Characteristics
3. Methods
3.1. Fly Ash Pre-Treatment Methods
3.1.1. Water-Washing
3.1.2. Washing by Ferrous Sulphate and Phosphoric Acid
3.1.3. Organic Chelation
3.1.4. Adding Sugarcane Ash
3.2. Experimental Methods
3.2.1. Sample Preparation
3.2.2. UCS Test
3.2.3. Heavy Metal Leaching Test
3.3. Analytical Methods
4. Results and Discussion
4.1. MSWIFA Behaviour in Cement Stabilisation of Sandy Soil
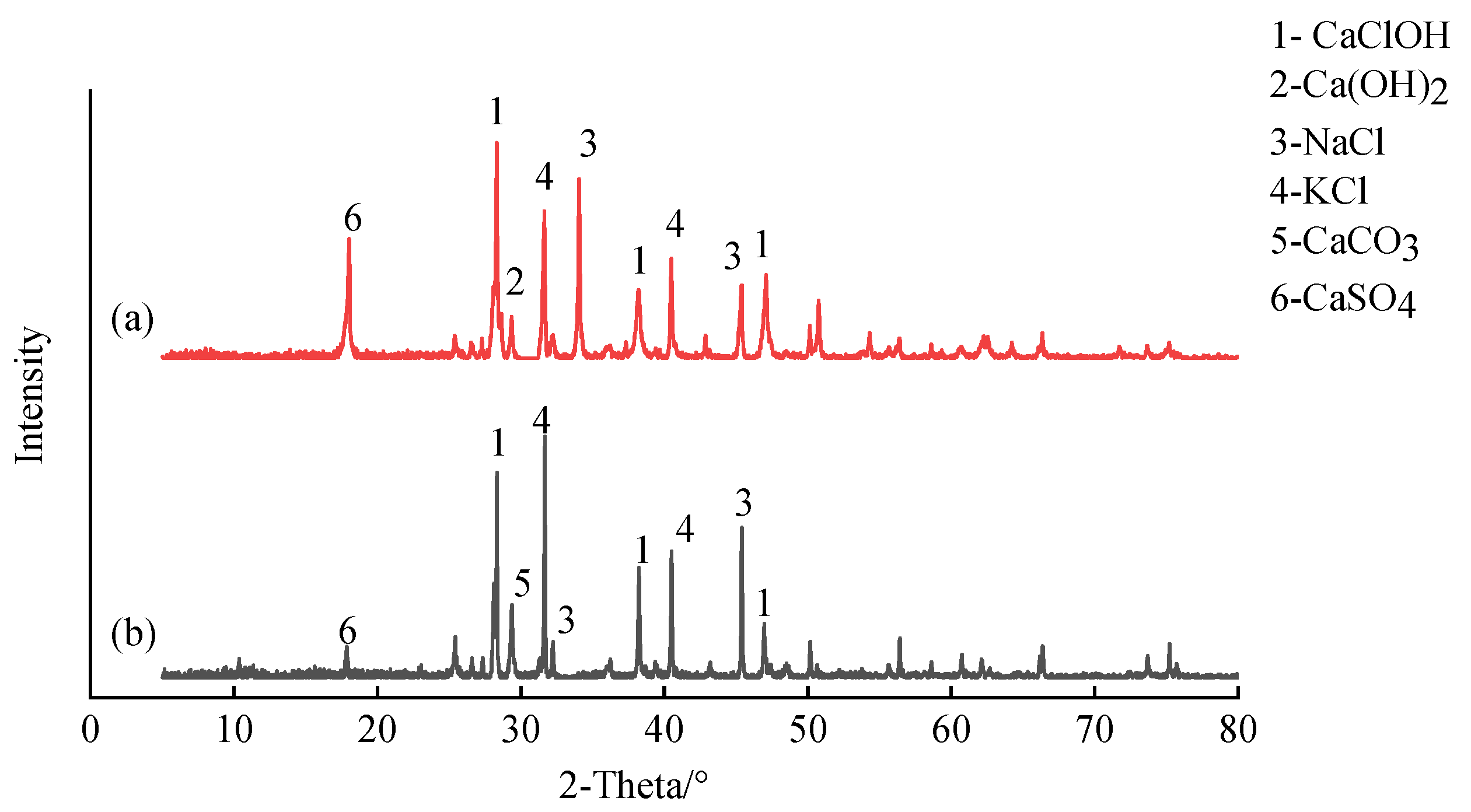
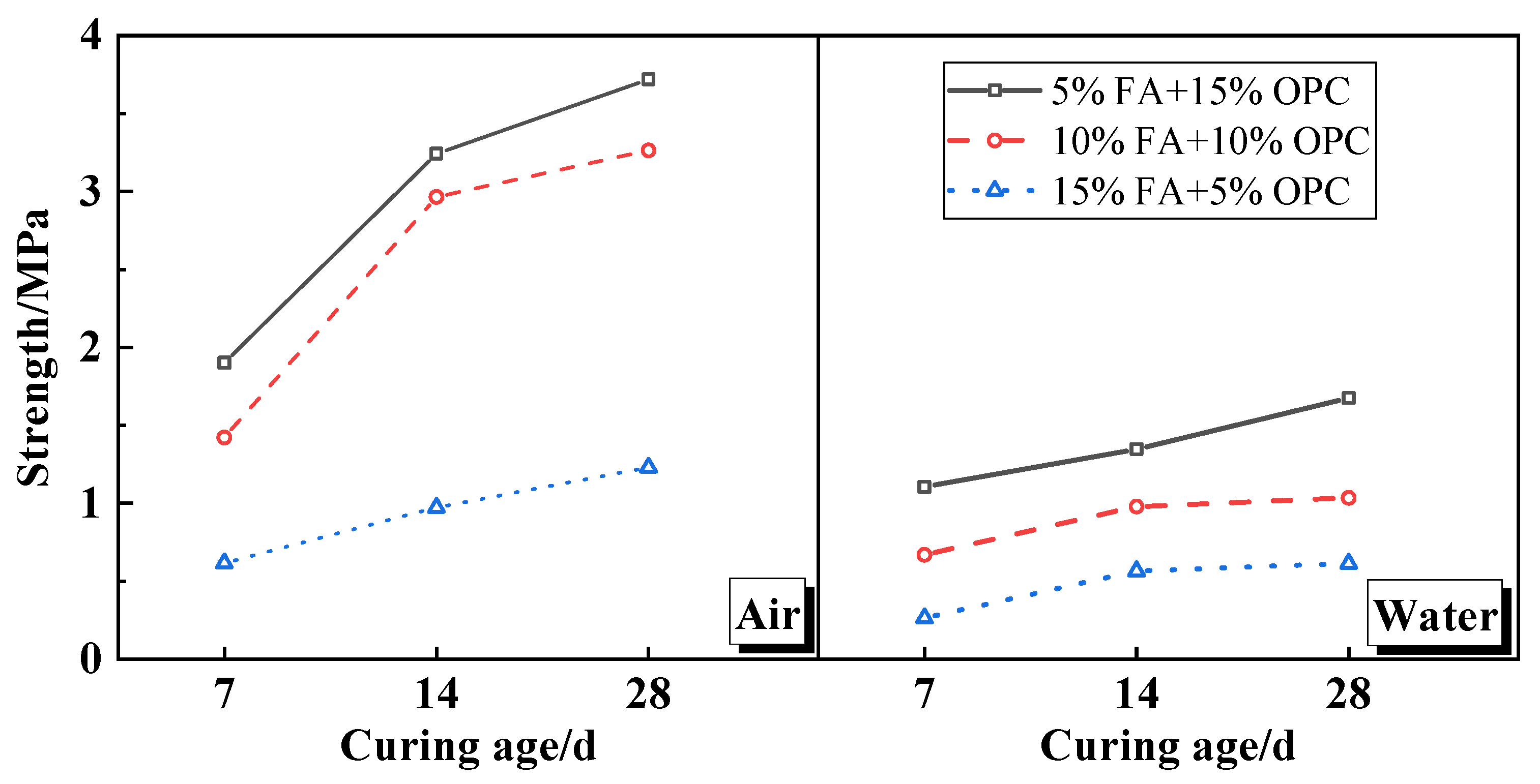
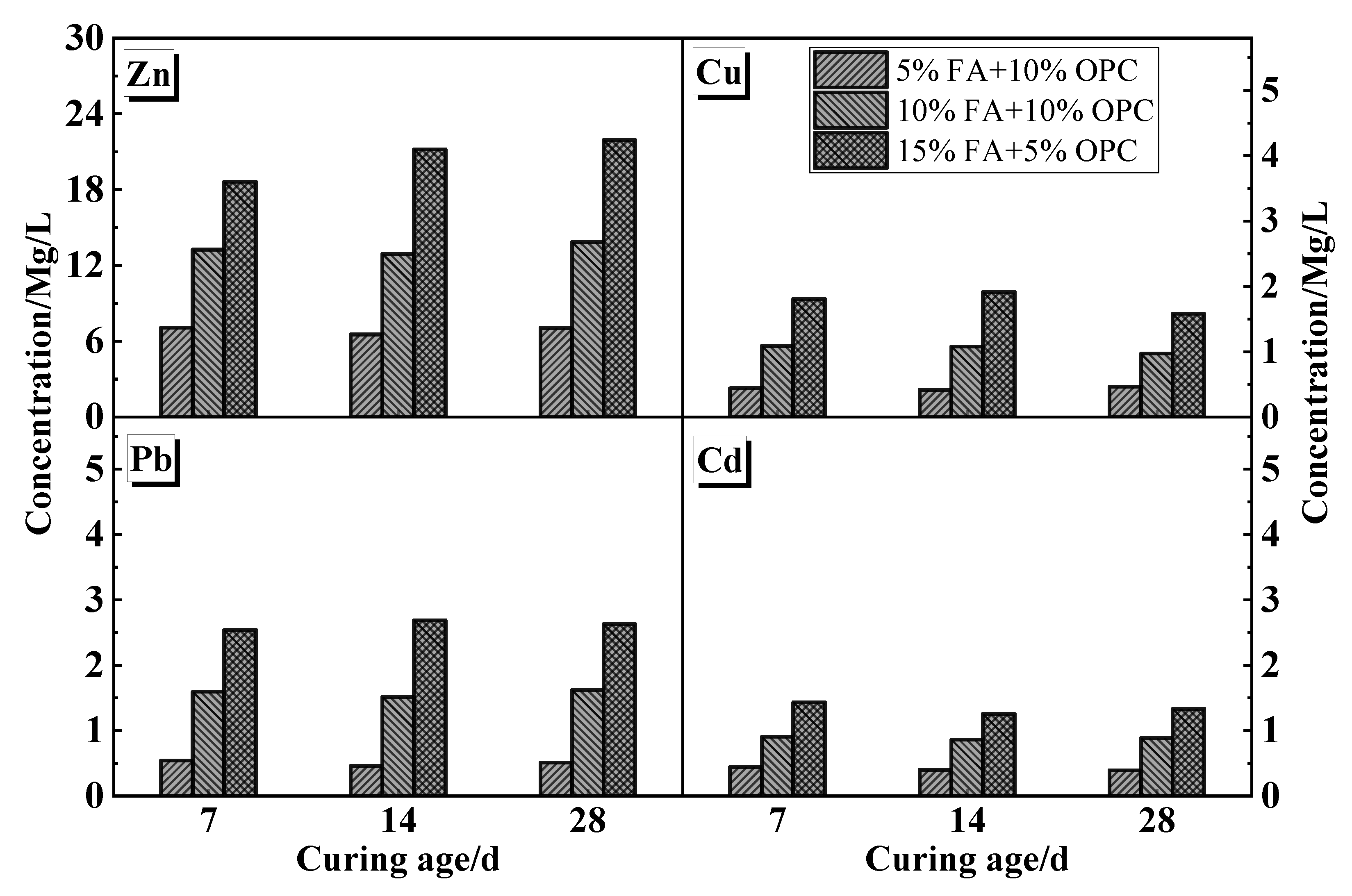
4.1.1. UCS and Leaching Characteristics
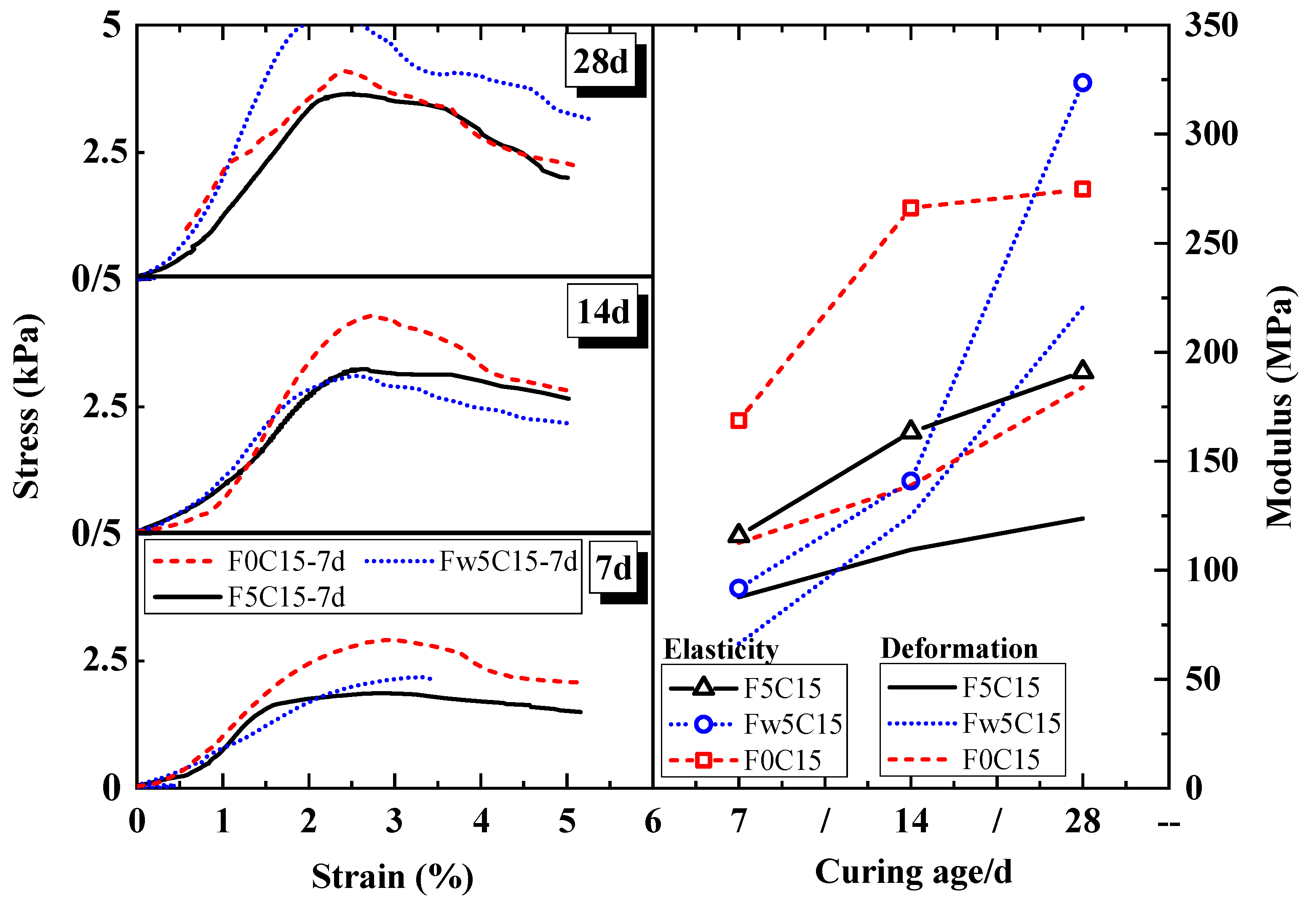
4.1.2. Engineering Characteristic Mechanisms
4.2. MSWIFA Pre-Treatment with High Chlorine
4.2.1. Pre-Treatment with Ferrous Sulphate
4.2.2. Pre-Treatment with Organic Chelating Agent
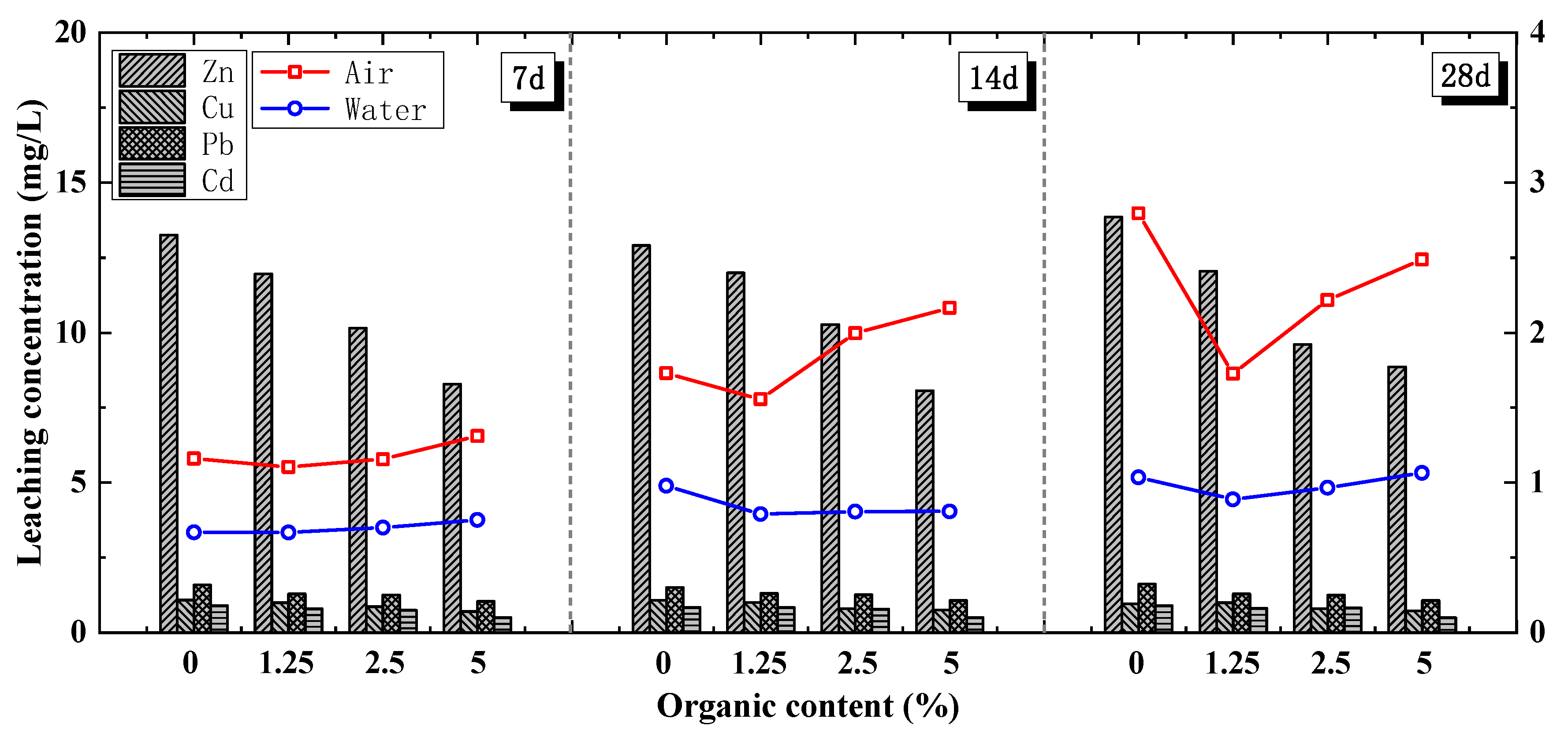
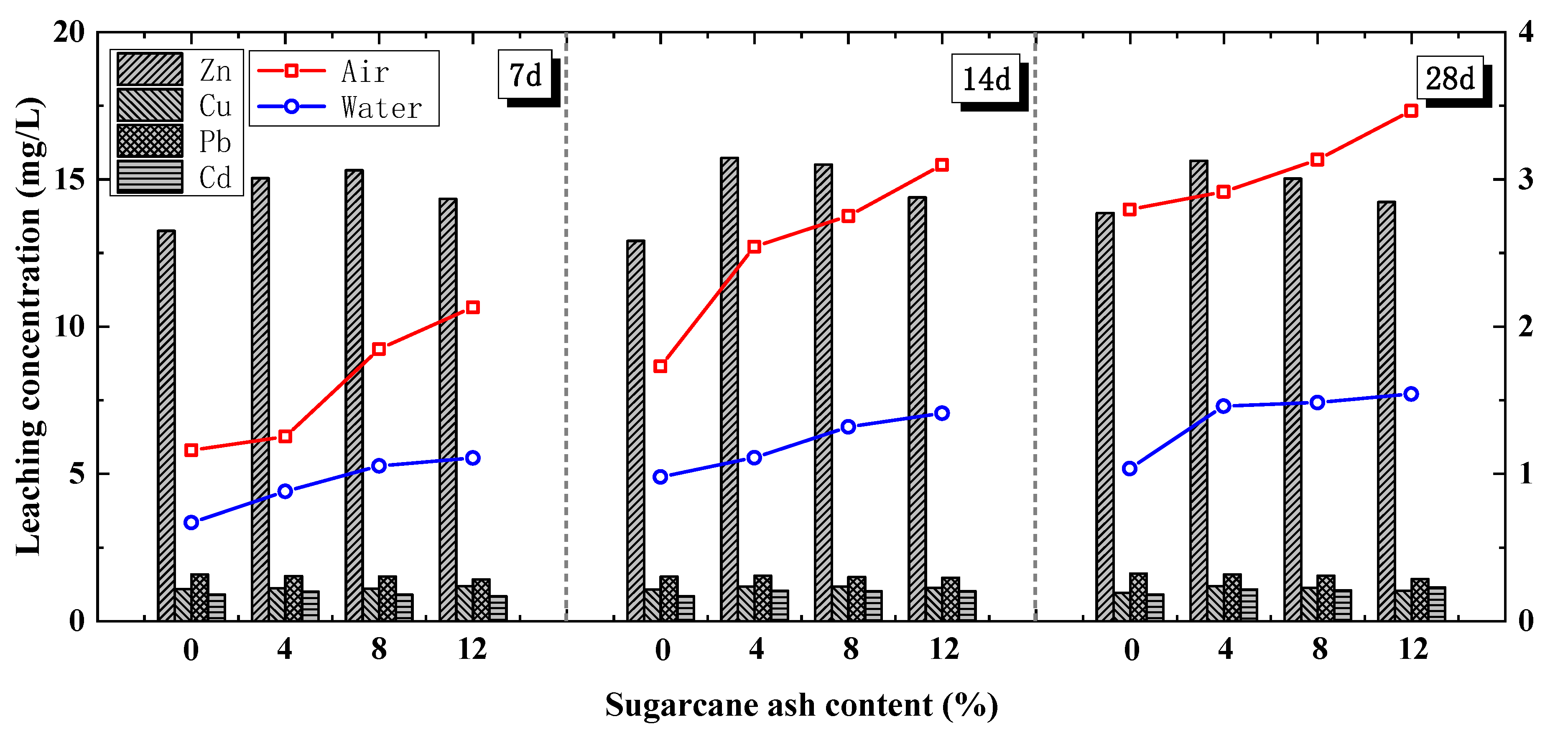
4.2.3. Addition of Sugarcane Ash
4.2.4. Pre-Treatment Summary
5. Conclusions
- (1)
- The basic physical, chemical, and environmental characteristics of FA from Guangxi were systematically tested and compared with samples from different countries and other Chinese cities. The FA had similar characteristics overall. Moreover, MSWIFA from Hezhou, China was slightly different from other sources of MSWIFA. The chloride ion content of Hezhou’s FA (31.5%) was significantly higher than that from most Chinese cities, as well as that from the United States, Japan, and Italy.
- (2)
- The peak strength of the FA cement soil typically increased along with the curing age and decreased as the FA content was increased. This is because the MSWIFA produced hydrating C–S–H and had a certain pozzolanic activity, which is lower than that of cement. Chloride ions and heavy metals hinder C–S–H development and production in the soil skeleton, meaning that the cement soil strength only grew slowly.
- (3)
- The leaching ion concentrations of Cu, Zn, Pb, and Cd generally increased along with the FA content. The Cu and Zn were well below the limit value of the entry standards of domestic landfill waste under all the mix proportions. In an acetic acid environment, Pb and Cd may leach when sand is solidified with untreated cement and FA.
- (4)
- After water washing and ferrous sulphate washing, FACS strength development speed obviously increased in the later stage of curing. Appropriate FA pre-treatment with chlorine enhanced MSWIFA resource recovery. This was because water washing and ferrous sulphate washing greatly decreased the chlorine salt and free-CaO in the FA, improved its potential cementitious activity, and reduced obstructions due to impurities in cement hydration.
- (5)
- Ferrous sulphate had a better stabilisation effect on Pb and Cd. As the ferrous sulphate concentration increased from 2% to 6%, the decrease in Pb concentration increased from 11.80% to 30.95%, while the decrease in Cd concentration increased from 32.15% to 39.93%. Moreover, the organic chelating agent significantly reduced each of the heavy metals. This process ensures that MSWIFA can be reused.
- (6)
- Although the cement soil strength was slightly hindered by the addition of an organic chelating agent, the FACS UCS obviously increased with the sugarcane ash content. The 28-day UCS results of the FACS with 4%, 8%, and 12% sugarcane ash content were 41.08%, 43.51%, and 49.07% higher, respectively. Thus, a portion of cement can be replaced with MSWIFA that has undergone suitable pre-treatments.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, J.; Chi, Y.; Zou, D.; Fu, C.; Huang, Q.; Ni, M. Energy-environment-economy assessment of waste management systems from a life cycle perspective: Model development and case study. Appl. Energy 2014, 114, 400–408. [Google Scholar] [CrossRef]
- Milbrath, M.O.; Wenger, Y.; Chang, C.-W.; Emond, C.; Garabrant, D.; Gillespie, B.W.; Jolliet, O. Apparent Half-Lives of Dioxins, Furans, and Polychlorinated Biphenyls as a Function of Age, Body Fat, Smoking Status, and Breast-Feeding. Environ. Health Perspect. 2009, 117, 417–425. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, L.; Zhou, J.; Liu, J.; Qian, G.; Ohtsuka, N.; Motegi, M.; Oh, K.; Hosono, S. Characteristics of dioxins content in fly ash from municipal solid waste incinerators in China. Chemosphere 2013, 92, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.; Min, X.-B.; Wang, Z.-X.; Pang, Z.-H.; Liang, Y.-J.; Ke, Y. Health and ecological risk assessment of heavy metals pollution in an antimony mining region: A case stu-dy from South China. Environ. Sci. Pollut. Res. 2017, 24, 27573–27586. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, J.; Zhu, G.; Zhang, D.; Zhu, Y.; Chen, Z.; Li, J.; Zhang, H.; Tang, J.; Nie, J.; et al. PCDD/Fs distribution characteristics and health risk assessment in fly ash discharged from MSWIs in China. Ecotoxicol. Environ. Saf. 2017, 139, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Huet, B.; L’Hostis, V.; Miserque, F.; Idrissi, H. Electrochemical behavior of mild steel in concrete: Influence of pH and carbonate content of concrete pore solution. Electrochim. Acta 2006, 51, 172–180. [Google Scholar] [CrossRef]
- Birninyauri, U.A.; Garba, S. Effect of Mechanism of Chloride Ion Attack on Portland Cement Concrete and the Structural Steel Reinforcement. Res. J. Appl. Sci. 2007, 8, 131–135. [Google Scholar]
- Inseok, Y. Binding Behavior of Chloride Ion to React with Cement Hydration Products. J. Am. Chem. Soc. 2010, 101, 1634–1635. [Google Scholar] [CrossRef]
- Jin, S.H.; Yang, H.J.; Hwang, J.P.; Ann, K.Y. Corrosion behaviour of steel in CAC-mixed concrete containing different concentrations of chloride. Constr. Build. Mater. 2016, 110, 227–234. [Google Scholar] [CrossRef]
- Ghazy, A.; Bassuoni, M.T. Resistance of concrete to different exposures with chloride-based salts. Cem. Concr. Res. 2017, 101, 144–158. [Google Scholar] [CrossRef]
- Gao, X.; Wang, W.; Ye, T.; Wang, F.; Lan, Y. Utilization of washed MSWI fly ash as partial cement substitute with the addition of dithiocarbamic chelate. J. Environ. Manag. 2008, 88, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Inoue, K.; Harada, H.; Kawakita, H.; Ohto, K. Leaching behavior of heavy metals with hydrochloric acid from fly ash generated in municipal waste incineration plants. Trans. Nonferr. Met. Soc. China 2011, 21, 1422–1427. [Google Scholar] [CrossRef]
- Bai, J.J.; Zhang, Z.Q.; Yan, D.H.; Li, L. Study on the removal of chlorine and heavy metals in incineration fly ash during water-washing process. Environ. Eng. 2012, 30, 104–108. (In Chinese) [Google Scholar] [CrossRef]
- Chen, W.-S.; Chang, F.-C.; Shen, Y.-H.; Tsai, M.-S.; Ko, C.-H. Removal of chloride from MSWI fly ash. J. Hazard. Mater. 2012, 237–238, 116–120. [Google Scholar] [CrossRef]
- Wang, L.; Li, R.D.; Li, Y.L.; Wei, L.H. Release of Soluble Salts and Heavy Metals during the Short-Time Washing Process of MSWI Fly Ash. Adv. Mater. Res. 2012, 518–523, 3247–3251. [Google Scholar] [CrossRef]
- Wang, X.; Li, A.; Zhang, Z. The Effects of Water Washing on Cement-based Stabilization of MWSI Fly Ash. Procedia Environ. Sci. 2016, 31, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Tian, S.; Liu, L.; Wang, X.; Zhang, Z. Application of washed MSWI fly ash in cement composites: Long-term environmental impacts. Environ. Sci. Pollut. Res. 2018, 25, 12127–12138. [Google Scholar] [CrossRef]
- Pan, J.R.; Huang, C.; Kuo, J.-J.; Lin, S.-H. Recycling MSWI bottom and fly ash as raw materials for Portland cement. Waste Manag. 2008, 28, 1113–1118. [Google Scholar] [CrossRef]
- Ning, S.; Haiyang, W.; Huanan, W.; Hac, K.J.; Qiyong, X. Study of compressive strength and leachability of cement mortars containing municipal solid waste incineration fly ash. Chin. J. Environ. Eng. 2016, 10, 3207–3214. (In Chinese) [Google Scholar] [CrossRef]
- Lee, T.-C.; Chang, C.-J.; Rao, M.-K.; Su, X.-W. Modified MSWI ash-mix slag for use in cement concrete. Constr. Build. Mater. 2011, 25, 1513–1520. [Google Scholar] [CrossRef]
- Tyrer, M. Municipal solid waste incinerator (MSWI) concrete. Eco-Effic. Concr. 2013, 59, 273–310. [Google Scholar] [CrossRef]
- Rafieizonooz, M.; Mirza, J.; Salim, M.R.; Hussin, M.W.; Khankhaje, E. Investigation of coal bottom ash and fly ash in concrete as replacement for sand and cement. Constr. Build. Mater. 2016, 116, 15–24. [Google Scholar] [CrossRef]
- Zheng, L.; Gao, X.; Wang, W.; Li, Z.; Zhang, L.; Cheng, S. Utilization of MSWI fly ash as partial cement or sand substitute with focus on cementing efficiency and health risk assessment. Front. Environ. Sci. Eng. 2019, 14, 5. [Google Scholar] [CrossRef]
- Hayashi, T.; Ono, S. Immobilization of Lead in Fly Ash by Phosphate Compounds. Bunseki Kagaku 2019, 68, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.H.; Chen, J.T.; Guo, M.X. Utilization of pretreated municipal solid waste incineration fly ash for cement-stabilized soil. Waste Manag. 2020, 105, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Marruzzo, G.; Medici, F.; Panei, L. Characteristics and properties of a mixture containing fly ash, hydrated lime, and an organic additive. Environ. Eng. Sci. 2001, 18, 159–166. [Google Scholar] [CrossRef]
- Huang, W.J.; Lo, J.S. Synthesis and efficiency of a new chemical fixation agent for stabilizing MSWI fly ash. J. Hazard. Mater. 2004, 112, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, H.B.; Jin, X. An Analysis Summary of Urban Waste Incineration Technology in Our and Foreign Countries. Ind. Boil. 2003, 5, 15–19. [Google Scholar]
- Qiu, Q.; Jiang, X.; Chen, Z.; Lu, S.; Ni, M. Microwave-Assisted Hydrothermal Treatment with Soluble Phosphate Added for Heavy Metals Solidification in MS-WI Fly Ash. Energy Fuels 2017, 31, 5222–5232. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Z.; Fang, Z.; Qian, Y.; Zhong, P.; Yan, J. Review of harmless treatment of municipal solid waste incineration fly ash. Waste Dispos. Sustain. Energy 2020, 2, 25. [Google Scholar] [CrossRef] [Green Version]
- An, J.; Golestani, B.; Nam, B.H.; Lee, J.L. Sustainable Utilization of MSWI Bottom Ash as Road Construction Materials, Part I: Physical and Mechanical Evaluation. Airfld. Highw. Pavements 2015, 225–235. [Google Scholar] [CrossRef]
- Zhang, Y.; Soleimanbeigi, A.; Likos, W.J.; Edil, T.B. Geotechnical and Leaching Properties of Municipal Solid Waste Incineration Fly Ash for Use as Embankment Fill Material. Transp. Res. Rec. J. Transp. Res. Board 2016, 2579, 70–78. [Google Scholar] [CrossRef]
- Zhipeng, T.; Bingru, Z.; Chengjun, H.; Rongzhi, T.; Huangpu, Z.; Fengting, L. The physiochemical properties and heavy metal pollution of fly ash from municipal solid waste incineration. Process Saf. Environ. Prot. 2015, 98, 333–341. [Google Scholar] [CrossRef]
- Tang, Q.; Pan, L.L.; Gao, Y.F.; Chen, S. Strength and environmental behaviors of solidified fly ash under carbonation effect. Chin. J. Geotech. Eng. 2018, 40, 645–654. (In Chinese) [Google Scholar] [CrossRef]
- Liu, J.; Hu, L.; Tang, L. Utilisation of Municipal Solid Waste Incinerator (MSWI) Fly Ash with Metakaolin for Preparation of Alkali-Activated Cementitious Material. J. Hazard. Mater. 2020, 402, 123451. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hu, Y.; Cheng, H. Municipal solid waste (MSW) incineration fly ash as an important source of heavy metal pollution in China. Environ. Pollut. 2019, 252, 461–475. [Google Scholar] [CrossRef]
- Suthatta, D.; Suched, L.; Dao, J. Mechanisms of chloride and sulfate removalfrommunicipal-solid-waste-incinerationfly ash (MSWIFA): Effect of acid-base solutions. Waste Manag. 2020, 101, 44–53. [Google Scholar]
- Saikia, N.; Kato, S.; Kojima, T. Production of cement clinkers from municipal solid waste incineration (MSWI) fly ash. Waste Manag. 2007, 27, 1178–1189. [Google Scholar] [CrossRef]
- Cobo, M.; Gálvez, A.; Conesa, J.A.; de Correa, C.M. Characterization of fly ash from a hazardous waste incinerator in Medellin, Colombia. J. Hazard. Mater. 2009, 168, 1223–1232. [Google Scholar] [CrossRef]
- Shi, H.S.; Kan, L.L. Leaching behavior of heavy metals from municipal solid wastes incineration (MSWI) fly ash used in concrete. J. Hazard. Mater. 2009, 164, 750–754. [Google Scholar] [CrossRef]
- Guo, X.L.; Shi, H.S.; Huang, J.B. Effects of Cement Additives on Alinite Cement-Based Materials from Municipal Solid Waste Incineration (MSW-I) Fly Ash. Key Eng. Mater. 2017, 727, 1046–1053. [Google Scholar] [CrossRef]
- Ebert, B.A.R.; Steenari, B.-M.; Geiker, M.R.; Kirkelund, G.M. Screening of untreated municipal solid waste incineration fly ash for use in cement-based materials: Chemical and physical properties. SN Appl. Sci. 2020, 2, 802. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Fan, C. Hydration behavior and immobilization mechanism of MgO-SiO2-H2O cementitious system blended with MSWI fly ash. Chemosphere 2020, 250, 126269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Chen, L.; Ma, B.; Zhang, Y.; Ni, W.; Tsang, D.C. Treatment of municipal solid waste incineration fly ash: State-of-the-art technologies and future perspectives—ScienceDirect. J. Hazard. Mater. 2021, 411, 125–132. [Google Scholar] [CrossRef]
- Shim, Y.S.; Rhee, S.W.; Lee, W.K. Comparison of leaching characteristics of heavy metals from bottom and fly ashes in Korea and Japan. Waste Manag. 2005, 25, 473–480. [Google Scholar] [CrossRef]
- Jin, M.T.; Huang, C.J.; Jin, Z.F. The Physical and Chemical Properties of Fly Ash fom Municipal Solid Wastes Incineration. Adv. Mater. Res. 2011, 194–196, 2065–2071. [Google Scholar] [CrossRef]
- Colangelo, F.; Cioffi, R.; Montagnaro, F.; Santoro, L. Soluble salt removal from MSWI fly ash and its stabilization for safer disposal and recovery as road basement material. Waste Manag. 2012, 32, 1179–1185. [Google Scholar] [CrossRef]
- Tasneem, K.M.; Nam, B.H.; Eun, J. Sustainable Utilization of MSWI Bottom Ash as Road Construction Materials, Part II: Chemical and Environmental Characterization. Airfld. Highw. Pavements 2015, 2015, 593–604. [Google Scholar] [CrossRef]
- Xinghua, H.; Shujing, Z.; Hwang, J.Y. Physical and Chemical Properties of MSWI Fly Ash; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 451–459. [Google Scholar]
- Aziz, M.; Sheikh, F.N.; Qureshi, M.U.; Rasool, A.M.; Irfan, M. Experimental Study on Endurance Performance of Lime and Cement-Treated Cohesive Soil. Geotech. Eng. 2021, 25, 3306–3318. [Google Scholar] [CrossRef]



| Country/ Region | Remarks | Moisture Content (%) | Specific Gravity (g/cm3) | Specific Surface Area (m2/g) | pH | Plasticity Index | Particle Size Distribution/mm | ||
|---|---|---|---|---|---|---|---|---|---|
| <0.005 | 0.005–0.075 | 0.075–2 | |||||||
| FA, Hezhou | China | 6.57 | 2.1 | 4.862 | 12.09 | 9.29 | 3.6% | 2.5% | 94.0% |
| FA, Nanning | 6.11 | 2.03 | 5.893 | 12.20 | 9.60 | 2.7% | 2.0% | 95.3% | |
| FA, Suzhou | 2.50 | 2.28 | 8.008 | 12.90 | 30.84 | 12.6% | 9.0% | 78.0% | |
| FA, Huizhou | 4.60 | 2.46 | 6.000 | 12.30 | - | 21.5% | 11.5% | 50.0% | |
| FA, American | - American | 10.1 | 2.61 | - | - | - | 4.8% | 17.2% | 48.3% |
| FA, Japan | - Janpan | 1.28 | 3.03 | 1.07 | - | - | 0.1% | 4.1% | 95.8% |
| Sand soil, Hezhou | China | 14.5 | 2.61 | - | 5.89 | 9 | 11.05% | 42.02% | 46.94% |
| Country/ Region | Remarks | Zn | Cu | Pb | Cd | Cr | Ni | Hg |
|---|---|---|---|---|---|---|---|---|
| (mg/kg) | ||||||||
| FA, Hezhou | China | 7400 | 730 | 2200 | 340 | 97 | 600 | - |
| FA, Nanning | 6600 | 400 | 1200 | 260 | 84 | - | - | |
| FA, Hangzhou | 4745 | 587 | 3084 | 125 | 161 | - | 5.8 | |
| FA, Shanghai | 3112 | 422 | 3720 | 21 | 232 | 20 | 18.8 | |
| FA, Japan | - | 5000 | 420 | 1100 | 90 | 89 | 18 | - |
| FA, Korean | - | 7300 | 1000 | 3000 | 280 | 160 | 33 | - |
| FA, Italy | - | 8400 | 815 | 8950 | 88 | 270 | 117 | - |
| FA, America | - | 15,500 | 775 | 5250 | 220 | 360 | 1.3–1.4 | 6.4 |
| Number | Content of MSWIFA (by Mass)/% | Content of OPC (by Mass)/% | Moisture Content of Soil (by Mass)/% | Ratio of Water-Cement |
|---|---|---|---|---|
| 1 | 5 | 15 | 30 | 0.5 |
| 2 | 10 | 10 | ||
| 3 | 15 | 5 |
| Characteristic | Raw Ash | Ferrous Sulphate | Organics Chelating Agent | Sugarcane Ash | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | Age | 2% | 4% | 6% | 1.25% | 2.50% | 5.00% | 4% | 8% | 12% | |||||||||||||||||||
| Day | Strength (MPa) Leaching (mg/L) | Strength (MPa) Leaching (mg/L) | Relative Growth Rate | Strength (MPa) Leaching (mg/L) | Relative Growth Rate | Strength (MPa) Leaching (mg/L) | Relative Growth Rate | Strength (MPa) Leaching (mg/L) | Relative Growth Rate | Strength (MPa) Leaching (mg/L) | Relative Growth Rate | Strength (MPa) Leaching (mg/L) | Relative Growth Rate | Strength (MPa) Leaching (mg/L) | Relative Growth Rate | Strength (MPa) Leaching (mg/L) | Relative Growth Rate | Strength (MPa) Leaching (mg/L) | Relative Growth Rate | ||||||||||
| Peak strength air | 7 | 1.16 | 1.15 | −1.29% | PE | 1.36 | 17.41% | PE | 1.52 | 31.03% | PE | 1.11 | −4.74% | NE | 1.16 | −0.26% | NS | 1.31 | 13.10% | NS | 1.25 | 8.10% | PE | 1.85 | 59.31% | PE | 2.13 | 83.79% | PE |
| 14 | 1.73 | 1.65 | −4.74% | 1.72 | −0.87% | 2.52 | 45.66% | 1.56 | −10.06% | 2.00 | 15.43% | 2.17 | 25.14% | 2.54 | 46.99% | 2.75 | 59.08% | 3.10 | 79.13% | ||||||||||
| 28 | 2.80 | 3.03 | 8.30% | 3.12 | 11.62% | 3.52 | 25.89% | 1.73 | −38.23% | 2.22 | −20.71% | 2.49 | −10.98% | 2.92 | 4.26% | 3.13 | 12.09% | 3.47 | 23.96% | ||||||||||
| IWA & IWC of FS | IWA & IWC of OCA | IWA & IWC of SA | |||||||||||||||||||||||||||
| Peak strength water | 7 | 0.67 | 0.90 | 34.08% | PE | 0.92 | 38.12% | PE | 1.00 | 49.03% | PE | 0.67 | −0.15% | NE | 0.70 | 4.78% | NE | 0.75 | 12.41% | NS | 0.88 | 31.69% | PE | 1.05 | 57.55% | PE | 1.11 | 65.62% | PE |
| 14 | 0.98 | 1.11 | 13.28% | 1.13 | 15.02% | 1.19 | 21.14% | 0.79 | −19.20% | 0.81 | −17.57% | 0.81 | −17.36% | 1.11 | 13.38% | 1.32 | 34.83% | 1.41 | 44.23% | ||||||||||
| 28 | 1.04 | 1.17 | 13.33% | 1.25 | 20.48% | 1.37 | 32.37% | 0.89 | −14.11% | 0.97 | −6.67% | 1.07 | 3.00% | 1.46 | 41.06% | 1.49 | 43.48% | 1.54 | 49.08% | ||||||||||
| IWA & IWC of FS | IWA & IWC of OCA | IWA & IWC of SA | |||||||||||||||||||||||||||
| Leaching concentration of Zn | 7 | 13.25 | 13.05 | −1.54% | LC | 12.75 | −3.80% | PE | 11.87 | −10.44% | PE | 11.96 | −9.74% | PE | 10.15 | −23.40% | PE | 8.29 | −37.47% | PE | 15.04 | 13.48% | NE | 15.31 | 15.51% | NE | 14.34 | 8.19% | NE |
| 14 | 12.91 | 13.17 | 2.03% | 12.15 | −5.87% | 11.07 | −14.24% | 12.00 | −7.03% | 10.27 | −20.44% | 8.08 | −37.44% | 15.73 | 21.86% | 15.50 | 20.08% | 14.39 | 11.48% | ||||||||||
| 28 | 13.86 | 13.86 | 0.00% | 12.19 | −12.04% | 11.30 | −18.46% | 12.05 | −13.05% | 9.61 | −30.67% | 8.86 | −36.05% | 15.63 | 12.78% | 15.03 | 8.45% | 14.23 | 2.68% | ||||||||||
| LCA & DWC of FS | LCA & DDWC of OCA | LCA & DDWC of SA | |||||||||||||||||||||||||||
| Leaching concentration of Cu | 7 | 1.09 | 0.99 | −9.01% | NS | 0.86 | −20.96% | PE | 0.74 | −31.99% | PE | 1.00 | −7.81% | NS | 0.86 | −20.59% | PE | 0.70 | −35.66% | PE | 1.11 | 2.02% | NE | 1.10 | 1.10% | NE | 1.19 | 9.37% | NE |
| 14 | 1.08 | 0.90 | −16.74% | 0.81 | −25.07% | 0.77 | −28.77% | 1.01 | −6.75% | 0.80 | −25.99% | 0.76 | −29.42% | 1.17 | 8.23% | 1.18 | 9.16% | 1.13 | 4.53% | ||||||||||
| 28 | 0.97 | 1.03 | 6.40% | 0.89 | −8.06% | 0.75 | −22.52% | 1.00 | 3.31% | 0.80 | −17.46% | 0.73 | −25.10% | 1.19 | 22.93% | 1.13 | 16.74% | 1.03 | 6.40% | ||||||||||
| LCA & DDWC of FS | LCA & DDWC of OCA | LCA & DDWC of SA | |||||||||||||||||||||||||||
| Leaching concentration of Pb | 7 | 1.59 | 1.41 | −11.80% | PE | 1.32 | −17.45% | PE | 1.10 | −30.95% | PE | 1.29 | −19.02% | PE | 1.26 | −20.90% | PE | 1.04 | −34.46% | PE | 1.53 | −3.95% | LC | 1.52 | −4.58% | PE | 1.42 | −10.86% | PE |
| 14 | 1.51 | 1.37 | −9.39% | 1.37 | −9.39% | 1.10 | −27.25% | 1.31 | −13.36% | 1.26 | −16.47% | 1.07 | −29.30% | 1.54 | 1.85% | 1.50 | −0.79% | 1.48 | −2.12% | ||||||||||
| 28 | 1.62 | 1.42 | −12.60% | 1.21 | −25.32% | 1.30 | −19.70% | 1.29 | −20.32% | 1.25 | −22.79% | 1.07 | −33.91% | 1.58 | −2.41% | 1.54 | −4.88% | 1.43 | −11.67% | ||||||||||
| LCA & DDWC of FS | LCA & DDWC OCA | LCA & DDWC of SA | |||||||||||||||||||||||||||
| Leaching concentration of Cd | 7 | 0.90 | 0.61 | −32.30% | PE | 0.59 | −34.52% | PE | 0.51 | −43.40% | PE | 0.80 | −11.21% | PE | 0.75 | −16.76% | PE | 0.51 | −43.40% | PE | 1.00 | 10.99% | NE | 0.90 | −0.11% | NE | 0.85 | −5.66% | NE |
| 14 | 0.90 | 0.66 | −26.75% | 0.60 | −33.41% | 0.54 | −40.07% | 0.84 | −6.99% | 0.79 | −12.54% | 0.51 | −43.84% | 1.03 | 14.32% | 1.02 | 13.21% | 1.02 | 13.21% | ||||||||||
| 28 | 0.90 | 0.62 | −31.19% | 0.57 | −36.74% | 0.57 | −36.74% | 0.81 | −9.77% | 0.83 | −8.44% | 0.50 | −44.28% | 1.08 | 19.87% | 1.04 | 15.43% | 1.14 | 26.53% | ||||||||||
| LCA & DDWC of FS | LCA & DDWC of OCA | LCA & DLC of SA | |||||||||||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Li, J.; Hu, L.; Zhang, X.; Ding, S.; Li, H. Strength and Environmental Behaviours of Municipal Solid Waste Incineration Fly Ash for Cement-Stabilised Soil. Sustainability 2023, 15, 364. https://doi.org/10.3390/su15010364
Liu Z, Li J, Hu L, Zhang X, Ding S, Li H. Strength and Environmental Behaviours of Municipal Solid Waste Incineration Fly Ash for Cement-Stabilised Soil. Sustainability. 2023; 15(1):364. https://doi.org/10.3390/su15010364
Chicago/Turabian StyleLiu, Zonghui, Jiaqi Li, Liqiang Hu, Xiaolei Zhang, Shiying Ding, and Haodong Li. 2023. "Strength and Environmental Behaviours of Municipal Solid Waste Incineration Fly Ash for Cement-Stabilised Soil" Sustainability 15, no. 1: 364. https://doi.org/10.3390/su15010364
APA StyleLiu, Z., Li, J., Hu, L., Zhang, X., Ding, S., & Li, H. (2023). Strength and Environmental Behaviours of Municipal Solid Waste Incineration Fly Ash for Cement-Stabilised Soil. Sustainability, 15(1), 364. https://doi.org/10.3390/su15010364








