Enhanced Rate of Enzymatic Saccharification with the Ionic Liquid Treatment of Corn Straw Activated by Metal Ion Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Metal Ion Solution Pretreatment Experiment
2.3. Ionic Liquid Treatment Experiment
2.4. Analysis Method
2.5. Structural and Morphological Analysis
2.6. Statistical Analysis Methods
3. Results
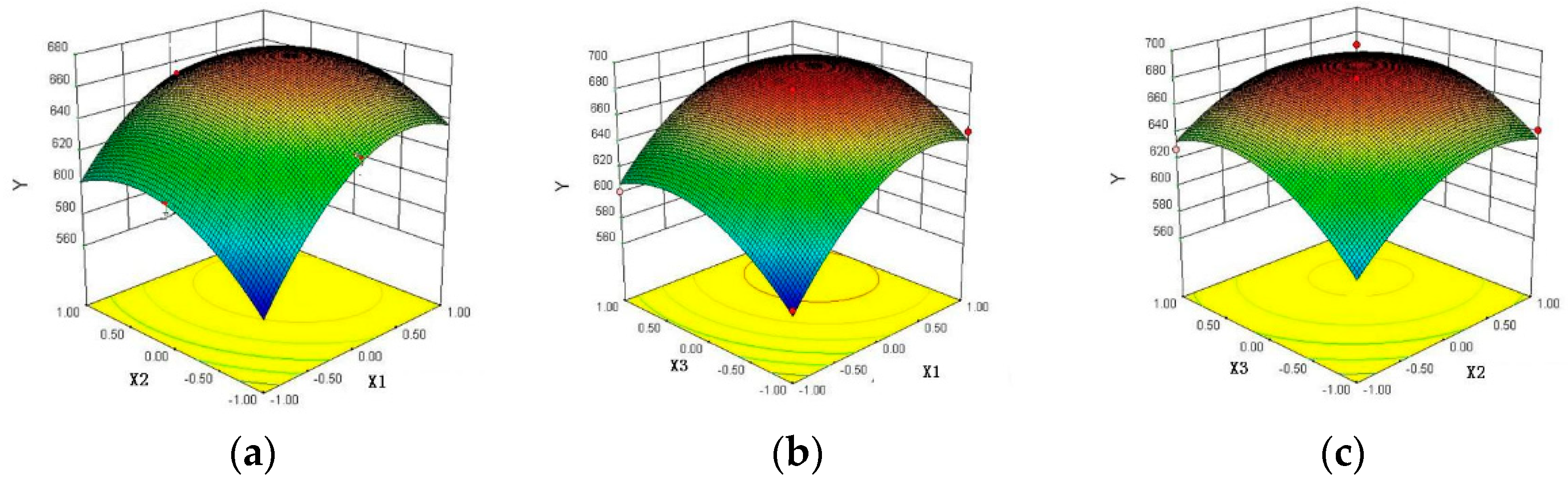
3.1. Optimization Experiment Results of Response Surface Method
3.2. Analysis of Components
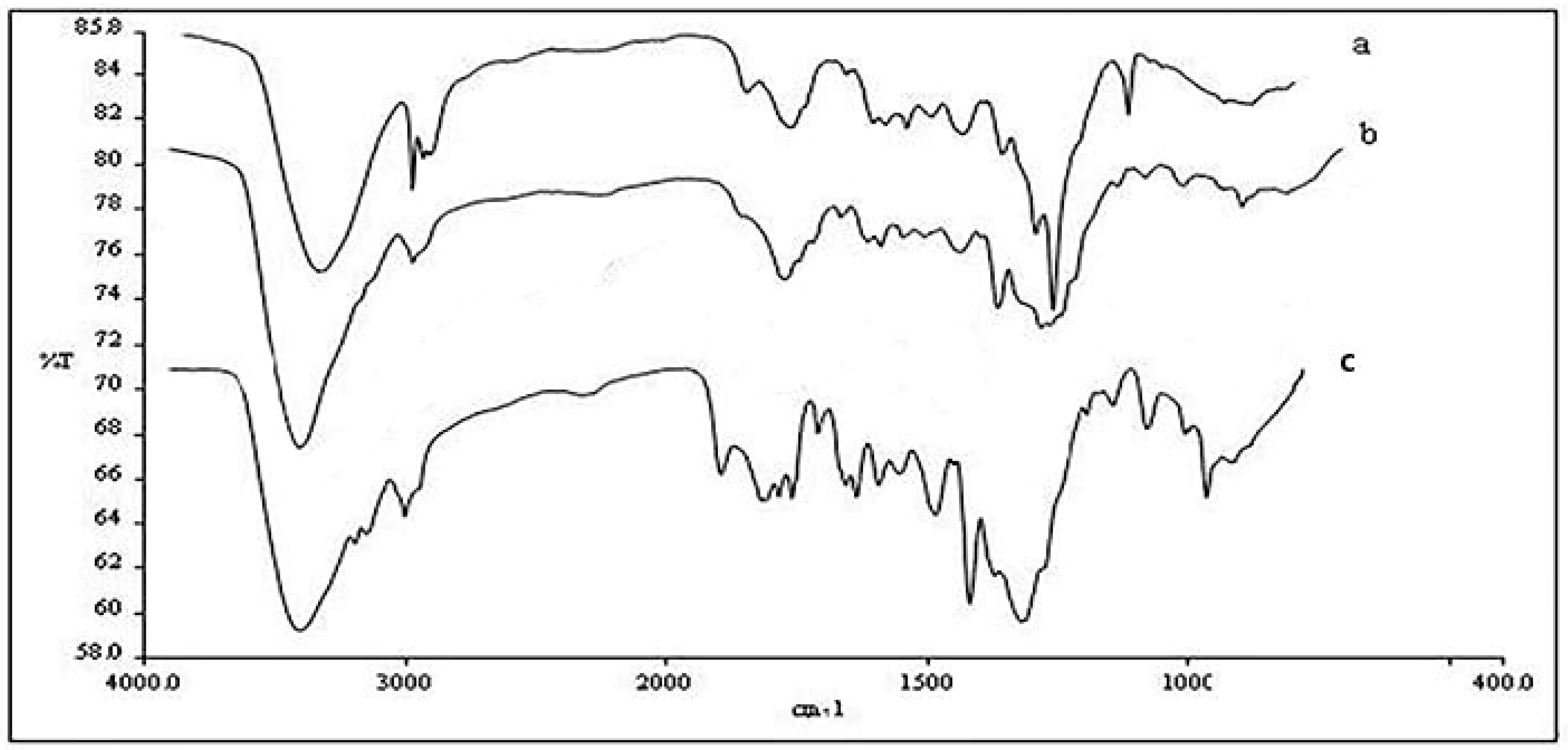
3.3. Fourier Transformation Infrared Spectroscopy Analysis
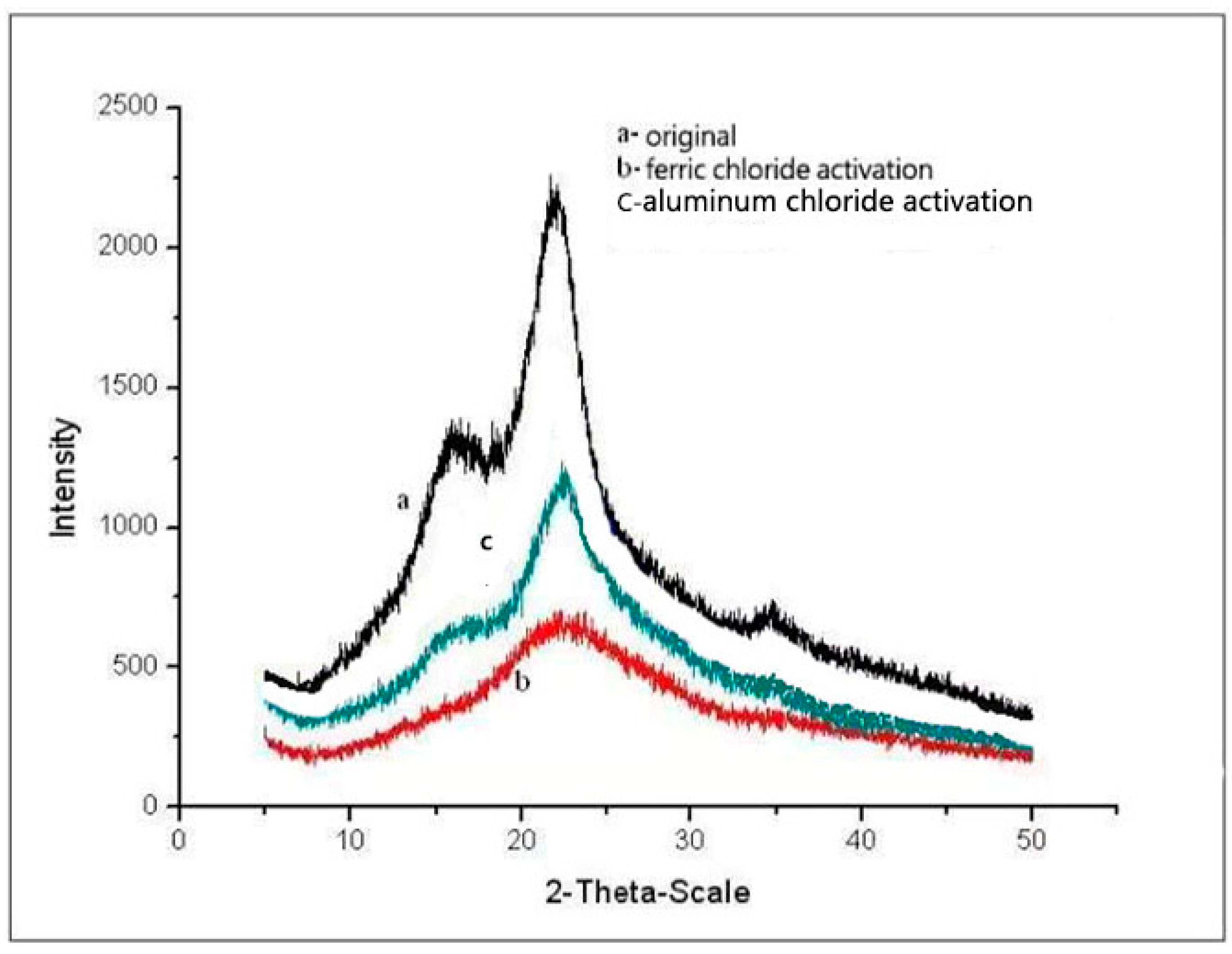
3.4. X-ray Diffraction Analysis
3.5. Scanning Electron Microscope Analysis
4. Discussion
4.1. Effect of Response Surface on Enzymatic Hydrolysis Results
4.2. Effect of Ionic Liquids on Components
4.3. Effect of Ionic Liquids on Crystallinity
4.4. Effect of Ionic Liquid on Morphology
5. Conclusions
- (1)
- Response surface methodology was used to optimize the enzymatic hydrolysis conditions of corn straw after metal ion activation by ionic liquid treatment. The optimization results are as follows: ionic liquid treatment of ferric chloride solution activation, substrate mass fraction of 4.2%, reaction temperature of 85 °C, reaction time of 185 min. The aluminum chloride solution was activated by ionic liquid treatment. The substrate mass fraction was 3.6%, the reaction temperature was 105 °C, and the reaction time was 230 min. Through the experiment of enzymatic hydrolysis, it was found that the enzymatic hydrolysis value of the material activated by ionic liquid treatment of ferric chloride solution was the largest, reaching 706.52 mg/g.
- (2)
- The results of component analysis showed that the cellulose content of corn straw was significantly increased after the activation of metal ions by ionic liquid treatment, and the yield of reducing sugar after cellulase hydrolysis was increased.
- (3)
- FTIR analysis showed that ionic liquids could dissolve more cellulose, so the hydrogen bonds of cellulose weakened and the spatial structure changed. The XRD results showed that the relative crystallinity of corn straw material was reduced to a certain extent compared with the raw material after the activation of metal ion solution by ionic liquid. The SEM results showed that the treated material became fluffy, the surface was uneven, and more holes appeared, which was more conducive to the enzymatic hydrolysis of cellulase.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, H.; Zheng, P.; Bilal, M.; Xie, F.; Zeng, Q.; Zhu, C.; Yang, R.; Wang, Z. Efficient Bio-Butanol Production from Lignocellulosic Waste by Elucidating the Mechanisms of Clostridium Acetobutylicum Response to Phenolic Inhibitors. Sci. Total Environ. 2020, 710, 136399. [Google Scholar] [CrossRef]
- Han, X.; Guo, Y.; Liu, X.; Xia, Q.; Wang, Y. Catalytic Conversion of Lignocellulosic Biomass into Hydrocarbons: A Mini Review. Catal. Today 2019, 319, 2–13. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, P.; Hu, C. Controlling the Cleavage of the Inter- and Intra-Molecular Linkages in Lignoc-ellulosic Biomass for Further Biorefining: A Review. Bioresour. Technol. 2018, 256, 466–477. [Google Scholar] [CrossRef]
- Pan, Q.; Shi, P.; Xu, W.; Li, X.; Shi, J. Research Progress on Preparation of Xylose Oligosaccharide from Corn Stalk. China For. Prod. Ind. 2020, 57, 8–12. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Yu, Z.; Shang, J. Study on Preparation and Properties of Sound Absorbing and Heat Insulating Corn Straw Pith Board. China For. Prod. Ind. 2019, 46, 27–31. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Li, X.; Zuo, Y. Research Status of Resource Utilization for Rice and Wheat Straw. China For. Prod. Ind. 2021, 58, 1–5. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, M.; Zhao, H.; Ma, L.; Xiao, J. Research Status on Liquid-Phase Catalytic Depolymerization of Lignin. China For. Prod. Ind. 2020, 57, 1–7. [Google Scholar] [CrossRef]
- Cheng, J.; Xu, W.; Shi, J.; Li, X.; Hong, Y. Study on the Synthesis Process of Soybean Protein Adhesive Modified by Bioethanol Lignin-Pae. China For. Prod. Ind. 2021, 58, 7–11. [Google Scholar] [CrossRef]
- Wang, M.; Wu, M.; Huo, H. Life-cycle energy and greenhouse gasemission impacts of different corn ethanol plant types. Environ. Res. Lett. 2007, 2, 024001. [Google Scholar] [CrossRef]
- Pang, B.; Sun, Z.H.; Wang, L.; Chen, W.J.; Sun, Q.; Cao, X.F.; Shen, X.J.; Xiao, L.; Yan, J.L.; Deuss, P.J.; et al. Improved value and carbon footprint by complete utilization of corncob lignocellulose. Chem. Eng. J. 2021, 419, 129565. [Google Scholar] [CrossRef]
- Liang, C.; Feng, Q.; Lu, S.; Wang, Q.; Hu, Y.; Wang, Z.; Wang, W.; Qi, W. Synergistic Enhancement Effect of Compound Additive of Organic Alcohols and Biosurfactant on Enzymatic Hydrolysis of Lignocellulose. Fermentation 2022, 8, 725. [Google Scholar] [CrossRef]
- Sankaran, R.; Parra-Cruz, R.A.; Pakalapati, H.; Show, P.L.; Ling, T.C.; Chen, W.-H.; Tao, Y. Recent advances in the pretreat-ment of microalgal and lignocellulosic biomass: A comprehensive review. Bioresour. Technol. 2022, 298, 122476. [Google Scholar] [CrossRef]
- Uju; Goto, M.; Kamiya, N. Powerful peracetic acid–ionic liquid pretreatment process for the efficient chemical hydrolysis of lignocellulosic biomass. Bioresour. Technol. 2016, 214, 487–495. [Google Scholar] [CrossRef]
- Wang, S.Q.; Li, F.; Wu, D.; Zhang, P.Y.; Wang, H.J.; Tao, X.; Ye, J.P.; Nabi, M. Enzyme pretreatment enhancing biogas yield from corn stover: Feasibility, optimization, and mechanism analysis. J. Agric. Food Chem. 2018, 66, 10026–10032. [Google Scholar] [CrossRef]
- Sun, X.; Liu, S.; Zhang, X.Y.; Tao, Y.; Boczkaj, G.; Yoon, J.Y.; Xuan, X.X. Recent advances in hydrodynamic cavitation-based pretreatments of lignocellulosic biomass for valorization. Bioresour. Technol. 2021, 214, 126251. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, P. Progress of Ionic Liquids-Green Preparation and Application Research in Environmental Remediation. Chin. J. Org. Chem. 2018, 38, 3176–3188. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Guo, W. Preparation Carbon Fibers from Cellulose by Ionic Liquid Method. J. Funct. Mater. 2019, 50, 7172–7175. [Google Scholar] [CrossRef]
- Polaskova, M.; Cermak, R.; Verney, V.; Ponizil, P.; Commereuc, S.; Gomes, M.F.C.; Padua, A.A.; Mokrejs, P.; Machovsky, M. Preparation of Microfibers from Wood/Ionic Liquid Solutions. Carbohydr. Polym. 2013, 92, 214–217. [Google Scholar] [CrossRef]
- Xiao, W.; Yin, W.; Xia, S.; Ma, P. The Study of Factors Affecting the Enzymatic Hydrolysis of Cellulose after Ionic Liquid Pretreatment. Carbohydr. Polym. 2012, 87, 2019–2023. [Google Scholar] [CrossRef]
- Iguchi, M.; Aida, T.M.; Watanabe, M.; Smith, R.L. Dissolution and Recovery of Cellulose from 1-Butyl-3-Methylimidazolium Chloride in Presence of Water. Carbohydr. Polym. 2013, 92, 651–658. [Google Scholar] [CrossRef]
- Xu, F.; Chen, Y.; You, T.; Mao, J.; Zhang, X. Research Progress on Mechanism of Cellulose Dissolution. J. For. Eng. 2019, 4, 1–7. [Google Scholar] [CrossRef]
- Ziembowicz, F.I.; Mattiazzi, L.M.; Bender, C.R.; Frizzo, C.P.; da Rosa, M.B.; Reichert, J.M.; Kloster, C.L.; Villetti, M.A. Thermodynamics of aggregation and modulation of Rheo-Thermal properties of hydroxypropyl cellulose by imidazolium ionic liquids. J. Mol. Liq. 2022, 359, 119314. [Google Scholar] [CrossRef]
- Erdmenger, T.; Haensch, C.; Hoogenboom, R.; Schubert, U.S. Homogeneous tritylation of cellulose in 1-butyl-3-methylimidazolium chloride. Macromol. Biosci. 2007, 7, 440–445. [Google Scholar] [CrossRef]
- FitzPatrick, M.; Champagne, P.; Cunningham, M.F. The effect of subcritical carbon dioxide on the dissolu-tion of cellulose in the ionic liquid 1-ethyl-3-methylimidazolium acetate. Cellulose 2012, 19, 37–44. [Google Scholar] [CrossRef]
- Wei, J.; Gao, H.; Li, Y.; Nie, Y. Research on the degradation behaviors of wood pulp cellulose in ionic liquids. J. Mol. Liq. 2022, 365, 119071. [Google Scholar] [CrossRef]
- Zhang, H.; Ionita, A.; Seriñan, P.F.; Ferrer, M.L.; Rodríguez, M.A.; Tamayo, A.; Alons, F.R.; Monte, F.D.; Gutiérrez, M.C. Easy and Efficient Recovery of EMIMCl from Cellulose Solutions by Addition of Acetic Acid and the Transition from the Original Ionic Liquid to an Eutectic Mixture. Molecules 2022, 27, 987. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Yin, D.; Zhang, J.; Mi, Q.; Lu, H.; Liang, D.; Zhang, J. The solution state and dissolution process of cellulose in ionic-liquid-based solvents with different hydrogen-bonding basicity and microstructures. Green Chem. 2022, 24, 3824–3833. [Google Scholar] [CrossRef]
- Taokaew, S.; Kriangkrai, W. Recent Progress in Processing Cellulose Using Ionic Liquids as Solvents. Polysaccharides 2022, 3, 671–691. [Google Scholar] [CrossRef]
- Chaban, V.V.; Prezhdo, O.V. Nanoscale Carbon Greatly Enhances Mobility of a Highly Viscous Ionic Liquid. ACS Nano 2014, 8, 8190–8197. [Google Scholar] [CrossRef]
- Ohba, T.; Hata, K.; Chaban, V.V. Nanocrystallization of Imidazolium Ionic Liquid in Carbon Nanotubes. J. Phys. Chem. C 2015, 119, 28424–28429. [Google Scholar] [CrossRef]
- Ohba, T.; Chaban, V.V. A Highly Viscous Imidazolium Ionic Liquid inside Carbon Nanotubes. J. Phys. Chem. B 2014, 118, 6234–6240. [Google Scholar] [CrossRef]
- Xie, J.; Cheng, Z.; Zhu, S.; Xu, J. Lewis Base Enhanced Neutral Deep Eutectic Solvent Pretreatment for Enzymatic Hydrolysis of Corn Straw and Lignin Characterization. Renew. Energy 2022, 188, 320–328. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of Cellulase Activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Adekunle, A.E.; Rabeya, T.; Jehadin, F.; Asad, M.A.; Ayodele, O.O.; Islam, M.S. Compositional and Micr-ostructural Changes in Compressed Hot Water Pretreated Corn Stalk. Curr. Res. Green Sustain. Chem. 2021, 4, 100057. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Liu, C.G.; Wyman, C.E. Partial Flow of Compressed-Hot Water through Corn Stover to Enhance Hemicellulose Sugar Recovery and Enzymatic Digestibility of Cellulose. Bioresour. Technol. 2005, 96, 1978–1985. [Google Scholar] [CrossRef]
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladisch, M.R.; Lee, Y.Y. Comparative Sugar Recovery Data from Laboratory Scale Application of Leading Pretreatment Technologies to Corn Stover. Bioresour. Technol. 2005, 96, 2026–2032. [Google Scholar] [CrossRef]



| Factor | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| X1 Substrate mass fraction (%) | 2 | 4 | 6 |
| X2 Reaction temperature (°C) | 80 | 100 | 120 |
| X3 Reaction time (h) | 2 | 4 | 6 |
| Test Number | Factor | Reducing Sugar Yield (mg·g−1) | ||
|---|---|---|---|---|
| X1 | X2 | X3 | ||
| 1 | −1 | 1 | 0 | 623.76 a, 603.57 b |
| 2 | 0 | 0 | 0 | 700.67, 679.74 |
| 3 | −1 | −1 | 0 | 583.45, 574.12 |
| 4 | 0 | 1 | 1 | 691.23, 669.82 |
| 5 | 1 | −1 | 0 | 678.76, 656.52 |
| 6 | 1 | 1 | 0 | 685.48, 649.83 |
| 7 | −1 | 0 | −1 | 592.13, 566.92 |
| 8 | 0 | −1 | 1 | 663.21, 627.56 |
| 9 | 0 | 0 | 0 | 701.52, 678.12 |
| 10 | −1 | 0 | 1 | 623.87, 601.48 |
| 11 | 0 | 0 | 0 | 700.45, 681.25 |
| 12 | 1 | 0 | 1 | 672.43, 658.53 |
| 13 | 0 | 1 | −1 | 670.52, 641.79 |
| 14 | 0 | −1 | −1 | 599.78, 569.44 |
| 15 | 1 | 0 | −1 | 675.78, 647.52 |
| Item | Coefficient | Standard Error | t | P |
|---|---|---|---|---|
| constant | 2334.81 a, 679.703 b | 2592.76 a, 9.221 b | 16.19 a, 73.713 b | 0.0034 a, 0.0013 b |
| X1 | 1457.47, 33.289 | 1457.47, 5.647 | 65.31, 5.895 | 0.0005, 0.0021 |
| X2 | 2656.84, 17.171 | 2656.84, 5.647 | 16.59, 3.041 | 0.0096, 0.0032 |
| X3 | 1582.88, 16.465 | 1582.88, 5.647 | 9.88, 2.916 | 0.0256, 0.0294 |
| X1 X1 | −44.98, −33.617 | 3.286, 8.312 | −13.688, −4.045 | 0.0001, 0.0102 |
| X2 X2 | −17.74, −25.077 | 3.286, 8.312 | −5.398, −3.017 | 0.0017, 0.0211 |
| X3 X3 | −23.71, −27.474 | 3.286, 8.312 | −7.217, −3.305 | 0.0004, 0.0303 |
| X1 X2 | −8.32, −9.035 | 3.157, 7.986 | −2.635, −1.131 | 0.0388, 0. 0395 |
| X1 X3 | −10.42, −5.888 | 3.157, 7.986 | −3.300, −0.737 | 0.0164, 0.0441 |
| X2 X3 | −9.73, −7.522 | 3.157, 7.986 | −3.081, −0.942 | 0.0216, 0.0492 |
| Source of Variance | Degree of Freedom | Sum of Squares | Corrects the Sum of Squares | Correct Variance | F | P |
|---|---|---|---|---|---|---|
| Regression | 9 | 23,009.2 a, 22,159.3 b | 23,009.2 a, 22,159.3 b | 2556.58 a, 2462.14 b | 64.13 a, 9.65 b | 0.001 a, 0.011 b |
| Linear | 3 | 12,312.1, 13,392.7 | 12,312.1, 13,392.7 | 4104.02, 4464.24 | 102.95, 17.50 | 0.001, 0.004 |
| Square | 3 | 9607.8, 8075.0 | 9607.8, 8075.0 | 3202.61, 2691.68 | 80.34, 10.55 | 0.001, 0.013 |
| Interaction | 3 | 1089.3, 691.5 | 1089.3, 691.5 | 363.11, 230.51 | 9.11, 0.90 | 0.018, 0.501 |
| Residual Error | 5 | 199.3, 1275.4 | 199.3, 1275.4 | 39.86, 255.08 | ||
| Missing Fit | 3 | 197.5, 1270.5 | 197.5, 1270.5 | 65.84, 423.50 | 72.5, 172.84 | 0.014, 0.006 |
| Pure Error | 2 | 1.8, 4.9 | 1.8, 4.9 | 0.91, 2.45 | ||
| Total | 14 | 23208.5, 23434.7 |
| Corn Straw | Cellulose | Hemicellulose | Lignin | Soluble Material | Ash | |
|---|---|---|---|---|---|---|
| Not Processed | 0.600 | 0.185 | 0.189 | 0.046 | 0.175 | 0.004 |
| Iron Trichloride Solution + Ionic Liquid Treatment | 0.600 | 0.311 | 0.155 | 0.045 | 0.077 | 0.010 |
| Aluminum Trichloride Solution + Ionic Liquid Treatment | 0.600 | 0.297 | 0.157 | 0.052 | 0.081 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, G.; Fan, X.; Wang, F.; Luo, Y.; Liang, D.; Han, Y.; Gao, P.; Wang, Q.; Wang, J.; Yu, C.; et al. Enhanced Rate of Enzymatic Saccharification with the Ionic Liquid Treatment of Corn Straw Activated by Metal Ion Solution. Sustainability 2023, 15, 834. https://doi.org/10.3390/su15010834
Zeng G, Fan X, Wang F, Luo Y, Liang D, Han Y, Gao P, Wang Q, Wang J, Yu C, et al. Enhanced Rate of Enzymatic Saccharification with the Ionic Liquid Treatment of Corn Straw Activated by Metal Ion Solution. Sustainability. 2023; 15(1):834. https://doi.org/10.3390/su15010834
Chicago/Turabian StyleZeng, Guoming, Xuanhao Fan, Fei Wang, Yang Luo, Dong Liang, Yongguang Han, Pei Gao, Quanfeng Wang, Jiale Wang, Chunyi Yu, and et al. 2023. "Enhanced Rate of Enzymatic Saccharification with the Ionic Liquid Treatment of Corn Straw Activated by Metal Ion Solution" Sustainability 15, no. 1: 834. https://doi.org/10.3390/su15010834
APA StyleZeng, G., Fan, X., Wang, F., Luo, Y., Liang, D., Han, Y., Gao, P., Wang, Q., Wang, J., Yu, C., Jin, L., & Sun, D. (2023). Enhanced Rate of Enzymatic Saccharification with the Ionic Liquid Treatment of Corn Straw Activated by Metal Ion Solution. Sustainability, 15(1), 834. https://doi.org/10.3390/su15010834








