Stability of Functionally Modified Biochar: The Role of Surface Charges and Surface Homogeneity
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Modification of Biochars
2.2. Determination of Biochar Stability
2.3. Batch Sorption Experiments
2.3.1. Batch Sorption Isotherm Experiments
2.3.2. Kinetic Study
2.4. Characterization of Biochars
2.5. Boehm Titration
2.6. Statistics
3. Results and Discussion
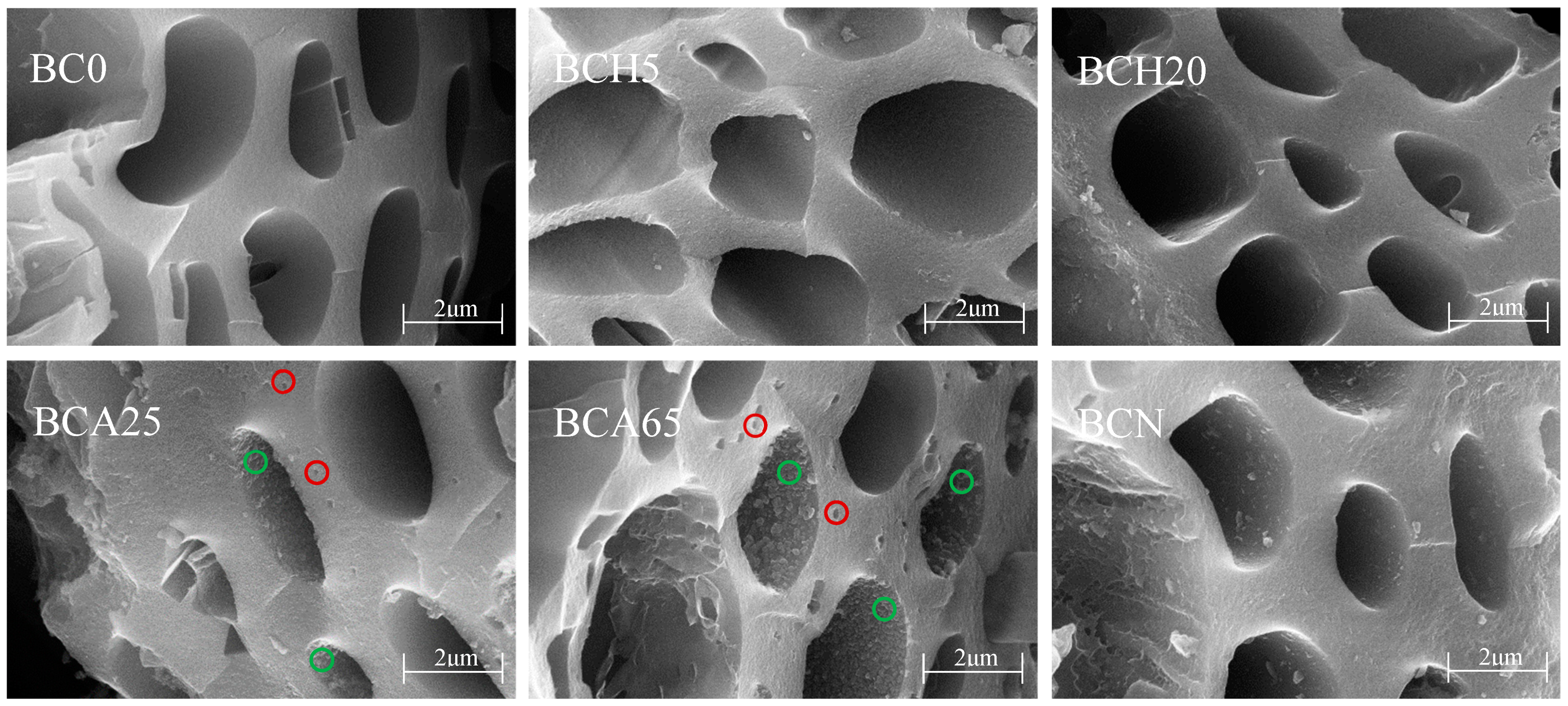
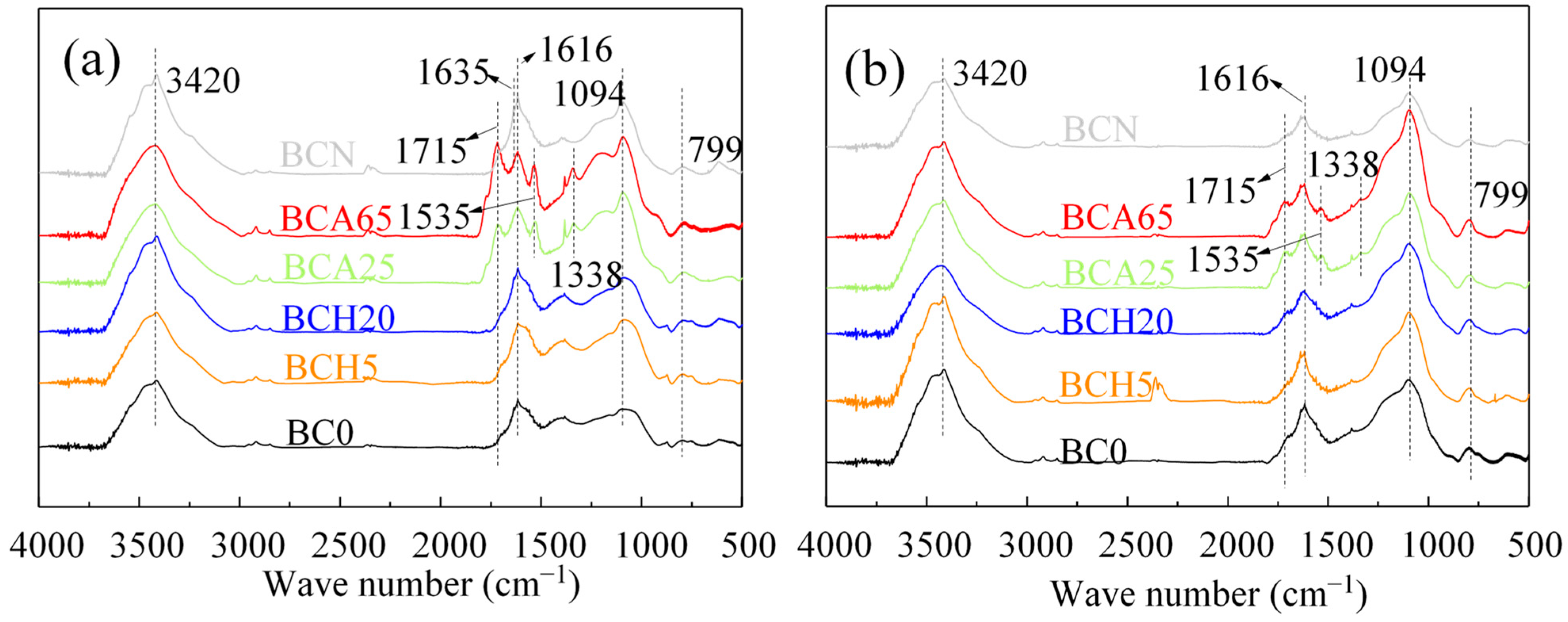
3.1. The Properties of Modified Biochars
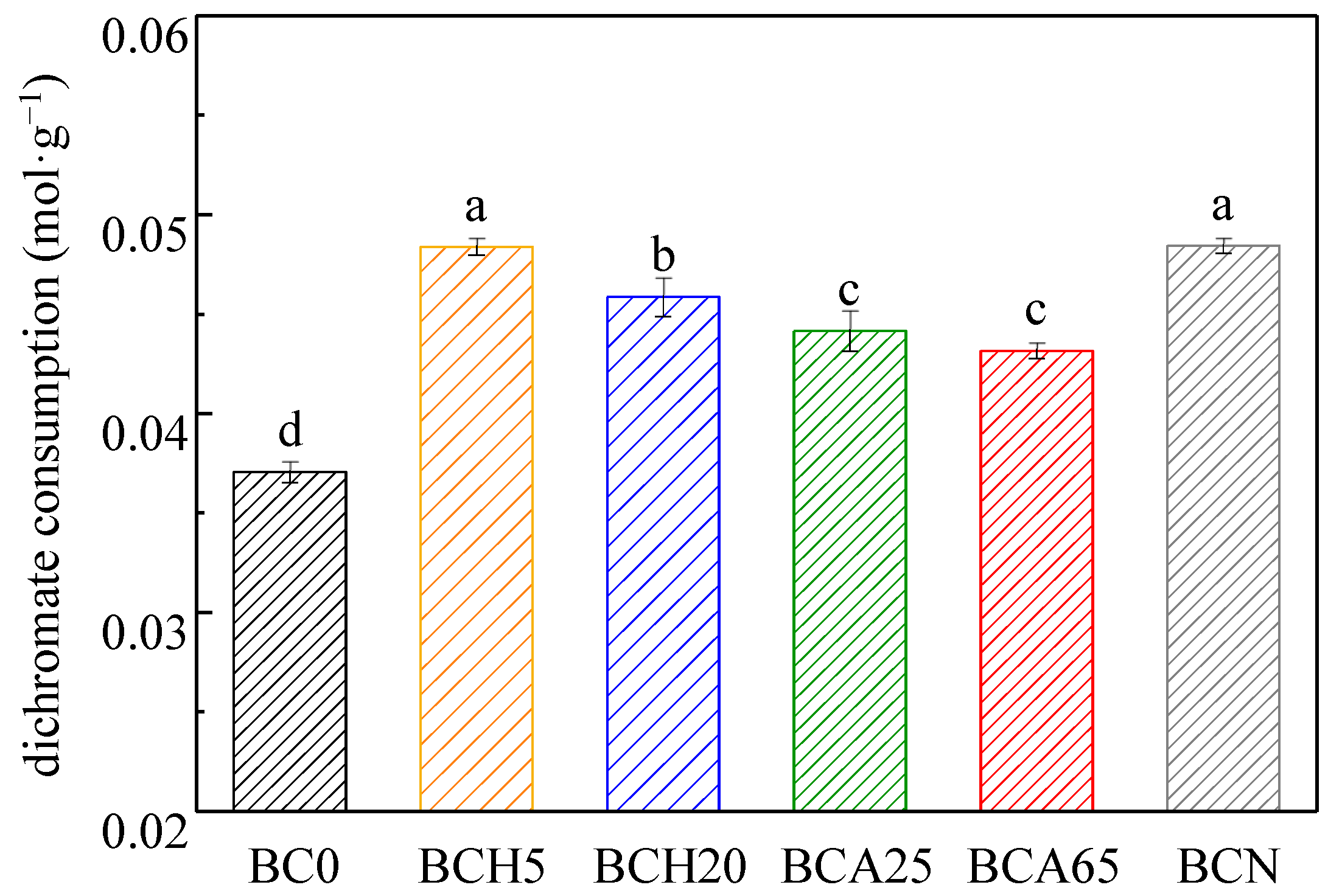
3.2. Chemical Oxidation Resistance of Modified Biochars
3.3. Decisive Roles in the Stability of the Modified Biochar
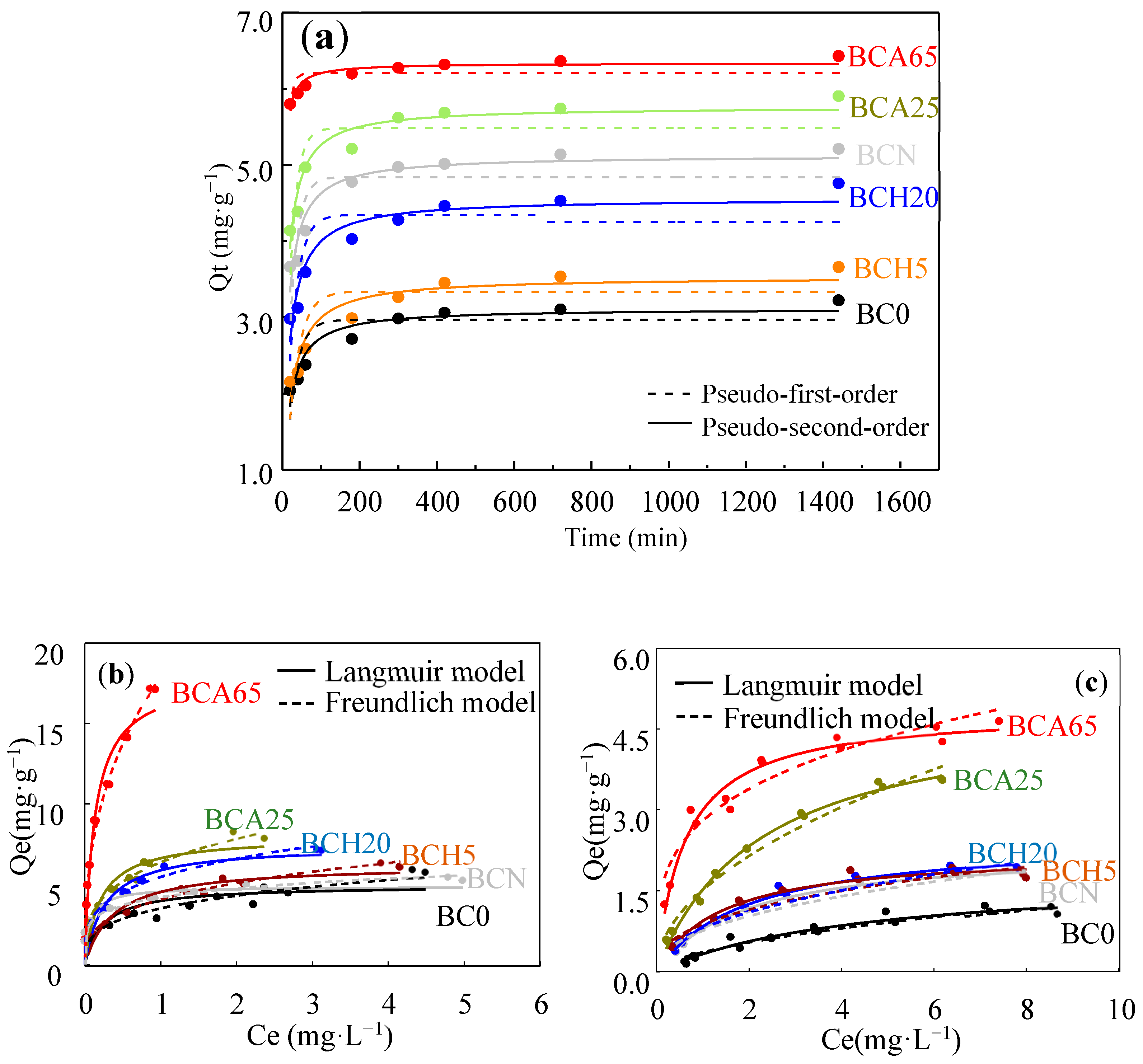
3.4. Chemical Aging Reduced the Adsorption Capacity of Heavy Metals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, Y.; Wang, L.; Li, H.; Westholm, L.J.; Carvalho, L.; Thorin, E.; Yu, Z.; Yu, X.; Skreiberg, O. A critical review on production, modification and utilization of biochar. J. Anal. Appl. Pyrolysis 2022, 161, 105405. [Google Scholar] [CrossRef]
- Jin, J.; Sun, K.; Wang, Z.; Han, L.; Du, P.; Wang, X.; Xing, B. Effects of chemical oxidation on phenanthrene sorption by grass- and manure-derived biochars. Sci. Total Environ. 2017, 598, 789–796. [Google Scholar] [CrossRef]
- Wang, B.; Gao, B.; Fang, J. Recent advances in engineered biochar productions and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2158–2207. [Google Scholar] [CrossRef]
- Wang, H.; Gao, B.; Wang, S.; Fang, J.; Xue, Y.; Yang, K. Removal of Pb(II), Cu(II), and Cd(II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood. Bioresour. Technol. 2015, 197, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yue, X.; Li, F.; Xiao, R.; Zhang, Y.; Gu, D. Preparation of rice straw-derived biochar for efficient cadmium removal by modification of oxygen-containing functional groups. Sci. Total Environ. 2018, 631–632, 795–802. [Google Scholar] [CrossRef]
- Leng, L.; Huang, H.; Li, H.; Li, J.; Zhou, W. Biochar stability assessment methods: A review. Sci. Total Environ. 2019, 647, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.P.; Cowie, A.L.; Smernik, R.J. Biochar carbon stability in a clayey soil as a function of feedstock and pyrolysis temperature. Environ. Sci. Technol. 2012, 46, 11770–11778. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.R. Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Baronti, S.; Vaccari, F.P.; Miglietta, F.; Calzolari, C.; Lugato, E.; Orlandini, S.; Pini, R.; Zulian, C.; Genesio, L. Impact of biochar application on plant water relations in Vitis vinifera (L.). Eur. J. Agron. 2014, 53, 38–44. [Google Scholar] [CrossRef]
- LeCroy, C.; Masiello, C.A.; Rudgers, J.A.; Hockaday, W.C.; Silberg, J.J. Nitrogen, biochar, and mycorrhizae: Alteration of the symbiosis and oxidation of the char surface. Soil Biol. Biochem. 2013, 58, 248–254. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Lehmann, J.; Engelhard, M.H. Natural oxidation of black carbon in soils: Changes in molecular form and surface charge along a climosequence. Geochim. Cosmochim. Acta 2008, 72, 1598–1610. [Google Scholar] [CrossRef]
- Spokas, K.A. Review of the stability of biochar in soils: Predictability of O:C molar ratios. Carbon Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef]
- Schmidt, H.P.; Bucheli, T.; Kammann, C.; Glaser, B.; Abiven, S.; Leifeld, J. European Biochar Certificate-Guidelines for a Sustainable Production of Biochar; European Biochar Foundation (EBC): Arbaz, Switzerland, 2016. [Google Scholar] [CrossRef]
- Han, L.F.; Ro, K.S.; Wang, Y.; Sun, K.; Sun, H.R.; Libra, J.A.; Xing, B.S. Oxidation resistance of biochars as a function of feedstock and pyrolysis condition. Sci. Total Environ. 2018, 616, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, J.A.; Cui, L.Q.; Kammann, C.; Wrage-Monnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K.; et al. Feedstock choice, pyrolysis temperature and type influence biochar characteristics: A comprehensive meta-data analysis review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- Tan, L.; Ma, Z.; Yang, K.; Cui, Q.; Wang, K.; Wang, T.; Wu, G.-L.; Zheng, J. Effect of three artificial aging techniques on physicochemical properties and Pb adsorption capacities of different biochars. Sci. Total Environ. 2019, 699, 134223. [Google Scholar] [CrossRef]
- Zheng, X.; Ma, X.; Hua, Y.; Li, D.; Xiang, J.; Song, W.; Dong, J. Nitric acid-modified hydrochar enhance Cd2+ sorption capacity and reduce the Cd2+ accumulation in rice. Chemosphere 2021, 284, 131261. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; He, M.; Xu, X.; Cao, X.; Tsang, D.C. Impacts of different activation processes on the carbon stability of biochar for oxidation resistance. Bioresour. Technol. 2021, 338, 125555. [Google Scholar] [CrossRef]
- Siatecka, A.; Rozylo, K.; Ok, Y.S.; Oleszczuk, P. Biochars ages differently depending on the feedstock used for their production: Willow- versus sewage sludge-derived biochars. Sci. Total Environ. 2021, 789, 147458. [Google Scholar] [CrossRef]
- Jin, J.; Li, S.; Peng, X.; Liu, W.; Zhang, C.; Yang, Y.; Han, L.; Du, Z.; Sun, K.; Wang, X. HNO3 modified biochars for uranium (VI) removal from aqueous solution. Bioresour. Technol. 2018, 256, 247–253. [Google Scholar] [CrossRef]
- Yang, G.-X.; Jiang, H. Amino modification of biochar for enhanced adsorption of copper ions from synthetic wastewater. Water Res. 2014, 48, 396–405. [Google Scholar] [CrossRef]
- Liu, G.; Pan, X.; Ma, X.; Xin, S.; Xin, Y. Effects of feedstock and inherent mineral components on oxidation resistance of biochars. Sci. Total Environ. 2020, 726, 138672. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yu, X.; Song, C.; Pang, X.; Huang, J.; Li, Y. Effect of pyrolysis temperature on the chemical oxidation stability of bamboo biochar. Bioresour. Technol. 2016, 218, 1303–1306. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, X.; Zhang, Y.; Yu, Y.; Cao, X. Pyrolysis-temperature depended electron donating and mediating mechanisms of biochar for Cr(VI) reduction. J. Hazard. Mater. 2020, 388, 121794. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Gui, X.; Ji, W.; Zhou, C. Capacity and potential mechanisms of Cd(II) adsorption from aqueous solution by blue algae-derived biochars. Chemosphere 2020, 251, 126335. [Google Scholar] [CrossRef]
- Feizbakhshan, M.; Hashisho, Z.; Crompton, D.; Anderson, J.E.; Nichols, M. Effect of activated carbon’s pore size distribution on oxygen induced heel build-up. J. Hazard. Mater. 2021, 126905. [Google Scholar] [CrossRef]
- Fernando, J.C.; Peiris, C.; Navarathna, C.M.; Gunatilake, S.R.; Welikala, U.; Wanasinghe, S.T.; Madduri, S.B.; Jayasinghe, S.; Mlsna, T.E.; Hassan, E.B.; et al. Nitric acid surface pre-modification of novel Lasia spinosa biochar for enhanced methylene blue remediation. Groundw. Sustain. Dev. 2021, 14, 100603. [Google Scholar] [CrossRef]
- Peiris, C.; Nayanathara, O.; Navarathna, C.M.; Jayawardhana, Y.; Nawalage, S.; Burk, G.; Karunanayake, A.G.; Madduri, S.B.; Vithanage, M.; Kaumal, M.N.; et al. The influence of three acid modifications on the physicochemical characteristics of tea-waste biochar pyrolyzed at different temperatures: A comparative study. RSC Adv. 2019, 9, 17612–17622. [Google Scholar] [CrossRef]
- Nzediegwu, C.; Naeth, M.A.; Chang, S.X. Feedstock type drives surface property, demineralization and element leaching of nitric acid-activated biochars more than pyrolysis temperature. Bioresour. Technol. 2022, 344, 126316. [Google Scholar] [CrossRef]
- Fan, Q.; Sun, J.; Chu, L.; Cui, L.; Quan, G.; Yan, J.; Hussain, Q.; Iqbal, M. Effects of chemical oxidation on surface oxygen-containing functional groups and adsorption behavior of biochar. Chemosphere 2018, 207, 33–40. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Ding, H.-S.; Wang, Y.-Y.; Liu, W.-J.; Jiang, H. Pyrolytic temperature dependent and ash catalyzed formation of sludge char with ultra-high adsorption to 1-naphthol. Environ. Sci. Technol. 2016, 50, 2602–2609. [Google Scholar] [CrossRef] [PubMed]
- Yakout, S.M.; Daifullah, A.M.; El-Reefy, S.A. Pore structure characterization of chemically modified biochar derived from rice straw. Environ. Eng. Manag. J. 2015, 14, 473–480. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, B.; Yao, Y.; Inyang, M.; Zhang, M.; Zimmerman, A.R.; Ro, K.S. Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: Batch and column tests. Chem. Eng. J. 2012, 200–202, 673–680. [Google Scholar] [CrossRef]
- Oliveira, G.A.; Gevaerd, A.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. Biochar obtained from spent coffee grounds: Evaluation of adsorption properties and its application in a voltammetric sensor for lead (II) ions. Microchem. J. 2021, 165, 106114. [Google Scholar] [CrossRef]
- Qian, L.; Chen, B. Interactions of aluminum with biochars and oxidized biochars: Implications for the biochar aging process. J. Agric. Food. Chem. 2014, 62, 373–380. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, S.; Ding, X.; Khan, A.; Alam, M. The effects of biochar and rice husk on adsorption and desorption of cadmium on to soils with different water conditions (upland and saturated). Chemosphere 2018, 193, 1120–1126. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Ge, L.; Jaroniec, M.; Qiao, S.Z. Two-step boron and nitrogen doping in graphene for enhanced synergistic catalysis. Angew. Chem. Int. Edit. 2013, 52, 3110–3116. [Google Scholar] [CrossRef]
- Qu, X.; Fu, H.; Mao, J.; Ran, Y.; Zhang, D.; Zhu, D. Chemical and structural properties of dissolved black carbon released from biochars. Carbon 2016, 96, 759–767. [Google Scholar] [CrossRef]
- Nan, H.; Zhao, L.; Yang, F.; Liu, Y.; Xiao, Z.; Cao, X.; Qiu, H. Different alkaline minerals interacted with biomass carbon during pyrolysis: Which one improved biochar carbon sequestration? J. Clean. Prod. 2020, 255, 120162. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Q.; Wang, X.; Bei, Q.; Zhang, Y.; Lin, Z.; Liu, G.; Zhu, J.; Hu, T.; Jin, H.; et al. A fast chemical oxidation method for predicting the long-term mineralization of biochar in soils. Sci. Total Environ. 2020, 718, 137390. [Google Scholar] [CrossRef]
- Chen, J.; Wang, P.; Ding, L.; Yu, T.; Leng, S.; Chen, J.; Fan, L.; Li, J.; Wei, L.; Li, J.; et al. The comparison study of multiple biochar stability assessment methods. J. Anal. Appl. Pyrolysis 2021, 156, 105070. [Google Scholar] [CrossRef]
- Manyà, J.J.; Ortigosa, M.A.; Laguarta, S.; Manso, J.A. Experimental study on the effect of pyrolysis pressure, peak temperature, and particle size on the potential stability of vine shoots-derived biochar. Fuel 2014, 133, 163–172. [Google Scholar] [CrossRef]
- Fang, Y.; Singh, B.; Singh, B.P.; Krull, E. Biochar carbon stability in four contrasting soils. Eur. J. Soil. Sci. 2014, 65, 60–71. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, L.; Gao, B.; Xu, X.; Cao, X. The Interfacial Behavior between Biochar and Soil Minerals and Its Effect on Biochar Stability. Environ. Sci. Technol. 2016, 50, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Rosas, J.M.; Ruiz-Rosas, R.; Rodriguez-Mirasol, J.; Cordero, T. Kinetic study of the oxidation resistance of phosphorus-containing activated carbons. Carbon 2012, 50, 1523–1537. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B. A direct observation of the fine aromatic clusters and molecular structures of biochars. Environ. Sci. Technol. 2017, 51, 5473–5482. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.; Zheng, W.; Kan, Y. Phosphorus-assisted biomass thermal conversion: Reducing carbon loss and improving biochar stability. PLoS ONE 2014, 9, e115373. [Google Scholar] [CrossRef]
- Ren, X.; Sun, H.; Wang, F.; Cao, F. The changes in biochar properties and sorption capacities after being cultured with wheat for 3 months. Chemosphere 2016, 144, 2257–2263. [Google Scholar] [CrossRef]
- Zhang, C.; Shan, B.; Tang, W.; Zhu, Y. Comparison of cadmium and lead sorption by Phyllostachys pubescens biochar produced under a low-oxygen pyrolysis atmosphere. Bioresour. Technol. 2017, 238, 352–360. [Google Scholar] [CrossRef]
- Chen, D.; Wang, X.; Wang, X.; Feng, K.; Su, J.; Dong, J. The mechanism of cadmium sorption by sulphur-modified wheat straw biochar and its application cadmium-contaminated soil. Sci. Total Environ. 2020, 714, 136550. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, B.; Lin, L.; Qiu, W.; Song, Z. Adsorption of Cu(II) and Cd(II) from aqueous solutions by ferromanganese binary oxide–biochar composites. Sci. Total Environ. 2018, 615, 115–122. [Google Scholar] [CrossRef]
- Liu, T.; Lawluvy, Y.; Shi, Y.; Ighalo, J.O.; He, Y.; Zhang, Y.; Yap, P.-S. Adsorption of cadmium and lead from aqueous solution using modified biochar: A review. J. Environ. Chem. Eng. 2022, 10, 106502. [Google Scholar] [CrossRef]
- Sun, L.; Gong, P.; Sun, Y.; Qin, Q.; Song, K.; Ye, J.; Zhang, H.; Zhou, B.; Xue, Y. Modified chicken manure biochar enhanced the adsorption for Cd2+ in aqueous and immobilization of Cd in contaminated agricultural soil. Sci. Total Environ. 2022, 851, 158252. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Sohi, S.P.; Jing, F.; Liu, Y.; Chen, J. A comparative study on biochar properties and Cd adsorption behavior under effects of ageing processes of leaching, acidification and oxidation. Environ. Pollut. 2019, 254, 113123. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Lian, F.; Liu, Z.; Zhu, L.; Song, Z. Biochars derived from various crop straws: Characterization and Cd(II) removal potential. Ecotoxicol. Environ. Saf. 2014, 106, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xu, X.; Tsang, D.C.W.; Cao, X. Contrasting impacts of pre- and post-application aging of biochar on the immobilization of Cd in contaminated soils. Environ. Pollut. 2018, 242, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Cai, C.; Chi, H.; Reid, B.J.; Coulon, F.; Zhang, Y.; Hou, Y. Remediation of cadmium and lead polluted soil using thiol-modified biochar. J. Hazard. Mater. 2020, 388, 122037. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Huang, S.; Laird, D.A.; Wu, J.; Lin, Z. Identifying key processes driving Cd long-term adsorption and immobilization by biochar in soils and evaluating combined aging effects under simulated local climates. J. Environ. Chem. Eng. 2022, 10, 108636. [Google Scholar] [CrossRef]

| Biochar Type | BC0 | BCH5 | BCH20 | BCA25 | BCA65 | BCN |
|---|---|---|---|---|---|---|
| CHN H/C | 0.49 ± 0.01 b | 0.41 ± 0.02 c | 0.42 ± 0.02 c | 0.44 ± 0.01 c | 0.49 ± 0.00 b | 0.59 ± 0.02 a |
| CHN O/C | 0.16 ± 0.01 d | 0.15 ± 0.00 e | 0.16 ± 0.00 de | 0.33 ± 0.00 b | 0.47 ± 0.00 a | 0.22 ± 0.01 c |
| XPS O/C | 0.23 | 0.24 | 0.25 | 0.36 | 0.47 | 0.35 |
| Ash content | 25.36% | 17.80% | 17.08% | 17.56% | 17.55% | 14.78% |
| SSA (cm2·g−1) | 132.35 | 94.83 | 37.52 | 5.94 | 5.32 | 25.81 |
| Pore volume (cm3·g−1) | 0.089 | 0.071 | 0.039 | 0.015 | 0.013 | 0.025 |
| Average pore size (nm) | 2.70 | 2.99 | 4.18 | 10.22 | 9.84 | 3.91 |
| ID/IG | 0.71 | 0.76 | 0.78 | 0.83 | 0.85 | 0.83 |
| Zeta (mV) | 2.42 | −0.21 | −1.31 | −2.09 | −4.05 | −0.60 |
| Biochar Type | C-C/C=C% X | C-O% X | C=O% X | -COOH% X | -COOH B (mmol/g) |
|---|---|---|---|---|---|
| BC0 | 88.22 | 8.23 | 2.11 | 1.44 | 0.07±0.00 d |
| BCH5 | 72.52 | 15.57 | 7.72 | 4.19 | 0.09 ± 0.07 d |
| BCH20 | 67.84 | 19.25 | 7.00 | 5.91 | 0.43 ± 0.01 c |
| BCA25 | 66.94 | 13.75 | 10.01 | 9.30 | 1.03 ± 0.08 b |
| BCA65 | 62.70 | 11.57 | 10.43 | 15.30 | 2.01 ± 0.18 a |
| BCN | 63.29 | 19.36 | 12.58 | 4.78 | 0.39 ± 0.00 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Duan, W.; Chang, Z.; Du, W.; Chen, F.; Li, F.; Oleszczuk, P. Stability of Functionally Modified Biochar: The Role of Surface Charges and Surface Homogeneity. Sustainability 2023, 15, 7745. https://doi.org/10.3390/su15107745
Zhu Z, Duan W, Chang Z, Du W, Chen F, Li F, Oleszczuk P. Stability of Functionally Modified Biochar: The Role of Surface Charges and Surface Homogeneity. Sustainability. 2023; 15(10):7745. https://doi.org/10.3390/su15107745
Chicago/Turabian StyleZhu, Ziyang, Wenyan Duan, Zhaofeng Chang, Wei Du, Fangyuan Chen, Fangfang Li, and Patryk Oleszczuk. 2023. "Stability of Functionally Modified Biochar: The Role of Surface Charges and Surface Homogeneity" Sustainability 15, no. 10: 7745. https://doi.org/10.3390/su15107745
APA StyleZhu, Z., Duan, W., Chang, Z., Du, W., Chen, F., Li, F., & Oleszczuk, P. (2023). Stability of Functionally Modified Biochar: The Role of Surface Charges and Surface Homogeneity. Sustainability, 15(10), 7745. https://doi.org/10.3390/su15107745







