Predicting the Effects of Future Climate Change on the Potential Distribution of Eolagurus luteus in Xinjiang
Abstract
1. Introduction
2. Materials and Methods
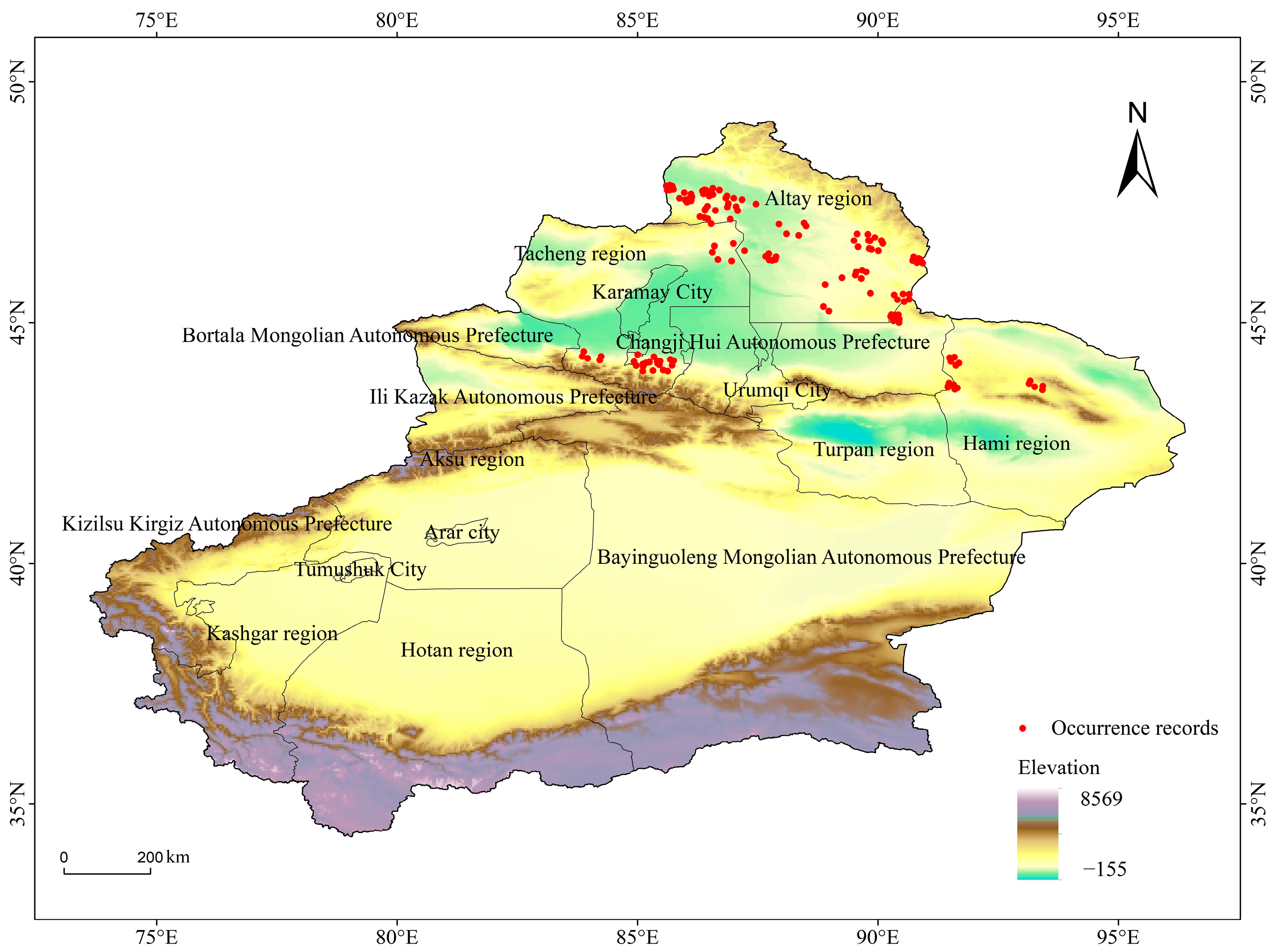
2.1. Study Area
2.2. Species Occurrence Data of E. luteus
2.3. Environment Variable Data
2.4. Modeling Process
2.5. Model Evaluation
3. Results
3.1. Model Evaluation and Contribution of Variables
3.2. Potential Geographical Distribution of E. luteus in Xinjiang under Current Climatic Conditions
3.3. Potential Suitable Areas for E. luteus in Xinjiang under Future Climate Scenarios
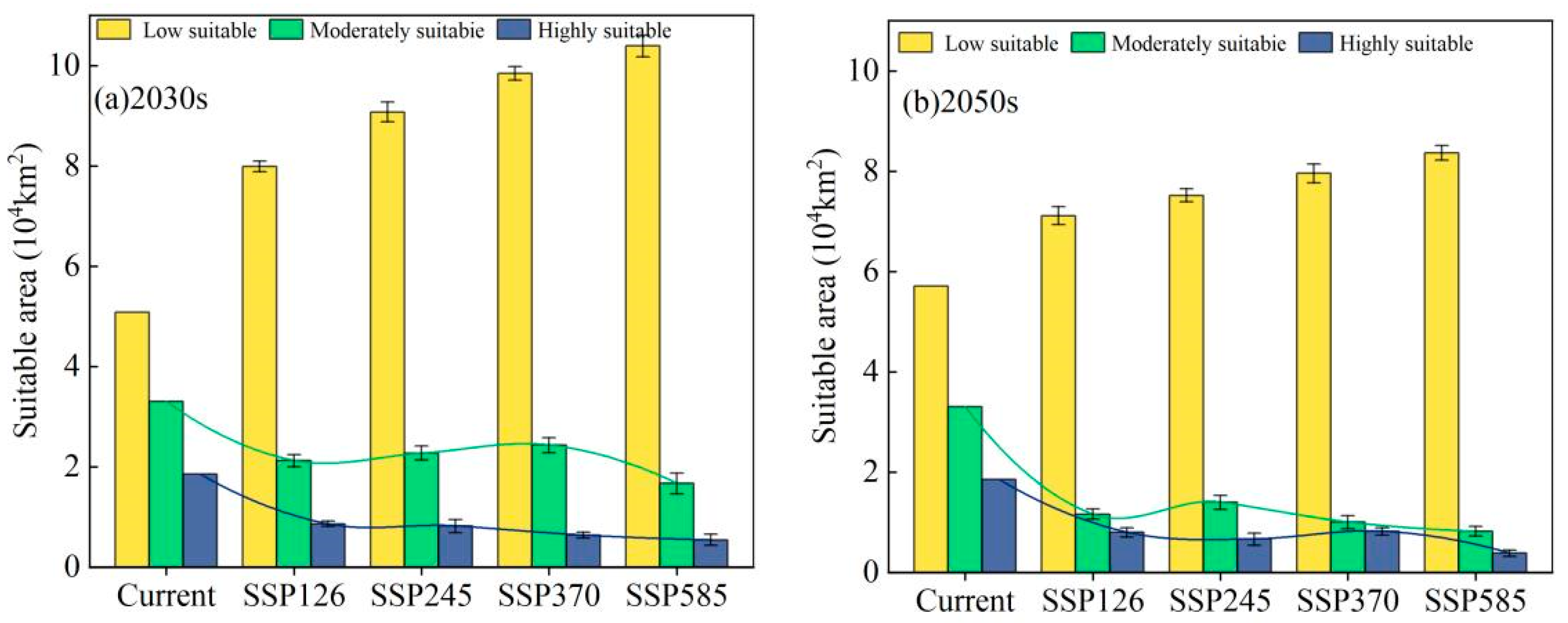
3.4. Acreage Change of Potential Habitat of E. luteus in Xinjiang under Future Climate Change Scenarios
4. Discussion
4.1. Effects of Environmental Factors on Potential Distribution of E. luteus in Xinjiang
4.2. Effects of Environmental Factors on Potential Habitat Areas of the E. luteus
4.3. Uncertainty and Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohammadi, S.; Ebrahimi, E.; Moghadam, M.S.; Bosso, L. Modelling current and future potential distributions of two desert jerboas under climate change in Iran. Ecol. Inform. 2019, 52, 7–13. [Google Scholar] [CrossRef]
- Addink, E.A.; De Jong, S.M.; Davis, S.A.; Dubyanskiy, V.; Burdelov, L.A.; Leirs, H. The use of high-resolution remote sensing for plague surveillance in Kazakhstan. Remote Sens. Environ. 2010, 114, 674–681. [Google Scholar] [CrossRef]
- Jäkel, T.; Mouaxengcha, K.; Nuber, U.; Douangboupha, B. Integrated rodent management in outbreak-prone upland rice growing areas of Northern Laos. Crop Prot. 2016, 79, 34–42. [Google Scholar] [CrossRef]
- World Health Organization. Human plague in 2002 and 2003. Wkly. Epidemiol. Rec. 2004, 79, 301–306. [Google Scholar]
- Davis, S.; Begon, M.; De Bruyn, L.; Ageyev, V.S.; Klassovskiy, N.L.; Pole, S.B.; Viljugrein, H.; Stenseth, N.C.; Leirs, H. Predictive thresholds for plague in Kazakhstan. Science 2004, 304, 736–738. [Google Scholar] [CrossRef]
- Linné Kausrud, K.; Viljugrein, H.; Frigessi, A.; Begon, M.; Davis, S.; Leirs, H.; Dubyanskiy, V.; Stenseth, N.C. Climatically driven synchrony of gerbil populations allows large-scale plague outbreaks. Proc. R. Soc. B Biol. Sci. 2007, 274, 1963–1969. [Google Scholar] [CrossRef]
- Prakash, I.; Ghosh, P.K. Rodents in Desert Environments; Springer: Berlin/Heidelberg, Germany, 2012; Volume 28. [Google Scholar]
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, H.; Xu, C. Study on Taiwania cryptomerioides under climate change: MaxEnt modeling for predicting the potential geographical distribution. Glob. Ecol. Conserv. 2020, 24, e1313. [Google Scholar] [CrossRef]
- Nameer, P.O. The expanding distribution of the Indian Peafowl (Pavo cristatus) as an indicator of changing climate in Kerala, southern India: A modelling study using MaxEnt. Ecol. Indic. 2020, 110, 105930. [Google Scholar]
- Scheper, J.; Holzschuh, A.; Kuussaari, M.; Potts, S.G.; Rundlöf, M.; Smith, H.G.; Kleijn, D. Environmental factors driving the effectiveness of European agri-environmental measures in mitigating pollinator loss—A meta-analysis. Ecol. Lett. 2013, 16, 912–920. [Google Scholar] [CrossRef]
- Fortunel, C.; Paine, C.T.; Fine, P.V.; Kraft, N.J.; Baraloto, C. Environmental factors predict community functional composition in A mazonian forests. J. Ecol. 2014, 102, 145–155. [Google Scholar] [CrossRef]
- Qin, A.; Liu, B.; Guo, Q.; Bussmann, R.W.; Ma, F.; Jian, Z.; Xu, G.; Pei, S. Maxent modeling for predicting impacts of climate change on the potential distribution of Thuja sutchuenensis Franch., an extremely endangered conifer from southwestern China. Glob. Ecol. Conserv. 2017, 10, 139–146. [Google Scholar] [CrossRef]
- Phillips, S.B.; Aneja, V.P.; Kang, D.; Arya, S.P. Modelling and analysis of the atmospheric nitrogen deposition in North Carolina. Int. J. Glob. Environ. Issues 2006, 6, 231–252. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef]
- Guan, J.; Li, M.; Ju, X.; Lin, J.; Wu, J.; Zheng, J. The potential habitat of desert locusts is contracting: Predictions under climate change scenarios. PeerJ 2021, 9, e12311. [Google Scholar] [CrossRef]
- Qi, Y.; Pu, X.; Li, Y.; Li, D.; Huang, M.; Zheng, X.; Guo, J.; Chen, Z. Prediction of Suitable Distribution Area of Plateau pika (Ochotona curzoniae) in the Qinghai–Tibet Plateau under Shared Socioeconomic Pathways (SSPs). Sustainability 2022, 14, 12114. [Google Scholar] [CrossRef]
- Bizhanova, N.; Steiner, M.; Rametov, N.; Grachev, A.; Grachev, Y.; Bespalov, M.; Zhaparkulov, T.; Saparbayev, S.; Sailaukhanuly, A.; Bespalov, S.; et al. The Elusive Turkestan Lynx at the Northwestern Edge of Geographic Range: Current Suitable Habitats and Distribution Forecast in the Climate Change. Sustainability 2022, 14, 9491. [Google Scholar] [CrossRef]
- Zhang, F.; Yushanjiang, A.; Wang, D. Ecological risk assessment due to land use/cover changes (LUCC) in Jinghe County, Xinjiang, China from 1990 to 2014 based on landscape patterns and spatial statistics. Environ. Earth Sci. 2018, 77, 491. [Google Scholar] [CrossRef]
- Wu, J.; Liu, L.; Xu, G.; Jiang, J.; Lian, W.; Zhou, H.; Nan, L.; Ren, H. Grape Planting Situation and Regional Spatial Analysis in Xinjiang, China; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 705, p. 12028. [Google Scholar]
- Guan, J.; Yao, J.; Li, M.; Zheng, J. Assessing the spatiotemporal evolution of anthropogenic impacts on remotely sensed vegetation dynamics in Xinjiang, China. Remote Sens. 2021, 13, 4651. [Google Scholar] [CrossRef]
- Guan, J.; Yao, J.; Li, M.; Li, D.; Zheng, J. Historical changes and projected trends of extreme climate events in Xinjiang, China. Clim. Dyn. 2022, 59, 1753–1774. [Google Scholar] [CrossRef]
- Mei, Z.; Mahan, N.; Alimu; Xiao-heng, J.; Ai-hua, L. Current Situation and Control Measures of Eolagurus luteus Eversmann in the North of Xinjiang. Xinjiang Agric. Sci. 2006, 43, 493–494. [Google Scholar]
- Yifei, N.; Guangqing, X. Distribution area and eco-geographical characteristics of the Eolagurus luteus in Xinjiang. Xinjiang Anim. Husb. 2012, 59–63. [Google Scholar]
- Di, S.; Yifei, N.; Jijun, C.; Abuduwali; Jianghua, Z. Application of UAV Low-altitude image on rathole monitoring of Eolagurus luteus. China Plant Prot. 2019, 39, 35–43. [Google Scholar]
- Yao, J.; Chen, Y.; Guan, X.; Zhao, Y.; Chen, J.; Mao, W. Recent climate and hydrological changes in a mountain–basin system in Xinjiang, China. Earth-Sci. Rev. 2022, 226, 103957. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Liu, T.; Zhang, C. The potential geographical distribution of Haloxylon across Central Asia under climate change in the 21st century. Agric. For. Meteorol. 2019, 275, 243–254. [Google Scholar] [CrossRef]
- Green, R.H. Sampling Design and Statistical Methods for Environmental Biologists; John Wiley & Sons: Hoboken, NJ, USA, 1979. [Google Scholar]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Liao, Z.; Zhang, L.; Nobis, M.P.; Wu, X.; Pan, K.; Wang, K.; Dakhil, M.A.; Du, M.; Xiong, Q.; Pandey, B. Climate change jointly with migration ability affect future range shifts of dominant fir species in Southwest China. Divers. Distrib. 2020, 26, 352–367. [Google Scholar] [CrossRef]
- Li, X.; Xu, D.; Jin, Y.; Zhuo, Z.; Yang, H.; Hu, J.; Wang, R. Predicting the current and future distributions of Brontispa ongissimi (Coleoptera: Chrysomelidae) under climate change in China. Glob. Ecol. Conserv. 2021, 25, e1444. [Google Scholar]
- Zhang, K.; Liu, H.; Pan, H.; Shi, W.; Zhao, Y.; Li, S.; Liu, J.; Tao, J. Shifts in potential geographical distribution of Pterocarya stenoptera under climate change scenarios in China. Ecol. Evol. 2020, 10, 4828–4837. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Xin, W.; Yizhao, R.; Qin, H.; Xiaobing, D.; Caiwen, C. Habitat suitability assessment of endangered plant Alsophila spinulosa in Chishui River area based on GIS and Maxent model. Acta Ecol. Sin. 2021, 41, 6123–6133. [Google Scholar]
- Wang, D.; Cui, B.; Duan, S.; Chen, J.; Fan, H.; Lu, B.; Zheng, J. Moving north in China: The habitat of Pedicularis kansuensis in the context of climate change. Sci. Total Environ. 2019, 697, 133979. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Araújo, M.B. Niche properties and geographical extent as predictors of species sensitivity to climate change. Glob. Ecol. Biogeogr. 2005, 14, 347–357. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez Valverde, A.; Hortal, J. The uncertain nature of absences and their importance in species distribution modelling. Ecography 2010, 33, 103–114. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Hijmans, R.J. Cross-validation of species distribution models: Removing spatial sorting bias and calibration with a null model. Ecology 2012, 93, 679–688. [Google Scholar] [CrossRef]
- Ge, L.; Zhao, Y.; Zhou, K.; Mu, X.; Yu, H.; Wang, Y.; Wang, N.; Fan, H.; Guo, L.; Huo, X. Spatio-temporal pattern and influencing factors of hemorrhagic fever with renal syndrome (HFRS) in Hubei Province (China) between 2005 and 2014. PLoS ONE 2016, 11, e167836. [Google Scholar] [CrossRef]
- Tian, H.; Yu, P.; Cazelles, B.; Xu, L.; Tan, H.; Yang, J.; Huang, S.; Xu, B.; Cai, J.; Ma, C. Interannual cycles of Hantaan virus outbreaks at the human–animal interface in Central China are controlled by temperature and rainfall. Proc. Natl. Acad. Sci. USA 2017, 114, 8041–8046. [Google Scholar] [CrossRef]
- Jinna, W.; Jiahuii, L.; Juan, H.; Song, G.; Yuyan, W.; Xiao, M.; Rong, X.; Zhenyu, G. Effect of meteorological factors on rat density. J. Prev. Med. 2018, 30, 870–873, 878. [Google Scholar]
- Stige, L.C.; Ottersen, G.; Brander, K.; Chan, K.; Stenseth, N.C. Cod and climate: Effect of the North Atlantic Oscillation on recruitment in the North Atlantic. Mar. Ecol. Prog. Ser. 2006, 325, 227–241. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, Z.; Lu, L.; Li, X.; Liu, Q. Meteorological factors are associated with hemorrhagic fever with renal syndrome in Jiaonan County, China, 2006–2011. Int. J. Biometeorol. 2014, 58, 1031–1037. [Google Scholar] [CrossRef]
- Chonglu, S.; Shoushen, H.; Fulai, F.; Zhang, Q.; Yong, Z. Discussion on economic threshold in control of Eolagurus luteus. Acta Zool. Sin. 1986, 86–91, 89–94. [Google Scholar]
- Xiao, H.; Liu, H.; Gao, L.; Huang, C.; Li, Z.; Lin, X.; Chen, B.; Tian, H. Investigating the effects of food available and climatic variables on the animal host density of hemorrhagic fever with renal syndrome in Changsha, China. PLoS ONE 2013, 8, e61536. [Google Scholar] [CrossRef]
- Alexander, L.V.; Zhang, X.; Peterson, T.C.; Caesar, J.; Gleason, B.; Klein Tank, A.; Haylock, M.; Collins, D.; Trewin, B.; Rahimzadeh, F. Global observed changes in daily climate extremes of temperature and precipitation. J. Geophys. Res. Atmos. 2006, 111. [Google Scholar] [CrossRef]
- Donat, M.G.; Lowry, A.L.; Alexander, L.V.; Gorman, P.A.O.; Maher, N. More extreme precipitation in the world’s dry and wet regions. Nat. Clim. Chang. 2016, 6, 508–513. [Google Scholar] [CrossRef]
- Zhang, K.; Dai, S.; Dong, X. Dynamic variability in daily temperature extremes and their relationships with large-scale atmospheric circulation during 1960–2015 in Xinjiang, China. Chin. Geogr. Sci. 2020, 30, 233–248. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhong, F.; Wang, P. Potential linkages of extreme climate events with vegetation and large-scale circulation indices in an endorheic river basin in northwest China. Atmos. Res. 2021, 247, 105256. [Google Scholar] [CrossRef]
- Yao, J.; Chen, Y.; Zhao, Y.; Mao, W.; Xu, X.; Liu, Y.; Yang, Q. Response of vegetation NDVI to climatic extremes in the arid region of Central Asia: A case study in Xinjiang, China. Theor. Appl. Climatol. 2018, 131, 1503–1515. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, D.; Lang, X. Future extreme climate changes linked to global warming intensity. Sci. Bull. 2017, 62, 1673–1680. [Google Scholar] [CrossRef]
- Zhengqi, W.; Xuejie, G.; Yao, T.; Zhenyu, H.; Ying, X. Future Climate Change Projection over Xinjiang based on an Ensemble of Regional Climate Model Simulations. Chin. J. Atmos. Sci. 2021, 45, 407–423. [Google Scholar]
- Namgung, H.; Kim, M.; Baek, S.; Lee, J.; Kim, H. Predicting potential current distribution of Lycorma delicatula (Hemiptera: Fulgoridae) using MaxEnt model in South Korea. J. Asia-Pac. Entomol. 2020, 23, 291–297. [Google Scholar] [CrossRef]





| Category | Code | Description |
|---|---|---|
| Bioclimatic variables | Bio_1 | Annual mean temperature |
| Bio_2 | Mean diurnal range (mean of monthly (max temp–min temp)) | |
| Bio_3 | Isothermality (bio02/bio07) | |
| Bio_4 | Temperature seasonality (Standard deviation × 100) | |
| Bio_5 | Max temperature of warmest month | |
| Bio_6 | Min temperature of coldest month | |
| Bio_7 | Temperature annual range (bio05-bio06) | |
| Bio_8 | Mean temperature of wettest quarter | |
| Bio_9 | Mean temperature of driest quarter | |
| Bio_10 | Mean temperature of warmest quarter | |
| Bio_11 | Mean temperature of coldest quarter | |
| Bio_12 | Annual precipitation | |
| Bio_13 | Precipitation of wettest month | |
| Bio_14 | Precipitation of driest month | |
| Bio_15 | Precipitation seasonality | |
| Bio_16 | Precipitation of wettest quarter | |
| Bio_17 | Precipitation of driest quarter | |
| Bio_18 | Precipitation of warmest quarter | |
| Bio_19 | Precipitation of coldest quarter | |
| Topography variable | Altitude | Topographic elevation |
| Slope | The degree of steepness of the surface element | |
| Aspect | The direction the slope faces | |
| Soil factors of variables | T_BS | Topsoil basic saturation |
| T_ESP | Topsoil exchangeable sodium salt | |
| T_OC | Topsoil organic carbon content | |
| T_PH_H2O | Topsoil pH value | |
| T_SAND | Topsoil sand fraction | |
| T_TEXTURE | Topsoil texture |
| Scenarios | Time | Low Suitable Area (×104 km2) | Moderately Suitable Area (×104 km2) | Highly Suitable Area (×104 km2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gain | Loss | Unchanged | Gain | Loss | Unchanged | Gain | Loss | Unchanged | ||
| SSP126 | 2030s | 4.07 | 6.95 | 2.90 | 1.17 | 4.14 | 0.82 | 0.41 | 1.50 | 0.35 |
| 2050s | 4.28 | 7.80 | 1.22 | 0.59 | 4.81 | 0.14 | 0.50 | 1.70 | 0.15 | |
| SSP245 | 2030s | 5.21 | 7.73 | 2.79 | 0.58 | 4.38 | 1.72 | 0.47 | 1.64 | 0.21 |
| 2050s | 6.40 | 4.73 | 0.98 | 0.56 | 4.83 | 0.13 | 0.50 | 1.73 | 0.12 | |
| SSP370 | 2030s | 5.82 | 8.11 | 2.58 | 1.66 | 4.29 | 0.67 | 0.43 | 1.65 | 0.21 |
| 2050s | 7.16 | 5.63 | 1.74 | 0.93 | 4.81 | 0.15 | 0.37 | 1.75 | 0.11 | |
| SSP585 | 2030s | 6.64 | 8.87 | 2.25 | 1.27 | 4.51 | 0.44 | 0.24 | 1.71 | 0.14 |
| 2050s | 6.25 | 6.09 | 0.95 | 0.73 | 4.86 | 0.10 | 0.26 | 1.78 | 0.08 | |
| Variable | Details | Percent Contribution |
|---|---|---|
| Bio_1 | Annual mean temperature | 10.5 |
| Bio_2 | Mean diurnal range (mean of monthly (max temp–min temp)) | 4.8 |
| Bio_3 | Isothermality (bio02/bio07) | 32.7 |
| Bio_5 | Max temperature of warmest month | 4 |
| Bio_7 | Temperature annual range (bio05-bio06) | 2.2 |
| Bio_8 | Mean temperature of wettest quarter | 8.9 |
| Bio_9 | Mean temperature of driest quarter | 5.4 |
| Bio_13 | Precipitation of wettest month | 2.4 |
| Bio_15 | Precipitation seasonality | 5.3 |
| Bio_16 | Precipitation of wettest quarter | 2.5 |
| Bio_17 | Precipitation of driest quarter | 3.3 |
| Altitude | Altitude | 3.5 |
| Slope | Slope | 5.5 |
| Aspect | Aspect | 3.7 |
| T_OC | Organic carbon content | 1.7 |
| T_PH_H2O | ph | 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Q.; Zheng, J.; Guan, J.; Wu, J.; Lin, J.; Ju, X.; Wu, R. Predicting the Effects of Future Climate Change on the Potential Distribution of Eolagurus luteus in Xinjiang. Sustainability 2023, 15, 7916. https://doi.org/10.3390/su15107916
An Q, Zheng J, Guan J, Wu J, Lin J, Ju X, Wu R. Predicting the Effects of Future Climate Change on the Potential Distribution of Eolagurus luteus in Xinjiang. Sustainability. 2023; 15(10):7916. https://doi.org/10.3390/su15107916
Chicago/Turabian StyleAn, Qinghui, Jianghua Zheng, Jingyun Guan, Jianguo Wu, Jun Lin, Xifeng Ju, and Rui Wu. 2023. "Predicting the Effects of Future Climate Change on the Potential Distribution of Eolagurus luteus in Xinjiang" Sustainability 15, no. 10: 7916. https://doi.org/10.3390/su15107916
APA StyleAn, Q., Zheng, J., Guan, J., Wu, J., Lin, J., Ju, X., & Wu, R. (2023). Predicting the Effects of Future Climate Change on the Potential Distribution of Eolagurus luteus in Xinjiang. Sustainability, 15(10), 7916. https://doi.org/10.3390/su15107916





