Optimization of Engineering and Process Parameters for Vermicomposting
Abstract
1. Introduction
2. Materials and Methods
2.1. Waste Collection
2.2. Pre-Treatment of Fresh Different Solid Wastes for Vermicomposting
2.3. Types of Vermireactor Systems
2.3.1. Experimental Design for Vermicomposting Reactors
2.3.2. Earthworm (E. fetida and E. eugeniae)
2.3.3. Monoculture and Polyculture Vermicomposting of E. fetida and E. eugeniae
2.3.4. Control
2.4. Physico-Chemical Analysis
2.5. Plant Growth
Statistical Analysis
3. Results and Discussion
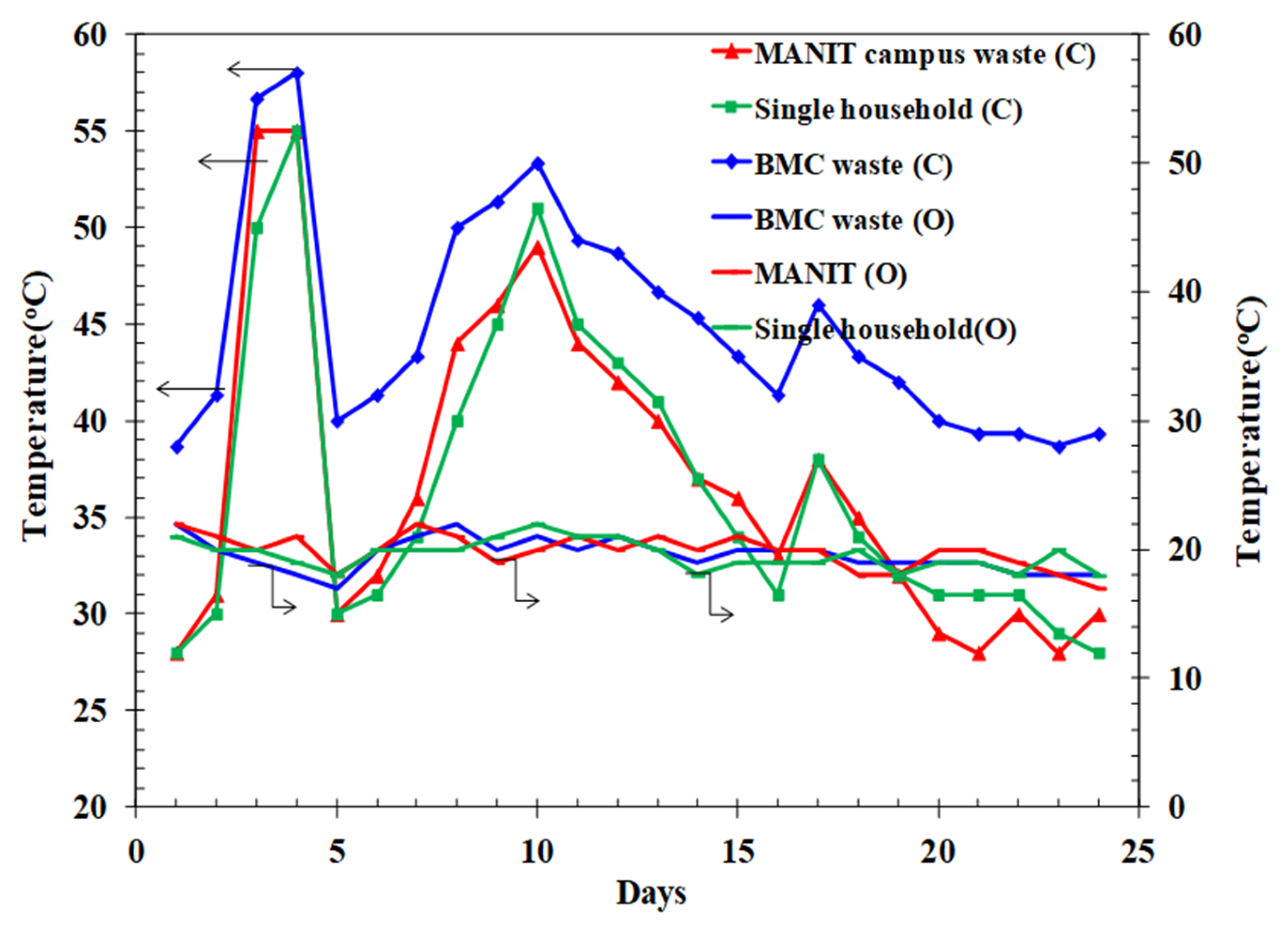
3.1. Variation in C:N Ratio
3.2. Vermicast and Zoomass Studies
3.3. Feasibility Study (Growing of Chilli and Brinjal Plants) over Composting/Vermicomposting Period
4. Conclusions and Scope of Future Work
4.1. Conclusions
4.2. Scope of Future Work
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suthar, S. Potential of domestic biogas digester slurry in vermitechnology. Bioresour. Technol. 2010, 101, 5419–5425. [Google Scholar] [CrossRef]
- Katiyar, R.B.; Suresh, S.; Sharma, A.K. Characterization of municipal solid waste generated by the city of Bhopal, India. Int. ChemTech Res. 2013, 3, 623–628. [Google Scholar]
- Kumari, K.; Suresh, S.; Arisutha, S.; Sudhakar, K. Anaerobic co-digestion of different wastes in a UASB reactor. Waste Manag. 2018, 77, 545–554. [Google Scholar] [CrossRef]
- Kaur, A.; Singh, J.; Vig, A.P.; Dhaliwal, S.S.; Rup, P.J. Cocomposting with and without Eisenia fetida for conversion of toxic paper mill sludge into soil conditioner. Bioresour. Technol. 2010, 3, 8192–8198. [Google Scholar] [CrossRef]
- Garg, V.K.; Kaushik, P. Vermistabilization of textile mill sludge spiked with poultry droppings by epigeic earthworm Eisenia fetida. Biores. Technol. 2005, 3, 1063–1071. [Google Scholar] [CrossRef]
- Suthar, S. Potential utilization of guargum industrial waste in vermicompost production. Bioresour. Technol. 2006, 3, 2474–2477. [Google Scholar] [CrossRef] [PubMed]
- Sen, B.; Chandra, T. Chemolytic and solid-state spectroscopic evaluation of organic matter transformation during vermicomposting of sugar industry wastes. Bioresour. Technol. 2007, 98, 1680–1683. [Google Scholar] [CrossRef] [PubMed]
- Suthar, S.; Singh, S. Feasibility of vermicomposting in biostabilization of sludge from a distillery industry. Sci. Total Environ. 2008, 394, 237–243. [Google Scholar] [CrossRef]
- Ravindran, B.; Dinesh, S.L.; Kennedy, L.J.; Sekaran, G. Vermicomposting of Solid Waste Generated from Leather Industries Using Epigeic Earthworm Eisenia foetida. Appl. Biochem. Biotechnol. 2008, 151, 480–488. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, A.; Vig, A.P.; Rup, P. Role of Eisenia fetida in rapid recycling of nutrients from bio sludge of beverage industry. Ecotoxicol. Environ. Saf. 2010, 73, 430–435. [Google Scholar] [CrossRef]
- Ravindran, B.; Sekaran, G. Bacterial composting of animal fleshing generated from tannery industries. Waste Manag. 2011, 30, 2622–2630. [Google Scholar] [CrossRef]
- Arisutha, S.; Baredar, P.; Deshpande, D.M.; Suresh, S. Evaluation of Methane from Sisal Leaf Residue and Palash Leaf Litter. J. Inst. Eng. (India) Ser. E 2014, 95, 105–110. [Google Scholar] [CrossRef]
- Arisutha, S.; Baredar, P.; Deshpande, D.; Suresh, S. Effects of Thermo-chemical Pre-treatment on Bamboo for Biogas Production. Indian Chem. Eng. 2016, 58, 79–88. [Google Scholar] [CrossRef]
- Colón, J.; Martínez-Blanco, J.; Gabarrell, X.; Artola, A.; Sánchez, A.; Rieradevall, J.; Font, X. Environmental assessment of home composting. Resour. Conserv. Recycl. 2010, 54, 893–904. [Google Scholar] [CrossRef]
- Ndegwa, P.; Thompson, S. Integrating composting and vermicomposting in the treatment and bioconversion of biosolids. Bioresour. Technol. 2001, 76, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Esteves, G.D.F.; de Souza, K.R.D.; Bressanin, L.A.; Andrade, P.C.C.; Júnior, V.V.; dos Reis, P.E.; da Silva, A.B.; Mantovani, J.R.; Magalhães, P.C.; Pasqual, M.; et al. Vermicompost improves maize, millet and sorghum growth in iron mine tailings. J. Environ. Manag. 2020, 264, 110468. [Google Scholar] [CrossRef]
- Alshehrei, F.; Ameen, F. Vermicomposting: A management tool to mitigate solid waste. Saudi J. Biol. Sci. 2021, 28, 3284–3293. [Google Scholar] [CrossRef]
- Aslam, Z.; Bashir, S.; Hassan, W.; Bellitürk, K.; Ahmad, N.; Niazi, N.K.; Khan, A.; Khan, M.I.; Chen, Z.; Maitah, M. Unveiling the Efficiency of Vermicompost Derived from Different Biowastes on Wheat (Triticum aestivum L.) Plant Growth and Soil Health. Agronomy 2019, 9, 791. [Google Scholar] [CrossRef]
- Barthod, J.; Rumpel, C.; Calabi-Floody, M.; Mora, M.-L.; Bolan, N.; Dignac, M.-F. Adding worms during composting of organic waste with red mud and fly ash reduces CO2 emissions and increases plant available nutrient contents. J. Environ. Manag. 2018, 222, 207–215. [Google Scholar] [CrossRef]
- Blouin, M.; Barrere, J.; Meyer, N.; Lartigue, S.; Barot, S.; Mathieu, J. Vermicompost significantly affects plant growth. A meta-analysis. Agron. Sustain. Dev. 2019, 39, 34. [Google Scholar] [CrossRef]
- Bottinelli, N.; Hedde, M.; Jouquet, P.; Capowiez, Y. An explicit definition of earthworm ecological categories—Marcel Bouché’s triangle revisited. Geoderma 2020, 372, 114361. [Google Scholar] [CrossRef]
- Bortolotti, A.; Kampelmann, S.; De Muynck, S. Decentralised Organic Resource Treatments—Classification and comparison through Extended Material Flow Analysis. J. Clean. Prod. 2018, 183, 515–526. [Google Scholar] [CrossRef]
- Ismail, S.A. Organic waste management. In Technology Appreciation Programme on Evaluation of Biotechnological Approaches to Waste Management Held on 26th October 2000; Industrial Association-Ship of IIT: Madras, India, 2000; pp. 28–30. [Google Scholar]
- Khwairakpam, M.; Bhargava, R. Vermitechnology for sewage sludge recycling. J. Hazard. Mater. 2009, 161, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, P.S.; Gajalakshmi, S.; Abbasi, S. Vermicomposting of the leaf litter of acacia (Acacia auriculiformis): Possible roles of reactor geometry, polyphenols, and lignin. Bioresour. Technol. 2009, 100, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, J.; Aira, M.; Kolbe, A.R.; Gómez-Brandón, M.; Pérez-Losada, M. Changes in the composition and function of bacterial communities during vermicomposting may explain beneficial properties of vermicompost. Sci. Rep. 2019, 9, 9657. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, R.; Malińska, K.; Marfà, O. Nitrification within composting: A review. Waste Manag. 2018, 72, 119–137. [Google Scholar] [CrossRef]
- Ducasse, V.; Capowiez, Y.; Peigné, J. Vermicomposting of municipal solid waste as a possible lever for the development of sustainable agriculture. A review. Agron. Sustain. Dev. 2022, 42, 89. [Google Scholar] [CrossRef]
- Eo, J.; Park, K.-C. Effect of vermicompost application on root growth and ginsenoside content of Panax ginseng. J. Environ. Manag. 2019, 234, 458–463. [Google Scholar] [CrossRef]
- Hřebečková, T.; Wiesnerová, L.; Hanč, A. Changes of enzymatic activity during a large-scale vermicomposting process with continuous feeding. J. Clean. Prod. 2019, 239, 118127. [Google Scholar] [CrossRef]
- Kelley, A.; Wilkie, A.C.; Maltais-Landry, G. Food-Based Composts Provide More Soil Fertility Benefits than Cow Manure-Based Composts in Sandy Soils. Agriculture 2020, 10, 69. [Google Scholar] [CrossRef]
- Lohri, C.R.; Diener, S.; Zabaleta, I.; Mertenat, A.; Zurbrügg, C. Treatment technologies for urban solid biowaste to create value products: A review with focus on low- and middle-income settings. Rev. Environ. Sci. Bio/Technol. 2017, 16, 81–130. [Google Scholar] [CrossRef]
- Martin, K.L.; Emanuel, R.E.; Vose, J.M. Terra incognita: The unknown risks to environmental quality posed by the spatial distribution and abundance of concentrated animal feeding operations. Sci. Total Environ. 2018, 642, 887–893. [Google Scholar] [CrossRef]
- Clarke, W.P.; Taylor, M.; Cossins, R. Evaluation by respirometry of the loading capacity of a high rate vermicompost bed for treating sewage sludge. Bioresour. Technol. 2007, 98, 2611–2618. [Google Scholar] [CrossRef]
- Ndegwa, P.; Thompson, S.; Das, K. Effects of stocking density and feeding rate on vermicomposting of biosolids. Bioresour. Technol. 2000, 71, 5–12. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and prepared modification of the chronic acid titration method. Soil Sci. 1934, 3, 29–38. [Google Scholar] [CrossRef]
- APHA; AWWA; WPCF. Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 1995. [Google Scholar]
- Kuo, S. Chapter 32: Phosphorus. In Methods of Soil Analysis 3; Bartels, J.M., Bigham, J.M., Eds.; Chemical Methods; Soil Science Society of America: Madison, WI, USA, 1996; pp. 869–919. [Google Scholar]
- Suresh, S.; Keshav, A. Textbook of Separation Processes; Studium Press (India) Pvt. Ltd.: New Delhi, India, 2012; pp. 1–459. ISBN 978-93-80012-32-2. [Google Scholar]
- Kaushik, P.; Garg, V. Vermicomposting of mixed solid textile mill sludge and cow dung with the epigeic earthworm Eisenia foetida. Bioresour. Technol. 2003, 90, 311–316. [Google Scholar] [CrossRef]
- Nigussie, A.; Bruun, S.; de Neergaard, A.; Kuyper, T.W. Earthworms change the quantity and composition of dissolved organic carbon and reduce greenhouse gas emissions during composting. Waste Manag. 2017, 62, 43–51. [Google Scholar] [CrossRef]
- Kalamdhad, A.S.; Singh, Y.K.; Ali, M.; Khwairakpam, M.; Kazmi, A. Rotary drum composting of vegetable waste and tree leaves. Bioresour. Technol. 2009, 100, 6442–6450. [Google Scholar] [CrossRef]
- Mitchell, A.; Edwards, C.A. Production of vermicompost using Eisenia fetida from cattle manure. Soil Biol. Biochem. 1997, 3, 763–766. [Google Scholar] [CrossRef]
- Obriot, F.; Stauffer, M.; Goubard, Y.; Cheviron, N.; Peres, G.; Eden, M.; Revallier, A.; Vieublé-Gonod, L.; Houot, S. Multi-criteria indices to evaluate the effects of repeated organic amendment applications on soil and crop quality. Agric. Ecosyst. Environ. 2016, 232, 165–178. [Google Scholar] [CrossRef]
- Elvira, C.; Sampedro, L.; Benítez, E.; Nogales, R. Vermicomposting of sludges from paper mill and dairy industries with Eisenia andrei: A pilot-scale study. Bioresour. Technol. 1998, 63, 205–211. [Google Scholar] [CrossRef]
- Mohee, R.; Mudhoo, A. Analysis of the physical properties of an in-vessel composting matrix. Powder Technol. 2005, 155, 92–99. [Google Scholar] [CrossRef]
- Kalamdhad, A.S.; Pasha, M.; Kazmi, A. Stability evaluation of compost by respiration techniques in a rotary drum composter. Resour. Conserv. Recycl. 2008, 52, 829–834. [Google Scholar] [CrossRef]
- Sahariah, B.; Das, S.; Goswami, L.; Paul, S.; Bhattacharyya, P.; Bhattacharya, S.S. An avenue for replacement of chemical fertilization under rice-rice cropping pattern: Sustaining soil health and organic C pool via MSW-based vermicomposts. Arch. Agron. Soil Sci. 2020, 66, 1449–1465. [Google Scholar] [CrossRef]
- Tedesco, D.E.; Conti, C.; Lovarelli, D.; Biazzi, E.; Bacenetti, J. Bioconversion of fruit and vegetable waste into earthworms as a new protein source: The environmental impact of earthworm meal production. Sci. Total Environ. 2019, 683, 690–698. [Google Scholar] [CrossRef]
- Tejada, M.; Benítez, C. Effects of different organic wastes on soil biochemical properties and yield in an olive grove. Appl. Soil Ecol. 2020, 146, 103371. [Google Scholar] [CrossRef]
- Garg, P.; Gupta, A.; Satya, S. Vermicomposting of different types of waste using Eisenia foetida: A comparative study. Bioresour. Technol. 2006, 97, 391–395. [Google Scholar] [CrossRef]
- Kaviraj; Sharma, S. Municipal solid waste management through vermicomposting employing exotic and local species of earthworms. Bioresour. Technol. 2003, 90, 169–173. [Google Scholar] [CrossRef]
- Samal, K.; Mohan, A.R.; Chaudhary, N.; Moulick, S. Application of vermitechnology in waste management: A review on mechanism and performance. J. Environ. Chem. Eng. 2019, 7, 103392. [Google Scholar] [CrossRef]
- Domínguez, J.; Edwards, C. Effects of stocking rate and moisture content on the growth and maturation of Eisenia andrei (Oligochaeta) in pig manure. Soil Biol. Biochem. 1997, 29, 743–746. [Google Scholar] [CrossRef]
- Edwards, C.A.; Dominguez, J.; Neuhauser, E.F. Growth and reproduction of Perionyx excavatus (Perr.) (Megascolecidae) as factors in organic waste management. Biol. Fertil. Soils 1998, 27, 155–161. [Google Scholar] [CrossRef]
- Pramanik, P.; Ghosh, G.K.; Ghosal, P.K.; Banik, P. Changes in organic—C, N, P and K and enzyme activities in ver-micompost of biodegradable organic wastes under liming and microbial inoculants. Bioresour. Technol. 2007, 3, 2485–2494. [Google Scholar] [CrossRef]
- Waqas, M.; Hashim, S.; Humphries, U.W.; Ahmad, S.; Noor, R.; Shoaib, M.; Naseem, A.; Hlaing, P.T.; Lin, H.A. Composting Processes for Agricultural Waste Management: A Comprehensive Review. Processes 2023, 11, 731. [Google Scholar] [CrossRef]
- Hartenstein, R.; Leaf, A.L.; Neuhauser, E.F.; Bickelhaupt, D.H. Composition of the earthworm Eisenia foetida (savigny) and assimilation of 15 elements from sludge during growth. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1980, 66, 187–192. [Google Scholar] [CrossRef]
- Hogg, D.; Eavoino, E.; Caimi, V.; Amlinger, F.; Devliegher, W.; Brinton, W.; Antler, S. Comparison of Compost Standards within the EU, North America and Australia; The Waste and Resources Action Programme (WARP): Oxford, UK, 2002. [Google Scholar]
- Gunadi, B.; Blount, C.; Edwards, C.A. The growth and fecundity of Eisenia fetida (Savigny) in cattle solids pre-composted for different periods. Pedobiologia 2002, 46, 15–23. [Google Scholar] [CrossRef]
- Hand, P.; Hayes, W.A.; Frankland, J.C.; Satchell, J.E. The vermicomposting of cow slurry. In Earthworms in Waste and Environmental Management; SPB Academic Publishing: The Hague, The Netherlands, 1988; pp. 49–63. [Google Scholar]
- Tripathi, G.; Bhardwaj, P. Decomposition of kitchen waste amended with cow manure using an epigeic species (Eisenia fetida) and an anecic species (Lampito mauritii). Bioresour. Technol. 2004, 92, 215–218. [Google Scholar] [CrossRef]
- Atiyeh, R.M.; Domínguez, J.; Subler, S.; Edwards, C.A. Changes in biochemical properties of cow manure during pro-cessing by earthworms (Eisenia andrei, Bouché) and the effects on seedling growth. Pedobiologia 2000, 3, 709–724. [Google Scholar] [CrossRef]
- Mansell, G.P.; Syers, J.K.; Gregg, P.E.H. Plant availability of phosphorous in dead herbage ingested by surface-casting earthworms. Soil Biol. Biochem. 1981, 3, 163–167. [Google Scholar] [CrossRef]
- Lee, K.E. Some trends opportunities in earthworm research or: Darwin’s children. The future of our discipline. Soil Biol. Biochem. 1992, 3, 1765–1771. [Google Scholar] [CrossRef]
- Benitez, E.; Nogales, R.; Elvira, C.; Masciandaro, G.; Ceccanti, B. Enzyme activities as indicators of the stabilization of sewage sludges composting with Eisenia foetida. Bioresour. Technol. 1999, 67, 297–303. [Google Scholar] [CrossRef]
- Suthar, S. Vermicomposting potential of Perionyx sansibaricus (Perrier) in different waste materials. Bioresour. Technol. 2007, 98, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Ndegwa, P.M.; Thompson, S. Effects of C-to-N ratio on vermicomposting of biosolids. Bioresour. Technol. 2000, 75, 7–12. [Google Scholar] [CrossRef]
- Butt, K.R. Utilization of solid paper mill sludge and spent brewery yeast as a food for soil-dwelling earthworms. Biores. Technol. 1993, 3, 105–107. [Google Scholar] [CrossRef]
- Garg, V.K.; Chand, S.; Chhillar, A.; Yadav, A. Growth and reproduction of Eisenia feotida in various animal wastes during vermicomposting. Appl. Ecol. Environ. Res. 2005, 3, 51–59. [Google Scholar] [CrossRef]
- Suthar, S. Bioconversion of post-harvest crop residues and cattle shed manure into value-added products using earth-worms Eudrilus eugeniae Kinberg. Ecol. Eng. 2008, 3, 206–214. [Google Scholar] [CrossRef]
- Edwards, C.A.; Fletcher, K. Interactions between earthworms and microorganisms in organic-matter breakdown. Agric. Ecosyst. Environ. 1988, 24, 235–247. [Google Scholar] [CrossRef]
- Buchanan, M.A.; Russell, G.; Block, S.D. Chemical characterization and nitrogen mineralization potentials of ver-micomposts derived from differing organic wastes. In Earthworms in Waste and Environmental Management; Edwards, C.A., Neuhauser, E.F., Eds.; SPB Academic Publishing: The Hague, The Netherlands, 1988. [Google Scholar]
- Jambhekar, H. Use of earthworm as a potential source of decompose organic wastes. In Proceedings of the National Seminar on Organic Farming, Coimbatore, India, 1992; pp. 52–53. [Google Scholar]
- Chaoui, H.I.; Zibilske, L.M.; Ohno, T. Effects of earthworm casts and compost on soil microbial activity and plant nutrient availability. Soil Biol. Biochem. 2003, 35, 295–302. [Google Scholar] [CrossRef]
- Gajalakshmi, S.; Ramasamy, E.; Abbasi, S. Composting–vermicomposting of leaf litter ensuing from the trees of mango (Mangifera indica). Bioresour. Technol. 2005, 96, 1057–1061. [Google Scholar] [CrossRef]
- Gajalakshmi, S.; Abbasi, S.A. Neem leaves as a source of fertilizer-cumpesticide vermicompost. Biores. Technol. 2004, 3, 291–296. [Google Scholar] [CrossRef]
- Crawford, J.H. Review of composting. Process. Biochem. 1983, 3, 14–15. [Google Scholar]
- Viel, M.; Sayag, D.; Andre, L. Optimization of agricultural, industrial waste management through in-vessel composting. In Compost: Production; Quality and Use; de Bertoldi, M., Ed.; Elsevier Applied Science: Essex, UK, 1987; pp. 230–237. [Google Scholar]
- Fadaee, R. A review on earthworm Eisenia foetida and its applications. Ann. Biol. Res. 2012, 3, 2500–2506. [Google Scholar]
- Flegel, M.; Schreder, S. Importance of food quality on selected enzyme activities in earthworm casts (Dendrobaena octaedra, Lumbricidae). Soil Biol. Biochem. 2000, 3, 1191–1196. [Google Scholar] [CrossRef]
- Senesi, N. Composted materials as organic fertilizers. Sci. Total Environ. 1989, 81–82, 521–542. [Google Scholar] [CrossRef]
- Gupta, R.; Garg, V.K. Vermiremediation and nutrient recovery of nonrecyclable paper waste employing Eisenia fetida. J. Hazard. Mater. 2009, 3, 430–439. [Google Scholar] [CrossRef]




| Parameter | Bhopal Municipal Waste | MANIT Campus Waste | Single-Household Waste |
|---|---|---|---|
| Composting | |||
| MC (%) | 40.50 ± 3.9 | 50.55 ± 4.2 | 60 ± 3 |
| C:N | 14.99 ± 0.35 | 14.30 ± 0.21 | 15.79 ± 0.33 |
| pH | 7.3 ± 0.8 | 6.7 ± 0.3 | 7.8 ± 0.34 |
| EC (dS/m) | 1.37 ± 0.08 | 1.69 ± 0.03 | 2.78 ± 0.02 |
| N (%) | 1.08 ± 0.6 | 1.102 ± 0.7 | 1.13 ± 0.5 |
| P (%) | 0.308 ± 0.2 | 0.411 ± 2.2 | 0.413 ± 0.6 |
| K (%) | 0.689 ± 0.2 | 0.611 ± 1.3 | 0.789 ± 0.5 |
| E. fetida | |||
| MC (%) | 55 ± 5.2 | 53 ± 6.3 | 58 ± 6.2 |
| C:N | 9.98 ± 0.41 | 9.73 ± 0.31 | 10.01 ± 0.23 |
| pH | 7.0 ± 0.2 | 6.5 ± 0.3 | 7.8 ± 0.6 |
| EC (dS/m) | 1.25 ± 0.08 | 1.06 ± 0.02 | 2.05 ± 0.02 |
| N (%) | 1.601 ± 4 | 1.57 ± 5 | 1.701 ± 4 |
| P (%) | 1.011 ± 1 | 1.19 ± 2 | 1.01 ± 1 |
| K (%) | 1.250 ± 3 | 1.109 ± 4 | 1.301 ± 4 |
| E. eugeniae | |||
| MC (%) | 53 ± 6.3 | 52 ± 6.1 | 55 ± 6.5 |
| C:N | 10.39 ± 0.41 | 10.89 ± 0.3 | 10.72 ± 0.32 |
| pH | 7.0 ± 0.02 | 6.99 ± 0.1 | 7.7 ± 0.02 |
| EC(dS/m) | 1.76 ± 0.01 | 1.07 ± 0.01 | 2.10 ± 0.02 |
| N (%) | 1.52 ± 1 | 1.57 ± 5 | 1.69 ± 2 |
| P (%) | 1.11 ± 3 | 1.02 ± 2 | 0.987 ± 1 |
| K (%) | 1.20 ± 3 | 1.109 ± 3 | 1.301 ± 1 |
| Parameter | Reactor 1 | Reactor 2 | Reactor 3 | Reactor 4 | Reactor 5 | Reactor 6 | Reactor 7 | Reactor 8 | Reactor 9 |
|---|---|---|---|---|---|---|---|---|---|
| E. fetida | |||||||||
| MC(%) | 62 ± 5.2 | 60 ± 0.2 | 63 ± 4.0 | 63 ± 5.6 | 65 ± 6.0 | 68 ± 6.0 | 67 ± 6.0 | 69 ± 4.0 | 70 ± 4.0 |
| C/N | 9.2 ± 0.3 | 9.0 ± 0.2 | 9.5 ± 0.4 | 10.1 ± 0.2 | 9.5 ± 0.3 | 9.6 ± 0.2 | 9.3 ± 0.1 | 9.2 ± 0.3 | 9.5 ± 0.3 |
| pH | 7.5 ± 0.3 | 7.8 ± 0.2 | 7.6 ± 0.3 | 7.5 ± 0.5 | 7.8 ± 0.6 | 7.0 ± 0.2 | 7.3 ± 0.2 | 7.2 ± 0.2 | 7.5 ± 0.3 |
| EC (dS/m) | 1.98 ± 0.07 | 1.68 ± 0.02 | 1.79 ± 0.03 | 1.86 ± 0.02 | 1.98 ± 0.07 | 1.93 ± 0.06 | 1.98 ± 0.03 | 1.97 ± 0.02 | 1.99 ± 0.02 |
| N (%) | 1.502 ± 0.02 | 1.313 ± 0.04 | 1.308 ± 0.03 | 1.302 ± 0.02 | 1.278 ± 0.03 | 1.379 ± 0.04 | 1.179 ± 3.0 | 1.220 ± 0.03 | 1.108 ± 0.02 |
| P (%) | 1.28 ± 0.02 | 1.27 ± 0.01 | 1.22 ± 0.02 | 1.108 ± 0.01 | 1.07 ± 0.01 | 1.09 ± 0.01 | 1.08 ± 0.02 | 1.07 ± 0.02 | 1.07 ± 0.03 |
| K (%) | 1.381 ± 0.01 | 1.38 ± 1.0 | 1.38 ± 0.02 | 1.27 ± 0.01 | 1.27 ± 0.01 | 1.2 ± 0.02 | 1.10 ± 0.03 | 1.08 ± 0.03 | 1.081 ± 0.04 |
| E. eugeniae | |||||||||
| MC (%) | 59 ± 2.5 | 58 ± 6.0 | 62 ± 5.0 | 65 ± 4.9 | 68 ± 4.0 | 72 ± 3.0 | 72 ± 2.9 | 73 ± 2.8 | 75 ± 4.0 |
| C/N | 10.2 ± 0.31 | 10.8 ± 0.41 | 10.7 ± 0.32 | 10.79 ± 0.35 | 10.72 ± 0.25 | 10.72 ± 0.22 | 10.71 ± 0.25 | 10.5 ± 0.27 | 10.7 ± 0.41 |
| pH | 7.2 ± 0.07 | 7.1 ± 0.02 | 7.5 ± 0.02 | 7.8 ± 0.03 | 7.8 ± 0.09 | 7.0 ± 0.07 | 7.8 ± 0.08 | 7.5 ± 0.02 | 7.0 ± 0.07 |
| EC (dS/m) | 1.23 ± 0.02 | 1.40 ± 0.01 | 1.52 ± 0.02 | 1.81 ± 0.07 | 1.52 ± 0.08 | 1.40 ± 0.02 | 1.87 ± 0.07 | 1.98 ± 0.01 | 1.7 ± 0.02 |
| N (%) | 1.113 ± 0.03 | 0.895 ± 0.05 | 0.878 ± 0.04 | 0.834 ± 0.03 | 0.798 ± 0.02 | 0.913 ± 0.03 | 0.940 ± 0.02 | 0.934 ± 0.02 | 0.838 ± 0.03 |
| P (%) | 1.090 ± 0.02 | 1.01 ± 0.03 | 0.98 ± 0.02 | 0.79 ± 0.02 | 0.775 ± 0.03 | 0.79 ± 0.02 | 0.708 ± 0.02 | 0.72 ± 0.03 | 0.71 ± 0.03 |
| K (%) | 1.19 ± 0.02 | 1.17 ± 0.03 | 1.12 ± 0.01 | 1.09 ± 0.03 | 1.02 ± 0.02 | 1.0 ± 0.03 | 0.979 ± 0.04 | 0.838 ± 0.04 | 0.923 ± 0.02 |
| E. fetida + E. eugeniae | |||||||||
| MC (%) | 62 ± 5.2 | 60 ± 0.2 | 63 ± 4.0 | 63 ± 5.6 | 65 ± 6.0 | 68 ± 6.0 | 67 ± 6.0 | 69 ± 4.0 | 70 ± 4.0 |
| C/N | 8.30 ± 0.3 | 9.32 ± 0.3 | 8.70 ± 0.3 | 9.98 ± 0.3 | 8.25 ± 0.2 | 8.99 ± 0.2 | 9.20 ± 0.2 | 8.10 ± 0.3 | 8.78 ± 0.2 |
| pH | 7.5 ± 0.2 | 7.9 ± 0.3 | 8.1 ± 0.4 | 8.0 ± 0.3 | 7.8 ± 0.4 | 8.5 ± 0.3 | 8.3 ± 0.7 | 7.9 ± 0.6 | 8.1 ± 0.2 |
| EC (dS/m) | 0.98 ± 0.07 | 1.32 ± 0.02 | 1.79 ± 0.02 | 1.20 ± 0.07 | 1.70 ± 0.02 | 2.10 ± 0.07 | 1.79 ± 0.02 | 0.98 ± 0.07 | 1.58 ± 0.04 |
| N (%) | 1.79 ± 0.03 | 1.686 ± 0.02 | 1.685 ± 0.03 | 1.58 ± 0.04 | 1.585 ± 0.02 | 1.68 ± 0.03 | 1.68 ± 0.04 | 1.67 ± 0.02 | 1.68 ± 0.03 |
| P (%) | 1.12 ± 0.02 | 1.19 ± 0.04 | 1.17 ± 0.04 | 1.2 ± 2.0 | 1.18 ± 0.01 | 1.19 ± 0.02 | 1.28 ± 0.03 | 1.17 ± 0.02 | 1.19 ± 0.01 |
| K (%) | 1.5 ± 0.03 | 1.48 ± 0.01 | 1.17 ± 0.02 | 1.28 ± 1.0 | 1.18 ± 0.03 | 1.08 ± 0.02 | 1.17 ± 0.03 | 1.08 ± 0.02 | 1.17 ± 0.01 |
| Days | Bhopal Municipal | MANIT Campus | Single-Household |
|---|---|---|---|
| Compost | |||
| 3 | 14.75 ± 0.5 | 14.2 ± 0.6 | 15.7 ± 0.3 |
| 6 | 11.6 ± 1.0 | 12.3 ± 0.2 | 14.5 ± 0.6 |
| 9 | 11.2 ± 0.35 | 11.1 ± 0.8 | 13.3 ± 0.3 |
| 12 | 10.1 ± 0.3 | 11.0 ± 0.7 | 11.3 ± 0.1 |
| 15 | 10.3 ± 0.4 | 10.9 ± 0.2 | 11.1 ± 0.3 |
| 18 | 10.0 ± 0.2 | 10.6 ± 0.2 | 10.9 ± 0.2 |
| 21 | 10.0 ± 0.25 | 10.6 ± 0.3 | 10.7 ± 0.4 |
| 24 | 10.1 ± 0.22 | 10.7 ± 0.3 | 10.9 ± 0.3 |
| E. fetida | |||
| 3 | 10.1 ± 0.16 | 10.6 ± 0.90 | 10.67 ± 0.81 |
| 6 | 10.1 ± 0.22 | 9.9 ± 0.155 | 10.5 ± 0.77 |
| 9 | 9.8 ± 0.3 | 10.2 ± 0.13 | 10.2 ± 0.85 |
| 12 | 9.7 ± 0.22 | 10.3 ± 0.80 | 10.1 ± 0.77 |
| 15 | 9.7 ± 0.12 | 10.2 ± 0.95 | 9.9 ± 0.53 |
| 18 | 9.7 ± 0.13 | 9.9 ± 0.86 | 9.7 ± 0.50 |
| 21 | 9.6 ± 0.02 | 9.7 ± 0.19 | 9.8 ± 0.95 |
| 24 | 10.1 ± 0.03 | 9.2 ± 0.79 | 9.0 ± 0.88 |
| E. eugeniae | |||
| 3 | 9.9 ± 0.12 | 10.71 ± 0.10 | 9.92 ± 0.16 |
| 6 | 9.8 ± 0.15 | 9.7 ± 0.13 | 9.7 ± 0.7 |
| 9 | 9.0 ± 0.27 | 9.2 ± 0.11 | 8.9 ± 0.6 |
| 12 | 9.7 ± 0.12 | 9.2 ± 0.8 | 9.5 ± 0.6 |
| 15 | 9.2 ± 0.31 | 9.1 ± 0.10 | 9.2 ± 0.31 |
| 18 | 9.1 ± 0.12 | 9.6 ± 0.11 | 8.8 ± 0.11 |
| 21 | 9.1 ± 0.11 | 9.5 ± 0.12 | 9.3 ± 0.7 |
| 24 | 9.9 ± 0.15 | 9.6 ± 0.10 | 9.5 ± 0.14 |
| Days | Reactor 1 | Reactor 2 | Reactor 3 | Reactor 4 | Reactor 5 | Reactor 6 | Reactor 7 | Reactor 8 | Reactor 9 |
|---|---|---|---|---|---|---|---|---|---|
| E. fetida | |||||||||
| 3 | 12.3 ± 0.16 | 11.2 ±0.6 | 10.8 ± 0.12 | 12.2 ± 0.15 | 12.9 ± 0.7 | 11.9 ± 0.15 | 11.9 ± 0.17 | 12.7 ± 0.18 | 10.8 ± 0.19 |
| 6 | 12.7 ± 0.12 | 12.9 ± 0.19 | 11.5 ± 0.11 | 11.7 ± 0.7 | 12.8 ± 0.4 | 10.2 ± 0.7 | 12.8 ± 0.9 | 10.2 ± 0.2 | 10.7 ± 0.2 |
| 9 | 11.7 ± 0.15 | 11.6 ± 0.13 | 10.7 ± 0.7 | 11.8 ± 0.2 | 11.2 ± 0.4 | 11.2 ± 0.2 | 12.2 ± 0.2 | 10.9 ± 0.6 | 11.8 ± 0.2 |
| 12 | 11.2 ± 0.01 | 10.8 ± 0.03 | 10.8 ± 0.2 | 11.2 ± 0.3 | 11.2 ± 0.5 | 12.9 ± 0.2 | 11.2 ± 0.7 | 11.2 ± 0.7 | 10.8 ± 0.3 |
| 15 | 10.2 ± 0.6 | 10.7 ± 0.7 | 11.9 ± 0.7 | 11.2 ± 0.4 | 10.2 ± 0.7 | 12.8 ± 0.3 | 10.2 ± 0.7 | 10.7 ± 0.7 | 11.8 ± 0.5 |
| 18 | 12.8 ± 0.17 | 11.9 ± 0.19 | 11.7 ± 0.3 | 11.8 ± 0.3 | 11.2 ± 0.1 | 10.2 ± 0.5 | 11.7 ± 0.8 | 10.8 ± 0.7 | 10.8 ± 0.7 |
| 21 | 11.8 ± 0.17 | 11.2 ± 0.18 | 12.8 ± 0.1 | 10.2 ± 0.2 | 10.2 ± 0.3 | 10.3 ± 0.7 | 10.9 ± 0.6 | 11.2 ± 0.9 | 10.2 ± 0.8 |
| 24 | 10.7 ± 0.16 | 10.8 ± 0.13 | 10.9 ± 0.9 | 10.2 ± 0.2 | 10.3 ± 0.2 | 10.9 ± 0.9 | 10.2 ± 0.5 | 10.7 ± 0.5 | 10.0 ± 0.5 |
| E. eugeniae | |||||||||
| 3 | 10.2 ± 0.12 | 11.8 ± 0.23 | 10.2 ± 0.5 | 11.5 ± 0.3 | 11.7 ± 0.4 | 10.8 ± 0.30 | 9.9 ± 0.17 | 10.8 ± 0.19 | 11.8 ± 0.17 |
| 6 | 10.7 ± 0.7 | 11.2 ± 0.7 | 9.0 ± 0.2 | 11.2 ± 0.7 | 11.2 ± 0.7 | 11.2 ± 0.3 | 10.8 ± 0.3 | 10.7 ± 0.3 | 10.2 ± 0.2 |
| 9 | 10.9 ± 0.2 | 10.2 ± 0.6 | 9.7 ± 0.3 | 10.9 ± 0.2 | 10.8 ± 0.6 | 11.2 ± 0.2 | 11.2 ± 0.3 | 11.8 ± 0.5 | 10.5 ± 0.1 |
| 12 | 11.2 ± 0.3 | 10.8 ± 0.7 | 10.2 ± 0.4 | 10.7 ± 0.2 | 10.2 ± 0.2 | 10.8 ± 0.2 | 10.2 ± 0.2 | 9.2 ± 0.4 | 10.6 ± 0.7 |
| 15 | 10.2 ± 0.2 | 11.2 ± 0.2 | 11.2 ± 0.2 | 11.2 ± 0.7 | 9.7 ± 0.2 | 9.9 ± 0.3 | 10.3 ± 0.2 | 11.2 ± 0.5 | 9.9 ± 0.6 |
| 18 | 9.3 ± 0.5 | 9.0 ± 0.4 | 9.8 ± 0.2 | 10.5 ± 0.2 | 9.3 ± 0.3 | 9.8 ± 0.2 | 10.0 ± 0.2 | 10.8 ± 0.7 | 9.5 ± 0.4 |
| 21 | 9.8 ± 0.9 | 9.2 ± 0.2 | 9.7 ± 0.7 | 10.2 ± 0.3 | 9.2 ± 0.3 | 9.7 ± 0.3 | 9.5 ± 0.4 | 10.7 ± 0.6 | 9.6 ± 0.3 |
| 24 | 9.2 ± 0.2 | 9.0 ± 0.2 | 9.5 ± 0.6 | 10.1 ± 0.2 | 9.5 ± 0.2 | 9.6 ± 0.2 | 9.3 ± 0.3 | 9.2 ± 0.2 | 9.5 ± 0.3 |
| E. fetida + E. eugeniae | |||||||||
| 3 | 13.4 ± 0.8 | 13.7 ± 0.2 | 14.1 ± 0.3 | 14.7 ± 0.2 | 14.2 ± 0.3 | 15.8 ± 0.3 | 15.8 ± 0.7 | 16.6 ± 0.6 | 16.9 ± 0.2 |
| 6 | 13.8 ± 0.9 | 10.2 ± 0.3 | 13.7 ± 0.4 | 13.2 ± 0.4 | 13.5 ± 0.3 | 14.3 ± 0.2 | 14.2 ± 0.5 | 15.3 ± 0.7 | 16.3 ± 0.3 |
| 9 | 12.7 ± 0.6 | 9.7 ± 0.4 | 12.8 ± 0.6 | 12.2 ± 0.5 | 12.1 ± 0.5 | 13.2 ± 0.1 | 13.6 ± 0.4 | 14.4 ± 0.8 | 15.2 ± 0.4 |
| 12 | 12.8 ± 0.3 | 9.4 ± 0.5 | 11.3 ± 0.3 | 11.7 ± 0.3 | 11.8 ± 0.2 | 12.8 ± 0.4 | 12.4 ± 0.8 | 13.2 ± 0.6 | 14.3 ± 0.5 |
| 15 | 10.8 ± 0.7 | 9.0 ± 0.3 | 10.2 ± 0.3 | 10.3 ± 0.2 | 11.3 ± 0.5 | 11.2 ± 0.7 | 12.8 ± 0.6 | 13.8 ± 0.5 | 13.9 ± 0.3 |
| 18 | 9.7 ± 0.6 | 8.9 ± 0.5 | 9.3 ± 0.5 | 9.8 ± 0.1 | 9.9 ± 0.2 | 11.2 ± 0.5 | 10.3 ± 0.8 | 11.7 ± 0.3 | 13.8 ± 0.6 |
| 21 | 8.2 ± 0.4 | 8.7 ± 0.6 | 8.4 ± 0.7 | 9.2 ± 0.5 | 9.9 ± 0.1 | 10.8 ± 0.7 | 9.8 ± 0.6 | 10.2 ± 0.2 | 12.2 ± 0.4 |
| 24 | 7.4 ± 0.3 | 8.9 ± 0.7 | 8.3 ± 0.8 | 8.1 ± 0.4 | 8.5 ± 0.4 | 9.3 ± 0.9 | 8.2 ± 0.7 | 9.4 ± 0.3 | 10.3 ± 0.3 |
| Process | Bhopal Municipality Waste | MANIT Campus Waste | Single-Household Waste | |||
|---|---|---|---|---|---|---|
| E. coli | E. faecalis | E. coli | E. faecalis | E. coli | E. faecalis | |
| Composting | >95 | >93 | >93 | >91 | >72 | >75 |
| Vermicomposting (E. fetida) | 5.4 | 5.6 | 5.0 | 5.5 | 5.0 | 5.7 |
| Vermicomposting (E. eugeniae) | 6.7 | 6.9 | 6.0 | 6.5 | 6.3 | 5.8 |
| Reactor 1 | Reactor 2 | Reactor 3 | Reactor 4 | Reactor 5 | Reactor 6 | Reactor 7 | Reactor 8 | Reactor 9 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Process | E. coli | E. faecalis | E. coli | E. faecalis | E. coli | E. faecalis | E. coli | E. faecalis | E. coli | E. faecalis | E. coli | E. faecalis | E. coli | E. faecalis | E. coli | E. faecalis | E. coli | E. faecalis |
| Vermicomposting (E. fetida) | 6.8 | 6.7 | 6.9 | 6.9 | 7.0 | 7.3 | 7.1 | 7.2 | 7.0 | 7.8 | 9.1 | 9.7 | 7.7 | 9.4 | 8.0 | 8.0 | 6.9 | 6.7 |
| Vermicomposting (E. eugeniae) | 9.9 | 9.8 | 9.0 | 8.6 | 8.2 | 8.0 | 8.1 | 7.4 | 8.4 | 8.6 | 6.0 | 5.9 | 8.0 | 8.0 | 9.5 | 8.0 | 7.0 | 8.0 |
| Vermicomposting (E. fetida + E. eugeniae) | 5.7 | 6.0 | 7.8 | 7.4 | 4.1 | 7.0 | 7.0 | 7.3 | 7.4 | 7.7 | 6.1 | 5.7 | 6.2 | 6.6 | 6.3 | 5.7 | 6.2 | 6.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katiyar, R.B.; Sundaramurthy, S.; Sharma, A.K.; Arisutha, S.; Khan, M.A.; Sillanpää, M. Optimization of Engineering and Process Parameters for Vermicomposting. Sustainability 2023, 15, 8090. https://doi.org/10.3390/su15108090
Katiyar RB, Sundaramurthy S, Sharma AK, Arisutha S, Khan MA, Sillanpää M. Optimization of Engineering and Process Parameters for Vermicomposting. Sustainability. 2023; 15(10):8090. https://doi.org/10.3390/su15108090
Chicago/Turabian StyleKatiyar, Rajesh Babu, Suresh Sundaramurthy, Anil Kumar Sharma, Suresh Arisutha, Moonis Ali Khan, and Mika Sillanpää. 2023. "Optimization of Engineering and Process Parameters for Vermicomposting" Sustainability 15, no. 10: 8090. https://doi.org/10.3390/su15108090
APA StyleKatiyar, R. B., Sundaramurthy, S., Sharma, A. K., Arisutha, S., Khan, M. A., & Sillanpää, M. (2023). Optimization of Engineering and Process Parameters for Vermicomposting. Sustainability, 15(10), 8090. https://doi.org/10.3390/su15108090








