Synthesis and Characterizations of Fe-Doped NiO Nanoparticles and Their Potential Photocatalytic Dye Degradation Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Fe-Doped NiO Nanoparticles
2.3. Characterization of Fe-NiO Nanoparticles
2.4. Photocatalytic Dye Degradation
3. Results and Discussion
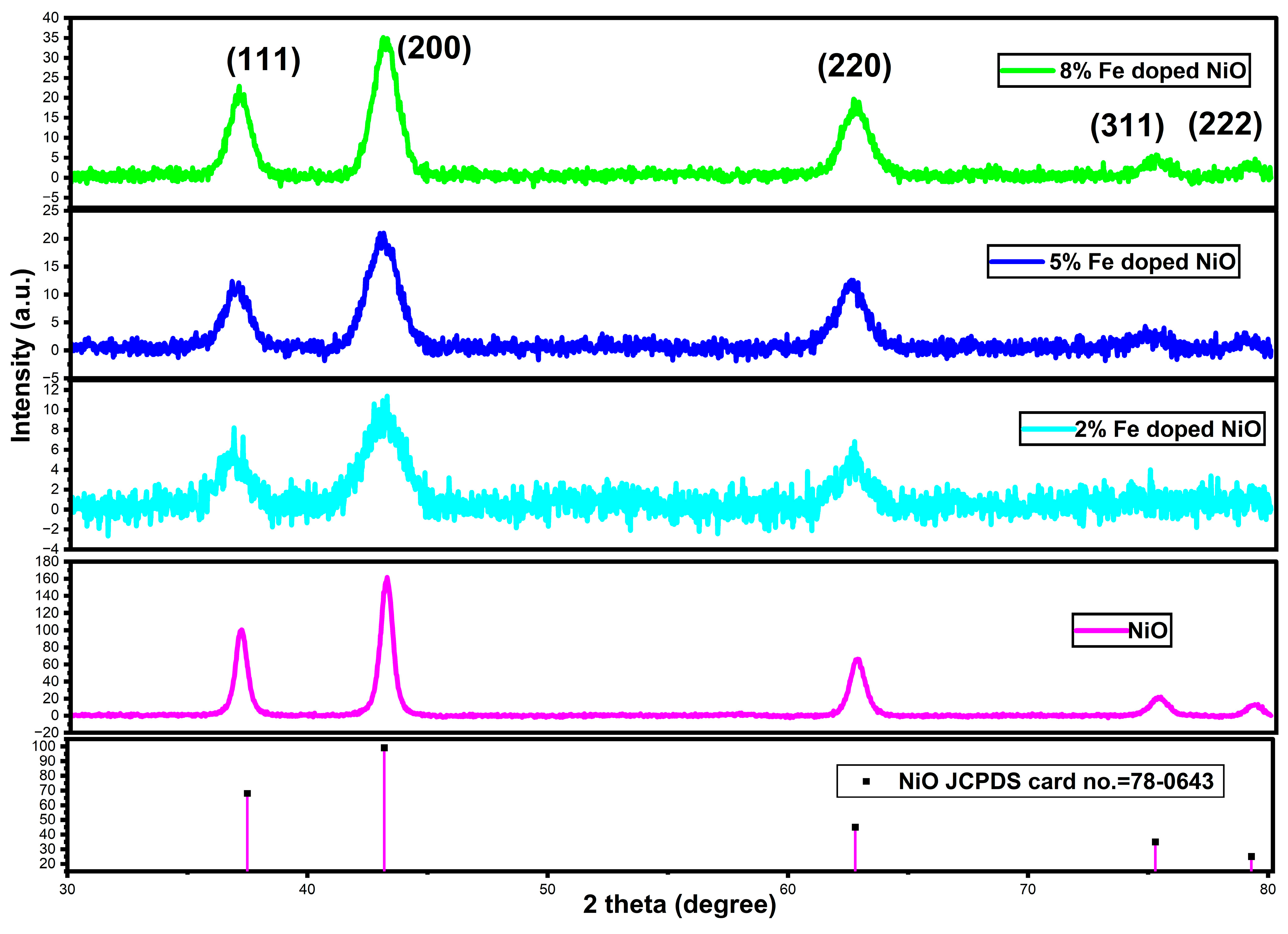
3.1. XRD Analysis
3.2. FTIR Analysis
3.3. FESEM Analysis
3.4. TEM with SAED Analysis
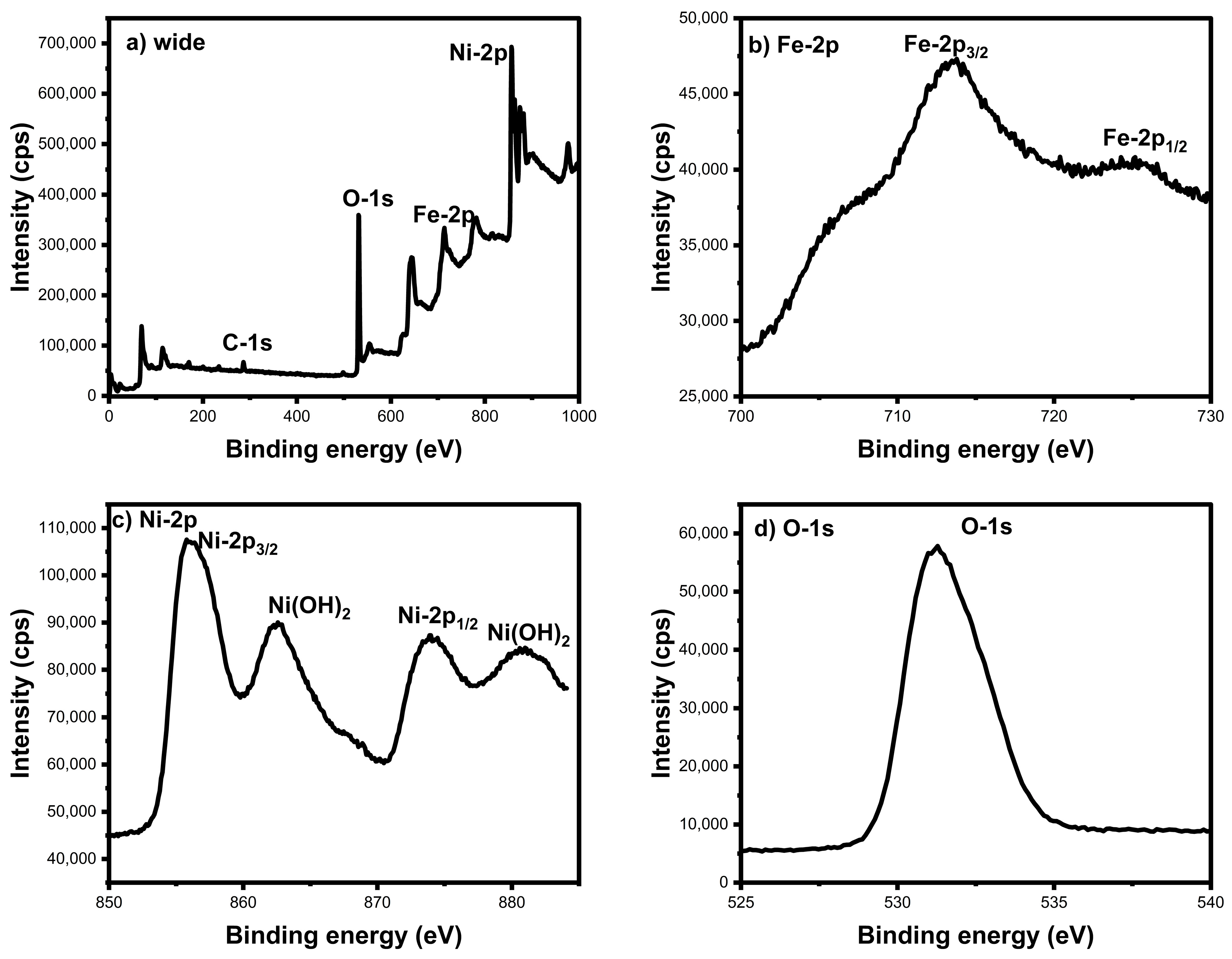
3.5. XPS Analysis
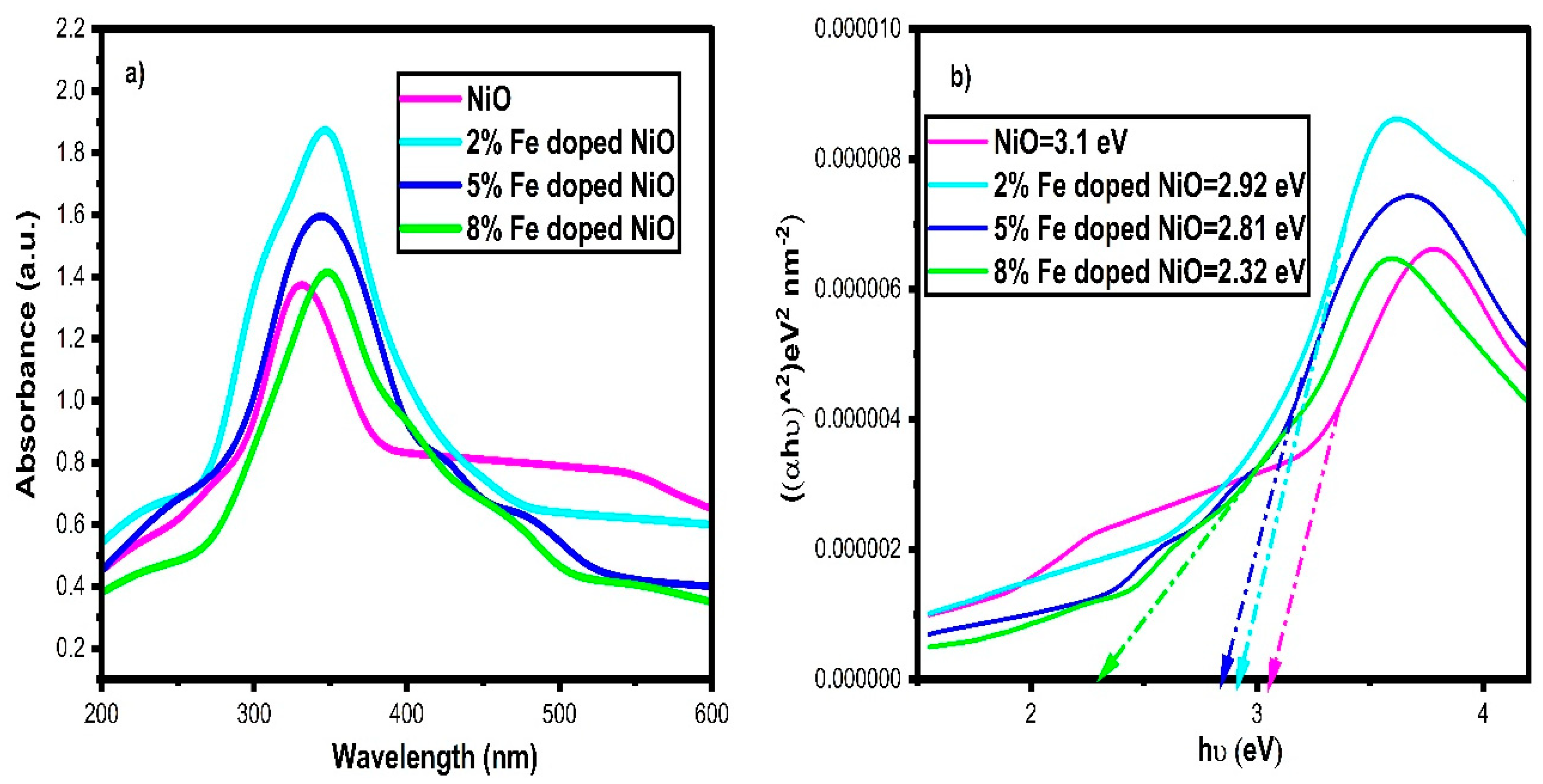
3.6. UV–Visible Analysis
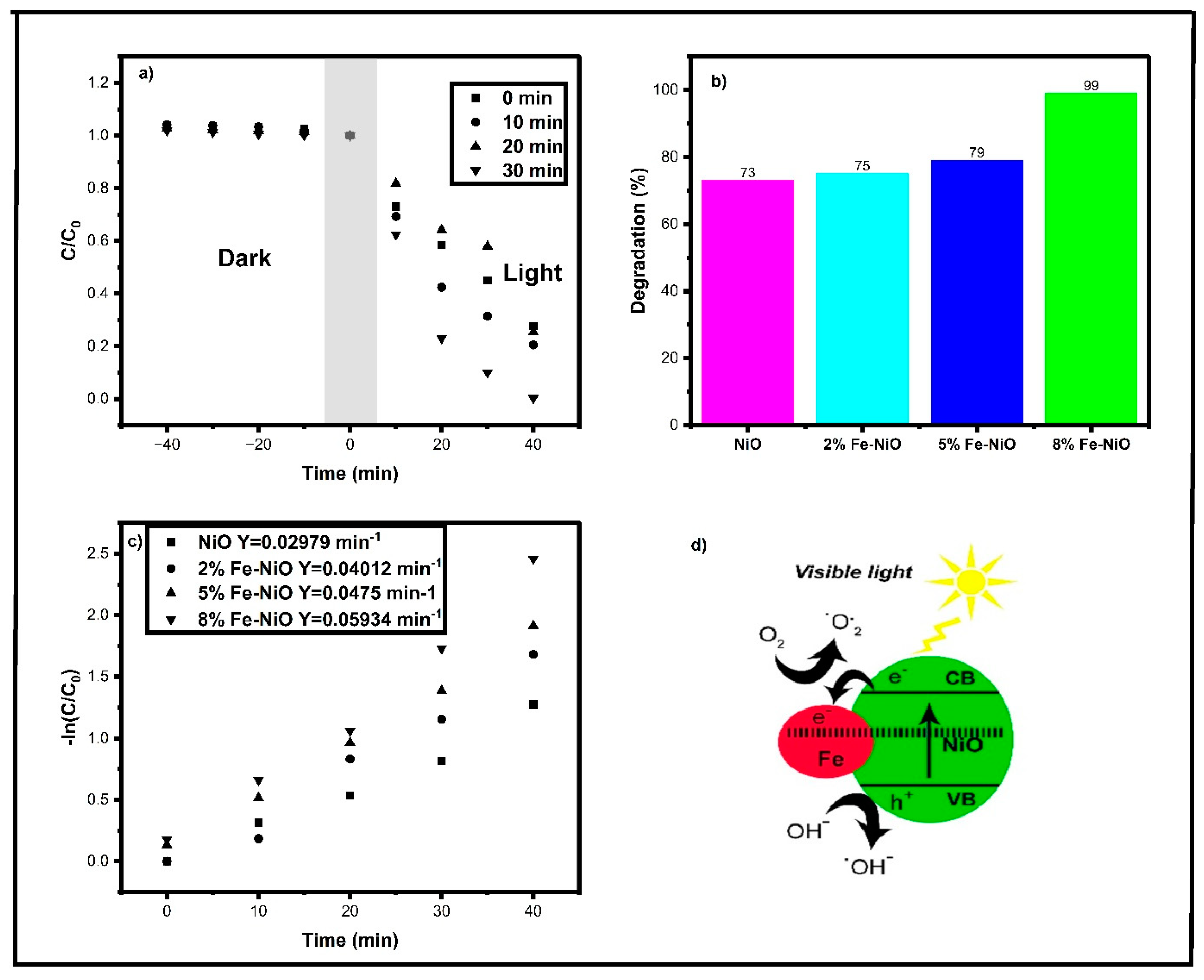
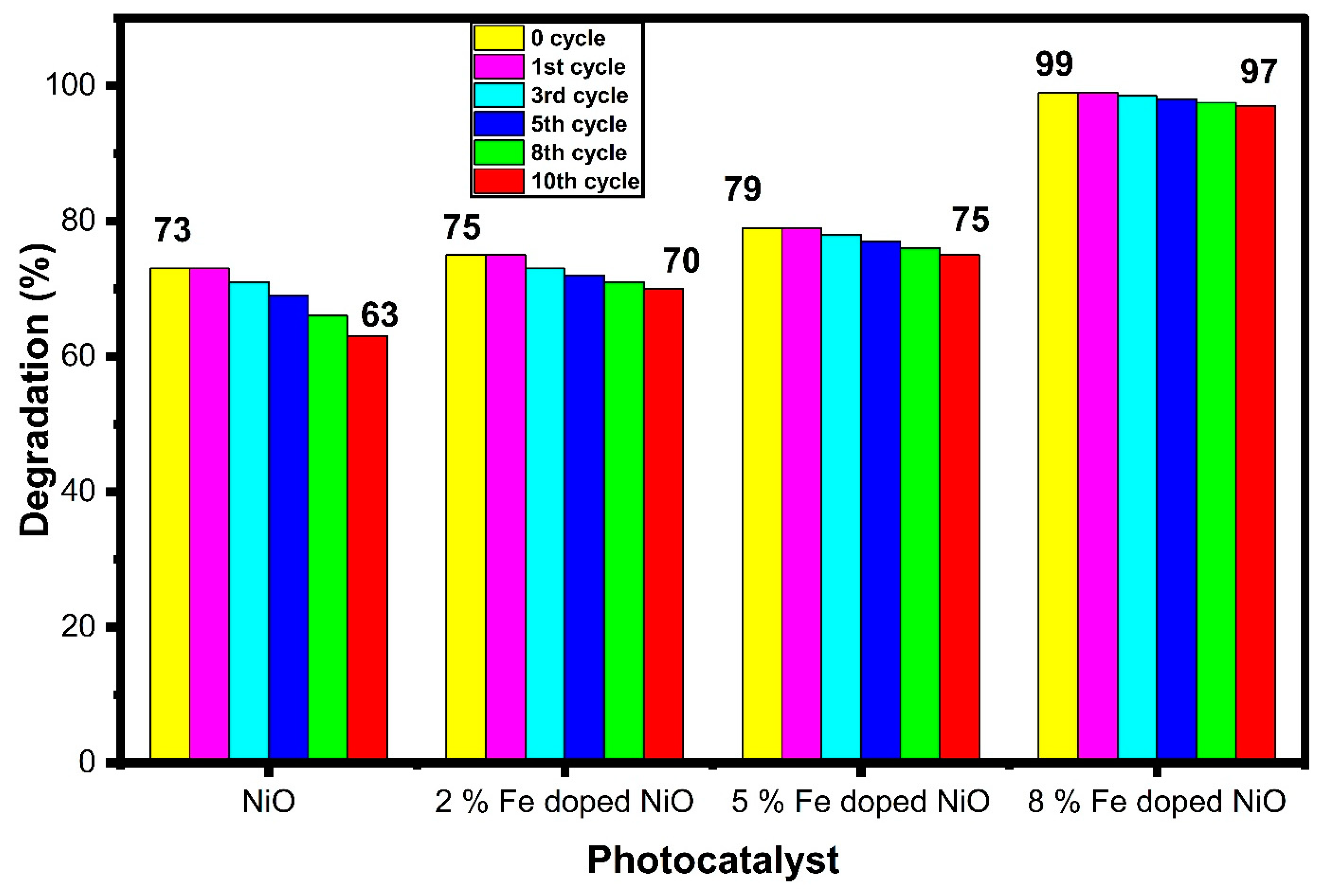
3.7. Photocatalytic Dye Degradation Activity
- Absorption of light: Fe-NiO absorbs photons with energy greater than its bandgap, which promotes electrons from the valence band to the conduction band.
- Generation of electron–hole pairs: The excited electrons and holes can migrate to the surface of the Fe-NiO nanoparticles and generate electron–hole pairs, which can participate in the following reactions:
- Formation of reactive species: The holes can react with water molecules or hydroxyl ions (OH−) adsorbed on the surface of Fe-NiO nanoparticles to produce hydroxyl radicals:
- 2.
- Oxidation of rhodamine B: The hydroxyl radicals can attack and break down the rhodamine B molecule, leading to its degradation into smaller, less toxic compounds:
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jha, S.; Gaur, R.; Shahabuddin, S.; Tyagi, I. Biochar as sustainable alternative and green adsorbent for the remediation of noxious pollutants: A comprehensive review. Toxics 2023, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- du Plessis, A. South Africa’s Impending Water Crises: Transforming Water Crises into Opportunities and the Way Forward. In South Africa’s Water Predicament: Freshwater’s Unceasing Decline; Springer International Publishing: Cham, Switzerland, 2023; pp. 143–170. [Google Scholar]
- Ranganathan, S.; Rajendran, S.; Ponce, L.C. Chemically Modified Carbon Nanotubes for Pollutants Adsorption. In Chemically Modified Carbon Nanotubes for Commercial Applications; Wiley: Hoboken, NJ, USA, 2023; pp. 129–150. [Google Scholar]
- Tazoe, H. Water quality monitoring. Anal. Sci. 2023, 39, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kiwanuka, M.; Mutanda, H.E.; Niyomukiza, J.B.; Nakasagga, E. Assessment of suitability of drinking water from the springs in Urban slums of Kampala. Environ. Chall. 2023, 10, 100667. [Google Scholar] [CrossRef]
- Verma, R.; Mishra, S.R.; Gadore, V.; Ahmaruzzaman, M. Hydroxyapatite-based composites: Excellent materials for environmental remediation and biomedical applications. Adv. Colloid Interface Sci. 2023, 315, 102890. [Google Scholar] [CrossRef]
- Khan, H.; Hussain, S.; Zahoor, R.; Arshad, M.; Umar, M.; Marwat, M.A.; Khan, A.; Khan, J.R.; Haleem, M.A. Novel modeling and optimization framework for Navy Blue adsorption onto eco-friendly magnetic geopolymer composite. Environ. Res. 2023, 216, 114346. [Google Scholar] [CrossRef] [PubMed]
- Vezzone, M.; Cesar, R.; Serrano, A.; Lourenço, R.; Rodrigues, A.P.; Castilhos, Z.; dos Anjos, R.M.; Polivanov, H. Mercury Contamination in Sediments and Fish from an Urban Tropical Estuary: Ecological and Human Health Risks. Water Air Soil Pollut. 2023, 234, 72. [Google Scholar] [CrossRef]
- Maleki, F.; Ghaemi, A.; Mir Mohamad Sadeghi, G. Synthesis and characterization of waste Styrofoam hypercrosslinked polymer as an adsorbent for CO2 capture. Environ. Prog. Sustain. Energy 2023, 42, e13954. [Google Scholar] [CrossRef]
- Larsen, J.J.; Lévy, L.; Asif, M.R. Removal of Powerline Noise in Geophysical Datasets with a Scientific Machine-Learning Based Approach. IEEE Trans. Geosci. Remote Sens. 2022, 60, 5923410. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, Y.; Zhang, Z.; Liao, S.; Deng, Y.; Wang, X.; Ye, Q.; Wang, K. Hydrothermal preparation of Sn3O4/TiO2 nanotube arrays as effective photocatalysts for boosting photocatalytic dye degradation and hydrogen production. Ceram. Int. 2023, 49, 5977–5985. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Wen, L.; Xi, J.; Liu, P.; Hansen, T.W.; Li, P. Ni-Pd-incorporated Fe3O4 yolk-shelled nanospheres as efficient magnetically recyclable catalysts for reduction of n-containing unsaturated compounds. Catalysts 2023, 13, 190. [Google Scholar] [CrossRef]
- Shakil, M.; Inayat, U.; Tanveer, M.; Nabi, G.; Gillani, S.S.A.; Rafique, M.; Tariq, N.H.; Shah, A.; Mahmood, A. NiO and Ag–Cd co-doped NiO nanoparticles: Study of photocatalytic degradation of rhodamine B dye for wastewater treatment. Int. J. Environ. Sci. Technol. 2023, 20, 2021–2036. [Google Scholar] [CrossRef]
- Wen, L.; Wang, D.; Xi, J.; Tian, F.; Liu, P.; Bai, Z.W. Heterometal modified Fe3O4 hollow nanospheres as efficient catalysts for organic transformations. J. Catal. 2022, 413, 779–785. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Dong, Z.; Zhan, Y.; Xi, J.; Xiao, J.; Huang, S.; Tian, F. Pd–Fe bimetallic nanoparticles anchored on N-doped carbon-modified graphene for efficient catalytic organic reactions. Carbon Lett. 2023, 33, 77–87. [Google Scholar] [CrossRef]
- Guo, T.; Fan, X.; Jiang, X.; Qi, Y.; Du, J.; Zhang, A.; Wang, H. Engineering shape of BiOClnanosheets with improved visible-light response for superior photocatalytic degradation of Rhodamine B. J. Alloys Compd. 2023, 948, 169586. [Google Scholar] [CrossRef]
- Cheng, H.; Li, X.; Huang, C.; Zhu, J.; Wang, P.; Cao, H.; Feng, C.; Ling, D.; Liu, H.; Cheng, M. Accelerated Fe (III)/Fe (II) cycle for rapid elimination of Rhodamine B by a novel Mo2C co-catalytic Fe2+/H2O2 system. J. Clean. Prod. 2023, 393, 136354. [Google Scholar] [CrossRef]
- Tanji, K.; El Mrabet, I.; Fahoul, Y.; Jellal, I.; Benjelloun, M.; Belghiti, M.; El Hajam, M.; Naciri, Y.; El Gaidoumi, A.; El Bali, B.; et al. Epigrammatic progress on the photocatalytic properties of ZnO and TiO2 based hydroxyapatite@ photocatlyst toward organic molecules photodegradation: A review. J. Water Process Eng. 2023, 53, 103682. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Y.; Zhao, P.; Bai, Y.; Cui, W.; Shen, S.; Liu, Y.; Wang, Z.; Yu, D.G. Insight into the superior piezophotocatalytic performance of BaTiO3//ZnO Janus nanofibrous heterostructures in the treatment of multi-pollutants from water. Appl. Catal. B Environ. 2023, 330, 122623. [Google Scholar] [CrossRef]
- Nowak, E.; Chłopocka, E.; Szybowicz, M. ZnO and ZnO-Based Materials as Active Layer in Resistive Random-Access Memory (RRAM). Crystals 2023, 13, 416. [Google Scholar] [CrossRef]
- Ameen, F.; Mostafazadeh, R.; Hamidian, Y.; Erk, N.; Sanati, A.L.; Karaman, C.; Ayati, A. Modeling of adsorptive removal of azithromycin from aquatic media by CoFe2O4@ NiO anchored microalgae-derived nitrogen-doped porous activated carbon adsorbent and colorimetric quantifying of azithromycin in pharmaceutical products. Chemosphere 2023, 329, 138635. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, Z.; Xu, T.; Jiang, H.; Ng, K.W.; Liao, C.; Danni, S.; Yanli, P.; Zimin, C.; Gang, W.; et al. Band alignment and electrical properties of NiO/β-Ga2O3 heterojunctions with different β-Ga2O3 orientations. Appl. Surf. Sci. 2023, 622, 156917. [Google Scholar] [CrossRef]
- Dejam, L.; Sabbaghzadeh, J.; Ghaderi, A.; Solaymani, S.; Matos, R.S.; Țălu, Ș.; da Fonseca Filho, H.D.; Sari, A.H.; Kiani, H.; Shayegan, A.H.S.; et al. Advanced nano-texture, optical bandgap, and Urbach energy analysis of NiO/Si heterojunctions. Sci. Rep. 2023, 13, 6518. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Kuo, T.Y.; Lin, Y.C.; Lin, H.C. Preparation and properties of p-type transparent conductive Cu-doped NiO films. Thin Solid Films 2011, 519, 4944–4947. [Google Scholar] [CrossRef]
- Amor, M.B.; Boukhachem, A.; Boubaker, K.; Amlouk, M. Structural, optical and electrical studies on Mg-doped NiO thin films for sensitivity applications. Mater. Sci. Semicond. Process. 2014, 27, 994–1006. [Google Scholar] [CrossRef]
- Amor, M.B.; Boukhachem, A.; Labidi, A.; Boubaker, K.; Amlouk, M. Physical investigations on Cd doped NiO thin films along with ethanol sensing at relatively low temperature. J. Alloys Compd. 2017, 693, 490–499. [Google Scholar] [CrossRef]
- Al Boukhari, J.; Zeidan, L.; Khalaf, A.; Awad, R. Synthesis, characterization, optical and magnetic properties of pure and Mn, Fe and Zn doped NiO nanoparticles. Chem. Phys. 2019, 516, 116–124. [Google Scholar] [CrossRef]
- Zhang, J.H.; Cai, G.F.; Zhou, D.; Tang, H.; Wang, X.L.; Gu, C.D.; Tu, J.P. Co-doped NiOnanoflake array films with enhanced electrochromic properties. J. Mater. Chem. C 2014, 2, 7013–7021. [Google Scholar] [CrossRef]
- Layek, S.; Verma, H.C. Room temperature ferromagnetism in Mn-doped NiO nanoparticles. J. Magn. Magn. Mater. 2016, 397, 73–78. [Google Scholar] [CrossRef]
- Peng, S.; Wang, C.; Xie, J.; Sun, S. Synthesis and stabilization of monodisperse Fe nanoparticles. J. Am. Chem. Soc. 2006, 128, 10676–10677. [Google Scholar] [CrossRef]
- Guo, J.; Wang, R.; Tjiu, W.W.; Pan, J.; Liu, T. Synthesis of Fe nanoparticles@ graphene composites for environmental applications. J. Hazard. Mater. 2012, 225, 63–73. [Google Scholar] [CrossRef]
- Pereira, C.; Pereira, A.M.; Fernandes, C.; Rocha, M.; Mendes, R.; Fernández-García, M.P.; Guedes, A.; Tavares, P.B.; Grenèche, J.-M.; Araújo, J.P.; et al. Superparamagnetic MFe2O4 (M = Fe, Co, Mn) nanoparticles: Tuning the particle size and magnetic properties through a novel one-step coprecipitation route. Chem. Mater. 2012, 24, 1496–1504. [Google Scholar] [CrossRef]
- Chelliah, P.; Wabaidur, S.M.; Sharma, H.P.; Jweeg, M.J.; Majdi, H.S.; Al Kubaisy, M.M.R.; Iqbal, A.; Lai, W.-C. Green Synthesis and Characterizations of Cobalt Oxide Nanoparticles and Their Coherent Photocatalytic and Antibacterial Investigations. Water 2023, 15, 910. [Google Scholar] [CrossRef]
- Chelliah, P.; Wabaidur, S.M.; Sharma, H.P.; Majdi, H.S.; Smait, D.A.; Najm, M.A.; Iqbal, A.; Lai, W.-C. Photocatalytic Organic Contaminant Degradation of Green Synthesized ZrO2 NPs and Their Antibacterial Activities. Separations 2023, 10, 156. [Google Scholar] [CrossRef]
- Gurugubelli, T.R.; Babu, B.; Kim, J.; Yoo, K. Hydrothermal synthesis of Fe-doped ZnAl2O4 nanosheets: Bandgap engineering and room temperature ferromagnetism. Chem. Pap. 2021, 75, 6407–6416. [Google Scholar] [CrossRef]
- Rao, G.T.; Babu, B.; Ravikumar, R.V.S.S.N.; Shim, J.; Reddy, C.V. Structural and optical properties of Fe-doped SnO2 quantum dots. Mater. Res. Express 2017, 4, 125021. [Google Scholar]
- Rana, P.S. Structural, optical, electrical, and photocatalytic application of NiFe2O4@ NiOnanocomposites for methylene blue dye. Ceram. Int. 2023, 49, 13520–13530. [Google Scholar]
- Vivek, P.; Sivakumar, R.; Esakki, E.S.; Deivanayaki, S. Fabrication of NiO/RGO nanocomposite for enhancing photocatalytic performance through degradation of RhB. J. Phys. Chem. Solids 2023, 176, 111255. [Google Scholar] [CrossRef]
- Motene, K.; Mahlaule-Glory, L.M.; Ngoepe, N.M.; Mathipa, M.M.; Hintsho-Mbita, N.C. Photocatalytic degradation of dyes and removal of bacteria using biosynthesised flowerlike NiO nanoparticles. Int. J. Environ. Anal. Chem. 2023, 103, 1107–1122. [Google Scholar] [CrossRef]
- Majeed, K.; Ambreen, J.; Khan, S.A.; Muhammad, S.; Shah, A.A.; Bhatti, M.A.; Batool, S.S.; Farooq, M.; Shah Bukhari, S.N.U.; Chandio, A.D.; et al. Effective Removal of Methylene Blue by Mn3O4/NiO Nanocomposite under Visible Light. Separations 2023, 10, 200. [Google Scholar] [CrossRef]
- Mandal, R.K.; Mondal, A.S.; Ghosh, S.; Halder, A.; Majumder, T.P. Synthesis, characterisation and optical studies of CdO-NiO NCs for comparative dye degradation study between two hazardous dyes Congo red and Rose Bengal. Results Chem. 2023, 5, 100810. [Google Scholar] [CrossRef]
- Huang, B.; Tong, X.; Zhang, X.; Feng, Q.; Rumyantseva, M.N.; Prakash, J.; Li, X. MXene/NiO Composites for Chemiresistive-Type Room Temperature Formaldehyde Sensor. Chemosensors 2023, 11, 258. [Google Scholar] [CrossRef]
- Bhat, M.A.; Rana, P.; Mir, F.A.; Pathak, D. A brief study on exploration of Ni doped PrFeO3 perovskite as multifunctional material. J. Mater. Sci. Mater. Electron. 2023, 34, 269. [Google Scholar] [CrossRef]
- Du, Y.; Li, Z.; Liu, H.; Qiao, S.; Chen, Y.; Zhu, Z.; Tang, Y.; Liu, C. Scalable oxygen-assisted-Fe2+ etching approach towards amorphous/crystalline structure Fe-Ni2P nanoarray for efficient water splitting. J. Alloys Compd. 2023, 936, 168073. [Google Scholar] [CrossRef]
- Kachhadiya, D.D.; Murthy, Z.V.P. Preparation and characterization of ZIF-8 and ZIF-67 engineered PVDF mixed-matrix membranes: Stability enhancement in pervaporation study. Environ. Sci. Water Res. Technol. 2023, 9, 1502–1517. [Google Scholar] [CrossRef]
- Ali, S.M.; Kassim, H.; Amer, M.S. Nanoarchitectonics of tin telluride: A novel pseudocapacitive material for energy storage application. Mater. Chem. Phys. 2023, 301, 127698. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, P.; Patidar, R.; Srivastava, V.C.; Lo, S.L.; Štangar, U.L. Catalytic oxidation of Bisphenol A with Co3+ rich spinel Co3O4: Performance evaluation with peroxymonosulfate activation and mineralization mechanism. J. Environ. Chem. Eng. 2023, 11, 110023. [Google Scholar] [CrossRef]
- Wei, Z.; Qiao, H.; Yang, H.; Zhang, C.; Yan, X. Characterization of NiO nanoparticles by anodic arc plasma method. J. Alloys Compd. 2009, 479, 855–858. [Google Scholar] [CrossRef]
- Qiao, H.; Wei, Z.; Yang, H.; Zhu, L.; Yan, X. Preparation and characterization of NiO nanoparticles by anodic arc plasma method. J. Nanomater. 2009, 2009, 795928. [Google Scholar] [CrossRef]
- Pebley, A.C.; Decolvenaere, E.; Pollock, T.M.; Gordon, M.J. Oxygen evolution on Fe-doped NiOelectrocatalysts deposited via microplasma. Nanoscale 2017, 9, 15070–15082. [Google Scholar] [CrossRef]
- Liu, S.; Jia, J.; Wang, J.; Liu, S.; Wang, X.; Song, H.; Hu, X. Synthesis of Fe-doped NiOnanofibers using electrospinning method and their ferromagnetic properties. J. Magn. Magn. Mater. 2012, 324, 2070–2074. [Google Scholar] [CrossRef]
- Bhoye, M.; Pansambal, S.; Basnet, P.; Lin, K.Y.A.; Gutierrez-Mercado, K.Y.; Pérez-Larios, A.; Chauhan, A.; Oza, R.; Ghotekar, S. Eco-Friendly Synthesis of Ni/NiO Nanoparticles Using Gymnemasylvestre Leaves Extract for Antifungal Activity. J. Compos. Sci. 2023, 7, 105. [Google Scholar] [CrossRef]
- Rad, L.R.; Anbia, M.; Vatanpour, V. Adsorption and Photocatalytic Degradation of Fluoxetine Using TiO2-Supported-Clinoptilolite, NaX and MIL-101 (Fe) Metal Organic Framework. J. Inorg. Organomet. Polym. Mater. 2023, 33, 2154–2171. [Google Scholar] [CrossRef]
- Ramasamy, N.; NagarasampattiPalani, K.; Mathew, A.; Natesan, B. Development of chitosan@ Fe2O3/rGO/Bi2S3 as a new eco-friendly photocatalyst for enhancing the catalytic stability and superior degradation of organic pollutants. Res. Chem. Intermed. 2023, 49, 2603–2624. [Google Scholar] [CrossRef]
- Mala, N.A.; Dar, M.A.; Rather, M.U.D.; Reshi, B.A.; Sivakumar, S.; Batoo, K.M.; Ahmad, Z. Supercapacitor and magnetic properties of NiO and manganese-doped NiO nanoparticles synthesized by chemical precipitation method. J. Mater. Sci. Mater. Electron. 2023, 34, 505. [Google Scholar] [CrossRef]
- Sharma, K.R.; Negi, N.S. Significant improvement in the structural, microstructural, and room-temperature magnetic properties of Fe-doped NiO nanoparticles prepared by the solution combustion method. J. Mater. Sci. Mater. Electron. 2022, 33, 22518–22540. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, H.; Zhang, H.H.; Yi, D.Q.; Zhu, J.F.; Qin, Y. Facile synthesis of NiFe2O4/Ni3Fe/LAS core-shell-like composites with improved impedance matching and microwave absorption properties. J. Phys. Chem. Solids 2023, 176, 111241. [Google Scholar] [CrossRef]
- Faramawy, A.; Elsayed, H.; Scian, C.; Mattei, G. Structural, optical, magnetic and electrical properties of sputtered ZnO and ZnO: Fe thin films: The role of deposition power. Ceramics 2022, 5, 1128–1153. [Google Scholar] [CrossRef]
- Che, H.; Huso, J.; Morrison, J.L.; Thapa, D.; Huso, M.; Yeh, W.J.; Tarun, M.C.; McCluskey, M.D.; Bergman, L. Optical properties of ZnO-alloyed nanocrystalline films. J. Nanomater. 2012, 2012, 7. [Google Scholar] [CrossRef]
- Marotti, R.E.; Guerra, D.N.; Bello, C.; Machado, G.; Dalchiele, E.A. Bandgap energy tuning of electrochemically grown ZnO thin films by thickness and electrodeposition potential. Sol. Energy Mater. Sol. Cells 2004, 82, 85–103. [Google Scholar] [CrossRef]
- Reddy, N.R.; Reddy, P.M.; Jyothi, N.; Kumar, A.S.; Jung, J.H.; Joo, S.W. Versatile TiO2 bandgap modification with metal, non-metal, noble metal, carbon material, and semiconductor for the photoelectrochemical water splitting and photocatalytic dye degradation performance. J. Alloys Compd. 2023, 935, 167713. [Google Scholar] [CrossRef]
- Nguyen, N.T.T.; Nguyen, L.M.; Nguyen, T.T.T.; Nguyen, N.H.; Nguyen, D.H.; Nguyen, D.T.C.; Van Tran, T. Green synthesis of ZnFe2O4@ ZnOnanocomposites using Chrysanthemum spp. floral waste for photocatalytic dye degradation. J. Environ. Manag. 2023, 326, 116746. [Google Scholar] [CrossRef]
- Beni, F.A.; Gholami, A.; Ayati, A.; Shahrak, M.N.; Sillanpää, M. UV-switchable phosphotungstic acid sandwiched between ZIF-8 and Au nanoparticles to improve simultaneous adsorption and UV light photocatalysis toward tetracycline degradation. Microporous Mesoporous Mater. 2020, 303, 110275. [Google Scholar] [CrossRef]
- Chahar, D.; Kumar, D.; Thakur, P.; Thakur, A. Visible light induced photocatalytic degradation of methylene blue dye by using Mg doped Co-Zn nanoferrites. Mater. Res. Bull. 2023, 162, 112205. [Google Scholar] [CrossRef]
- Moura, K.O.; Lima, R.J.S.; Coelho, A.A.; Souza-Junior, E.A.; Duque, J.G.S.; Meneses, C.T. Tuning the surface anisotropy in Fe-doped NiO nanoparticles. Nanoscale 2014, 6, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.; Nadeem, K.; Hassan, A.; Rahman, S.; Krenn, H. Enhanced photocatalytic activity of ferromagnetic Fe-doped NiO nanoparticles. Optik 2020, 202, 163637. [Google Scholar] [CrossRef]
- Ramesh, M. N and Fe doped NiO nanoparticles for enhanced photocatalytic degradation of azo dye methylene blue in the presence of visible light. SN Appl. Sci. 2021, 3, 817. [Google Scholar] [CrossRef]
- Khatri, A.; Rana, P.S. Visible light assisted photocatalysis of Methylene Blue and Rose Bengal dyes by iron doped NiO nanoparticles prepared via chemical co-precipitation. Phys. B Condens. Matter 2020, 579, 411905. [Google Scholar] [CrossRef]
- Sankar, S.; Sharma, S.K.; An, N.; Lee, H.; Kim, D.Y.; Im, Y.B.; Cho, Y.D.; Ganesh, R.S.; Ponnusamy, S.; Raji, P.; et al. Photocatalytic properties of Mn-doped NiO spherical nanoparticles synthesized from sol-gel method. Optik 2016, 127, 10727–10734. [Google Scholar] [CrossRef]
- Sudha, S.; Ramprasath, R.; Cholan, S.; Gokul, B.; Sridhar, S.; Ali, H.E.; Shkir, M. Enhanced triethylamine gas sensing and photocatalytic performance of Sn doped NiO (SNO) nanoparticles. Inorg. Chem. Commun. 2022, 136, 109104. [Google Scholar] [CrossRef]
- Zeng, L.; Peng, T.; Sun, H.; Yang, J.; Li, Y.; Qin, Y. Fe-doped LaNi1-xFexO3 perovskite oxides for enhanced visible-light-driven photocatalytic activity. J. Solid State Chem. 2021, 297, 122033. [Google Scholar] [CrossRef]
- Bawazeer, T.M.; Alsoufi, M.S.; Shkir, M.; Al-Shehri, B.M.; Hamdy, M.S. Excellent improvement in photocatalytic nature of ZnO nanoparticles via Fe doping content. Inorg. Chem. Commun. 2021, 130, 108668. [Google Scholar] [CrossRef]
- Mahendran, V.; Gogate, P.R. Degradation of Acid Scarlet 3R dye using oxidation strategies involving photocatalysis based on Fe doped TiO2 photocatalyst, ultrasound and hydrogen peroxide. Sep. Purif. Technol. 2021, 274, 119011. [Google Scholar] [CrossRef]
- Vijayaraghavan, T.; Althaf, R.; Babu, P.; Parida, K.M.; Vadivel, S.; Ashok, A.M. Visible light active LaFeO3 nanoperovskite-RGO-NiO composite for efficient H2 evolution by photocatalytic water splitting and textile dye degradation. J. Environ. Chem. Eng. 2021, 9, 104675. [Google Scholar] [CrossRef]
- Kahsay, M.H. Synthesis and characterization of ZnO nanoparticles using aqueous extract of Becium grandiflorum for antimicrobial activity and adsorption of methylene blue. Appl. Water Sci. 2021, 11, 45. [Google Scholar] [CrossRef]
- Selvi, S.; Rajendran, R.; Barathi, D.; Jayamani, N. Facile Synthesis of CeO2/CoWO4 Hybrid Nanocomposites for High Photocatalytic Performance and Investigation of Antimicrobial Activity. J. Electron. Mater. 2021, 50, 2890–2902. [Google Scholar] [CrossRef]
- Sierra-Fernandez, A.; De la Rosa-García, S.C.; Gomez-Villalba, L.S.; Gómez-Cornelio, S.; Rabanal, M.E.; Fort, R.; Quintana, P. Synthesis, Photocatalytic, and Antifungal Properties of MgO, ZnO and Zn/Mg Oxide Nanoparticles for the Protection of Calcareous Stone Heritage. ACS Appl. Mater. Interfaces 2017, 9, 24873–24886. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, G.; Velavan, R.; Batoo, K.M.; Raslan, E.H. Microstructure, optical and photocatalytic properties of MgO nanoparticles. Results Phys. 2020, 16, 103013. [Google Scholar] [CrossRef]
- Shahid, M.; Farrukh, M.A.; Umar, A.A.; Khaleeq-ur-Rahman, M. Solvent controlled synthesis of CaO-MgO nanocomposites and their application in the photodegradation of organic pollutants of industrial waste. Russ. J. Phys. Chem. A 2014, 88, 836–844. [Google Scholar] [CrossRef]
- George, S.E.; George, M.; Alex, J.; Joy, L.K.; Aravind, A.; Sajan, D.; Thakur, A.; Hussain, S.; Vinitha, G. Nonlinear optical and photocatalytic dye degradation of Co doped CeO2 nanostructures synthesized through a modified combustion technique. Ceram. Int. 2020, 46, 13932–13940. [Google Scholar] [CrossRef]
- Wang, H.; Li, G.; Fakhri, A. Fabrication and structural of the Ag2S-MgO/graphene oxide nanocomposites with high photocatalysis and antimicrobial activities. J. Photochem. Photobiol. B Biol. 2020, 207, 111882. [Google Scholar] [CrossRef]
- Monazzam, P.; Kisomi, B.F. Co/TiO2 nanoparticles: Preparation, characterization and its application for photocatalytic degradation of methylene blue. Desalination Water Treat. 2017, 63, 283–292. [Google Scholar]




| S.No | Nanoparticles | Source | Time (min) | Experimental Conditions | Degradation (%) | References |
|---|---|---|---|---|---|---|
| 1 | ZnO | UV light | 200 | 3 mL suspension/UV Batch reactor | 69 | [75] |
| 2 | CeO2 | Visible light | 105 | 20 ppm dye/10 mg/300 W Xe/λ = above 420 nm | 43 | [76] |
| 3 | MgO | UV light | 60 | 3 mg/50 mL/20 W Halogen/λ = 250 nm | 38 | [77] |
| 4 | MgO | UV light | 120 | 125 W/λ = 365 nm (HEBER MODELHVAR-MP400) | 75 | [78] |
| 6 | CaO-MgO | UV light | 100 | 10 ppm/1 mg | 44 | [79] |
| 7 | Co-CeO2 | Sunlight | 180 | 10 ppm/10 mg | 29 | [80] |
| 8 | Ag2O/MgO/GO | Visible light | 60 | 50 mL/100 mg 400 W/λ = above 420 nm | 65 | [81] |
| 9 | Co/TiO2 | UV light | 62 | 10 mg/100 mL/400 W Kr lamp | 150 | [82] |
| 10 | NiO | Visible light | 40 | 100 mL/10 mg 150 W/λ = above 400 nm | 73 | Present work |
| 11 | 2%Fe-NiO | Visible light | 40 | 100 mL/10 mg 150 W/λ = above 400 nm | 75 | Present work |
| 12 | 5%Fe-NiO | Visible light | 40 | 100 mL/10 mg 150 W/λ = above 400 nm | 79 | Present work |
| 13 | 8%Fe-NiO | Visible light | 40 | 100 mL/10 mg 150 W/λ = above 400 nm | 99 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minisha, S.; Johnson, J.; Mohammad Wabaidur, S.; Gupta, J.K.; Aftab, S.; Siddiqui, M.R.; Lai, W.-C. Synthesis and Characterizations of Fe-Doped NiO Nanoparticles and Their Potential Photocatalytic Dye Degradation Activities. Sustainability 2023, 15, 14552. https://doi.org/10.3390/su151914552
Minisha S, Johnson J, Mohammad Wabaidur S, Gupta JK, Aftab S, Siddiqui MR, Lai W-C. Synthesis and Characterizations of Fe-Doped NiO Nanoparticles and Their Potential Photocatalytic Dye Degradation Activities. Sustainability. 2023; 15(19):14552. https://doi.org/10.3390/su151914552
Chicago/Turabian StyleMinisha, S., J. Johnson, Saikh Mohammad Wabaidur, Jeetendra Kumar Gupta, Sikandar Aftab, Masoom Raza Siddiqui, and Wen-Cheng Lai. 2023. "Synthesis and Characterizations of Fe-Doped NiO Nanoparticles and Their Potential Photocatalytic Dye Degradation Activities" Sustainability 15, no. 19: 14552. https://doi.org/10.3390/su151914552
APA StyleMinisha, S., Johnson, J., Mohammad Wabaidur, S., Gupta, J. K., Aftab, S., Siddiqui, M. R., & Lai, W.-C. (2023). Synthesis and Characterizations of Fe-Doped NiO Nanoparticles and Their Potential Photocatalytic Dye Degradation Activities. Sustainability, 15(19), 14552. https://doi.org/10.3390/su151914552








