Experimental Study of MICP-Solidified Calcareous Sand Based on Ambient Temperature Variation in the South China Sea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiments
2.2. Test Method
3. Results
3.1. CaCO3 Formation Rate
3.2. Ca2+ Conversion Rate
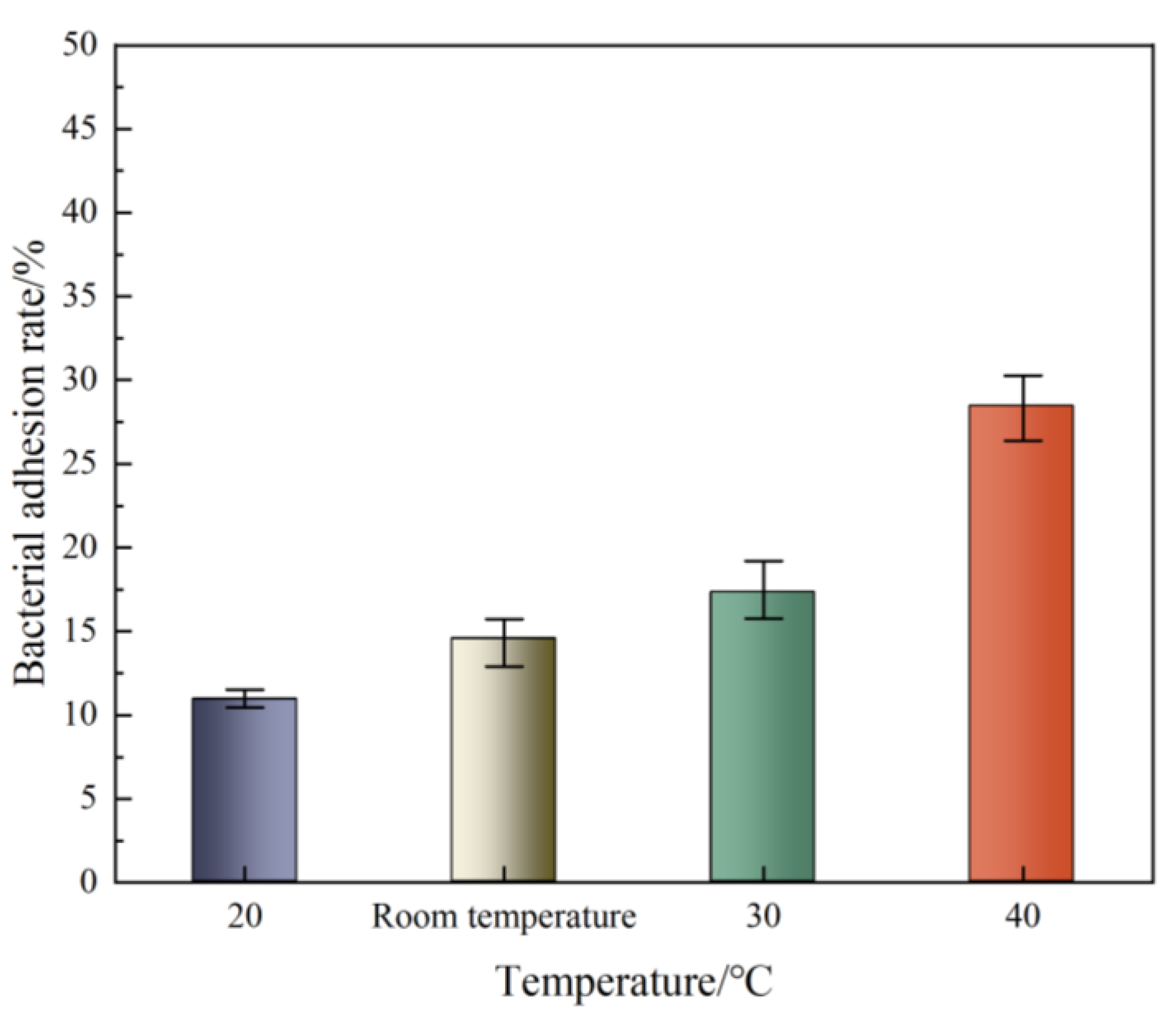
3.3. Bacterial Adhesion Rate
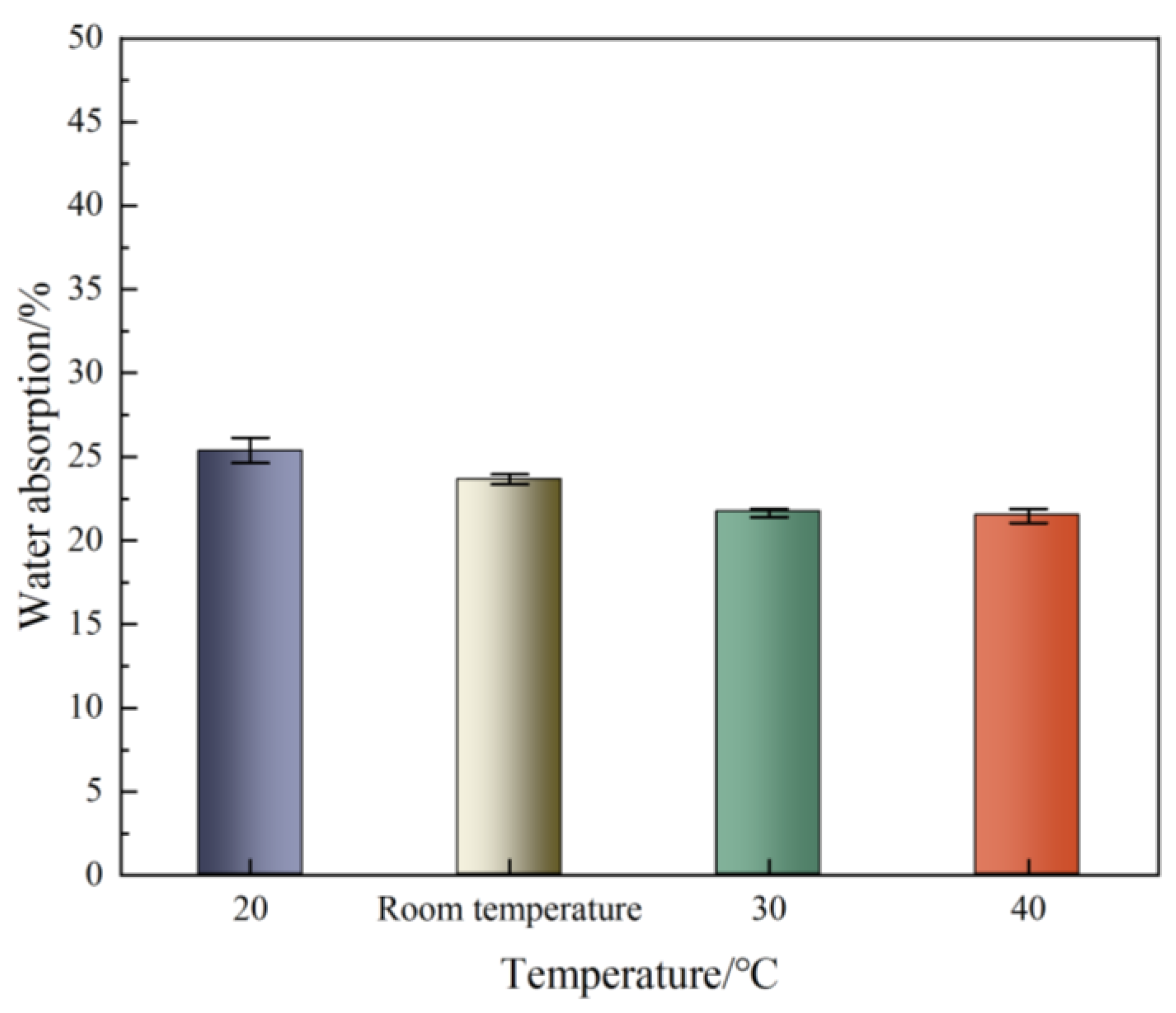
3.4. Water Absorption
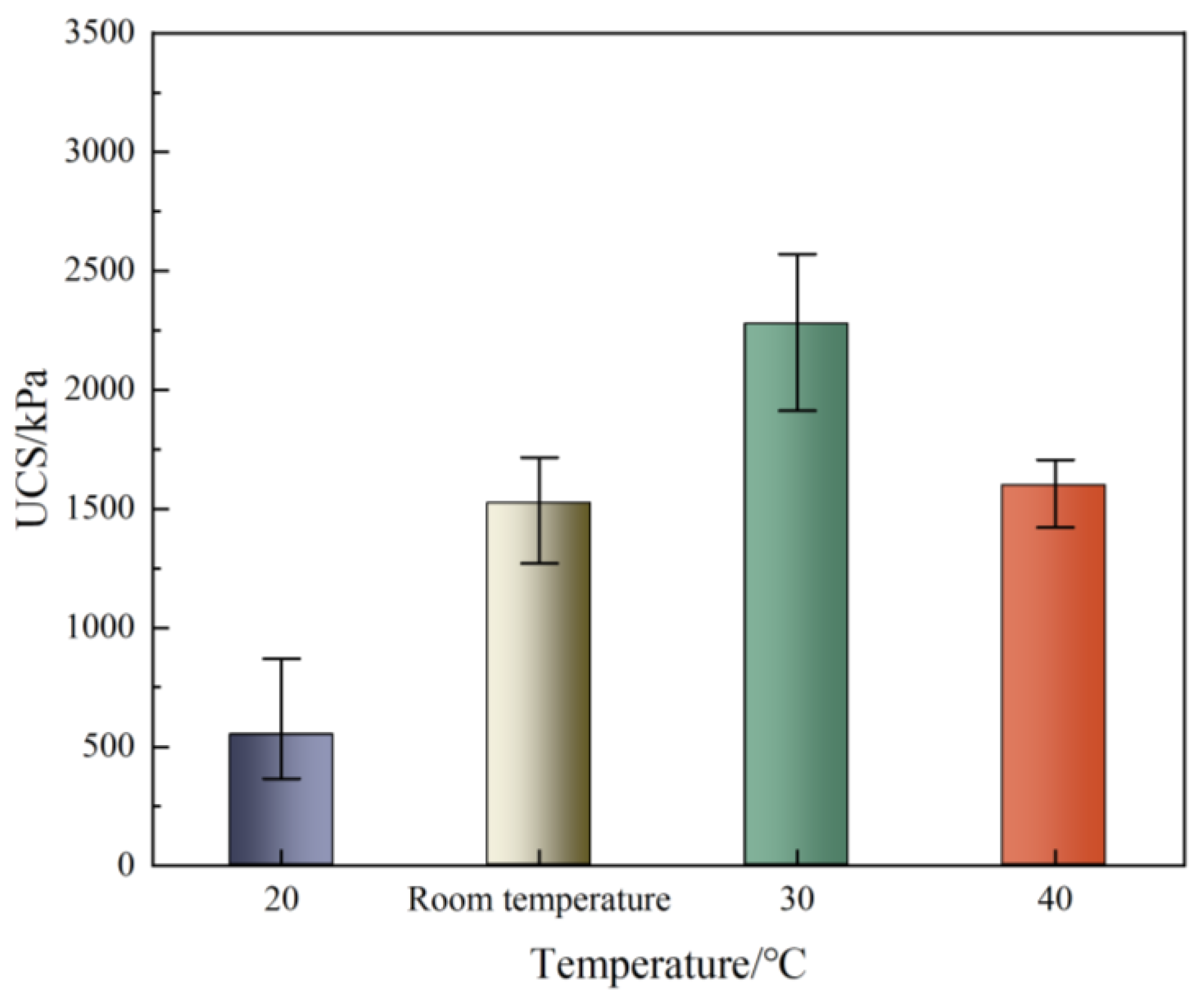
3.5. Unconfined Compressive Strength
3.6. Effect of Temperature on Test Index
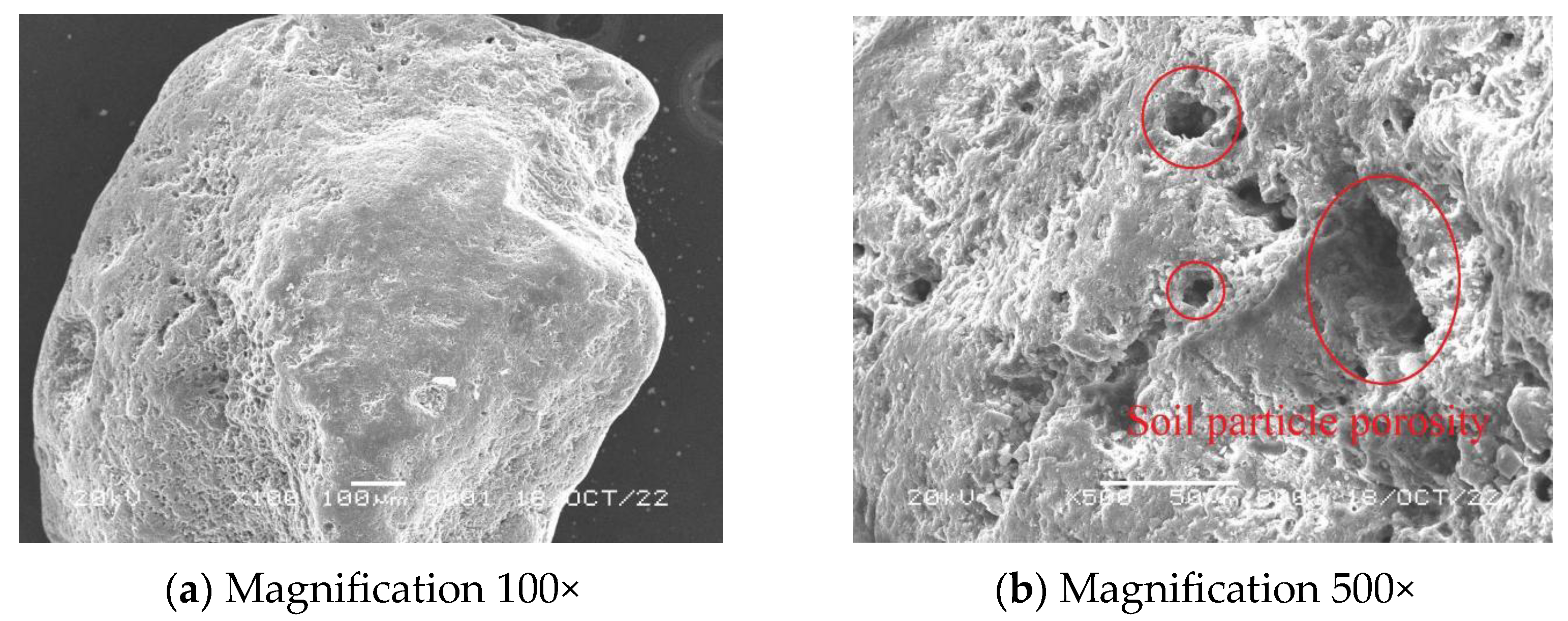
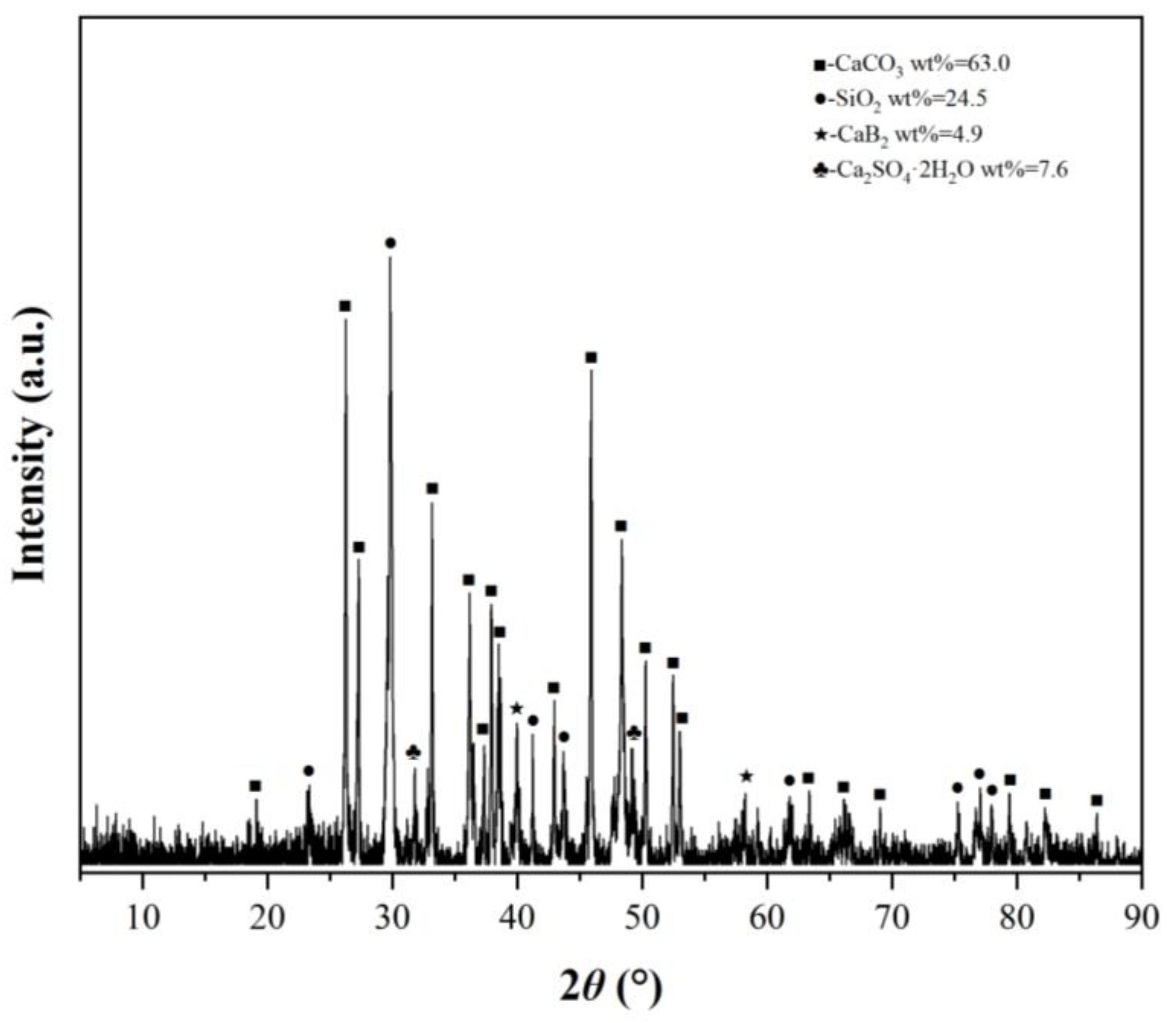
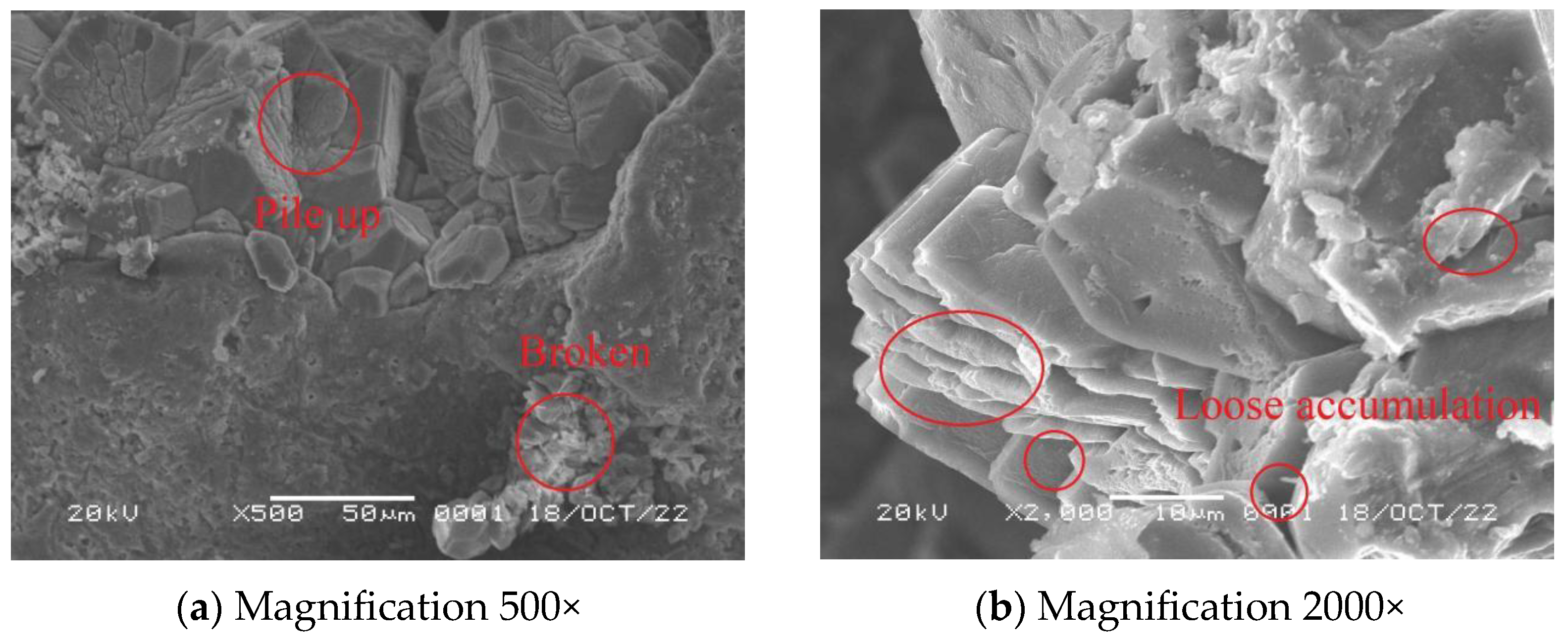
3.7. Scanning Electron Microscope Experiments
4. Conclusions
- (1)
- The CaCO3 formation rate, Ca2+ conversion rate, and unconfined compressive strength increased first and then decreased with increasing temperature, the UCS is 548 KPa at 20 °C and 2276.67 KPa at 30 °C, and the reinforcement effect was the best at 30 °C.
- (2)
- The adhesion rate of bacteria increased continuously with increasing temperature, from 10.91 at 20 °C to 28.44 at 40 °C, and an increase in temperature would affect the physiological state of bacterial cells.
- (3)
- The water absorption rate showed a continuous downward trend with increasing in temperature, from 25.32 at 20 °C to 21.49 at 40 °C, and the CaCO3 crystals formed at different temperatures were not the same.
- (4)
- When MICP reinforcement was carried out at 30 °C, the accumulation of CaCO3 was denser, the gap between adjacent sand particles was less, and the bonding was tight, which played a bridging role; the strength of the solidified sample was also higher.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jafarian, Y.; Javdanian, H. Dynamic Properties of Calcareous Sand from the Persian Gulf in Comparison with Siliceous Sands Database. Int. J. Civ. Eng. 2019, 18, 245–249. [Google Scholar] [CrossRef]
- Liu, L.; Yao, X.; Ji, Z.; Gao, H.; Wang, Z.; Shen, Z. Cyclic Behavior of Calcareous Sand from the South China Sea. J. Mar. Sci. Eng. 2021, 9, 1014. [Google Scholar] [CrossRef]
- Yang, S.; Shen, X.; Liu, H.; Ge, H.; Rui, X. Gradation affects basic mechanical characteristics of Chinese calcareous sand as airport subgrade of reefs. Mar. Georesour. Geotechnol. 2020, 38, 706–715. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.-Q.; Cui, J.; Wang, X.-Z.; Shen, J.-H.; Zhu, C.-Q. Particle breakage characteristics of a foundation filling material on island-reefs in the South China Sea. Constr. Build. Mater. 2021, 306, 124690. [Google Scholar] [CrossRef]
- Han, K.; Ju, J.W.; Lv, L.-Y.; Yan, Z.; Chen, X.; Jin, Y. Damage-healing analysis of microencapsulated self-healing concrete subjected to tensile loading using a 2D micromechanical model. Int. J. Damage Mech. 2023, 32, 579–599. [Google Scholar] [CrossRef]
- Hong, C.; Wang, X.; Han, K.; Su, D.; Chen, Z. Performance investigation of 3D printed clay soil using fiber Bragg grating technology. Acta Geotech. 2022, 17, 453–462. [Google Scholar] [CrossRef]
- e Portugal, C.R.M.; Fonyo, C.; Machado, C.C.; Meganck, R.; Jarvis, T. Microbiologically Induced Calcite Precipitation biocementation, green alternative for roads–is this the breakthrough? A critical review. J. Clean. Prod. 2020, 262, 121372. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, C.; Tang, C.S. Seawater based MICP cements two/one-phase cemented sand blocks. Eng. Geol. 2020, 264, 105389. [Google Scholar] [CrossRef]
- Lin, H.; Suleiman, M.T.; Brown, D.G. Investigation of pore-scale CaCO3 distributions and their effects on stiffness and permeability of sands treated by microbially induced carbonate precipitation (MICP). Soils Found. 2020, 60, 944–961. [Google Scholar] [CrossRef]
- Zhang, H.N.; Jia, C.Q.; Wang, G.H.; Su, F.; Sun, Y.S.; Fan, C.Y. Physical-mechanical properties of microbially induced calcite precipitation-treated loess and treatment mechanism. J. Mt. Sci 2022, 19, 2952–2967. [Google Scholar] [CrossRef]
- Liu, X.; Gao, X.; Pan, C. Experimental study on shear strength of cracks in MICP solidified soil sites. Chin. Civ. Eng. J. 2022, 55, 88–94. [Google Scholar]
- Yue, J.; Li, J.; Liu, D.; Wang, S.; Xing, X.; Chen, Y. Experimental study on the inhibitory effect of MICP technology on the cracking of site soil. J. Disas. Prev. Mitig. Eng. 2021, 41, 455–462. [Google Scholar]
- Liu, S.; Yu, J.; Zeng, W.; Peng, X.; Cai, Y.; Tu, B. Study on the effect of microbial induced calcium carbonate precipitation on repairing cracks in composite soil. Chin. J. Rock Mech. Eng. 2020, 39, 191–204. [Google Scholar]
- Liu, D. Experimental Study on Site Soil Restoration Based on Microbial Mineralization Technology. Master’s Thesis, Henan University, Henan, China, 2020. [Google Scholar]
- Xu, Y.; Qian, C.; Lu, Z. Study on the remediation of lead ion pollution by microbial mineralization. Chem. Ind. J. 2012, 26, 14–17. [Google Scholar]
- Xu, Y.; Qian, C.; Lu, Z. Microbial mineralization for remediation of heavy metal contaminated soil. Chin. J. Environ. Eng. 2013, 7, 2763–2768. [Google Scholar]
- Kumari, D.; Pan, X.; Lee, D.J.; Achal, V. Immobilization of cadmium in soil by microbially induced carbonate precipitation with Exiguobacterium undae at low temperature. Int. Biodeterior. Biodegrad. 2014, 94, 98–102. [Google Scholar] [CrossRef]
- Kang, C.H.; Han, S.H.; Shin, Y.J.; Oh, S.J.; So, J.S. Bioremediation of Cd by Microbially Induced Calcite Precipitation. Appl. Biochem. Biotechnol. 2014, 172, 1929–1937. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Zhou, C.; Wang, Y.; Chen, Q.; Zhong, Q. Soil and water conservation model test of MICP surface mineralized lattice fill in slope vegetation restoration. Chin. J. Geotech. Eng. 2022, 44, 29–34. [Google Scholar]
- Duan, J.; Wang, H.; Yao, J.; Wang, X.; Zhao, X.; Bai, X.; Tian, K. Experimental study on microbial mineralization reinforcement and erosion resistance of loess slope. Bull. Soil Water Conserv. 2022, 42, 33–40. [Google Scholar]
- Li, Y. Experimental Study on MICP Reinforcement of Sandy Slope Soil. Master’s Thesis, Zhejiang University, Zhejiang, China, 2022. [Google Scholar]
- Cheng, L.; Shahin, M.A.; Chu, J. Soil bio-cementation using a new one-phase low-pH injection method. Acta Geotech. 2019, 14, 615–626. [Google Scholar] [CrossRef]
- Zeybek, A.; Madabhushi, S. Influence of air injection on the liquefaction-induced deformation mechanisms beneath shallow foundations. Soil Dyn. Earthq. Eng. 2017, 97, 266–276. [Google Scholar] [CrossRef]
- Liu, H.; Xiao, P.; Xiao, Y.; Chu, J. State-of-the-art review of biogeotechnology and its engineering applications. J. Civ. Environ. Eng. 2019, 41, 1–14. [Google Scholar]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Muynck, W.D.; Belie, N.D.; Verstraete, W. Microbial carbonate precipitation in construction materials: A review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Wang, S.; Evanset, T.M.; Stuedlein, A.W.; Chu, K.; Zhao, C.; Wu, H.; Liu, H. Homogeneity and mechanical behaviors of sands improved by a temperature-controlled one-phase MICP method. Acta Geotech. 2021, 16, 1417–1427. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, Q.; Li, J.; Peng, J. Study on Microbial Induced Calcium Carbonate Production at Different Temperatures. Ind. Constr. 2019, 49, 88–92. [Google Scholar]
- Peng, J.; Feng, Q.; Sun, Y. Effect of temperature on sand reinforced by microbial induced calcium carbonate deposition. Chin. J. Geotech. Eng. 2018, 40, 1048–1055. [Google Scholar]
- Wang, Y.; Wang, Y.; Soga, K.; DeJong, J.T.; Kabla, A.J. Microscale investigations of temperature-dependent microbially induced carbonate precipitation (MICP) in the temperature range 4–50 °C. Acta Geotech. 2022, 18, 2239–2261. [Google Scholar] [CrossRef]
- Lv, C.; Tang, C.S.; Zhu, C.; Li, W.Q.; Chen, T.Y.; Zhao, L.; Pan, X.H. Environmental Dependence of Microbially Induced Calcium Carbonate Crystal Precipitations: Experimental Evidence and Insights. J. Geotech. Geoenviron. Eng. 2022, 148, 7. [Google Scholar] [CrossRef]
- Deng, J.; Deng, H.; Zhang, Y.; Luo, Y. Experimental Study on Microbial-Induced Calcium Carbonate Precipitation Repairing Fractured Rock under Different Temperatures. Sustainability 2022, 14, 11770. [Google Scholar] [CrossRef]
- Yin, L.; Tang, C.; Xie, J.; Lv, C.; Jiang, N.; Shi, B. Factors affecting the improvement of geotechnical material properties by microbial mineralization. Rock Soil Mech. 2019, 40, 2525–2546. [Google Scholar]
- Jin, C.; Liu, H.; Guo, M.; Wang, Y.; Zhu, J. Experimental study on tailings cementation by MICP technique with immersion curing. PLoS ONE 2022, 17, e0272281. [Google Scholar] [CrossRef]
- Keykha, A. Environmental Factors Affecting the Compressive Strength of Microbiologically Induced Calcite Precipitation-Treated Soil. Geomicrobiol. J. 2017, 34, 10. [Google Scholar] [CrossRef]
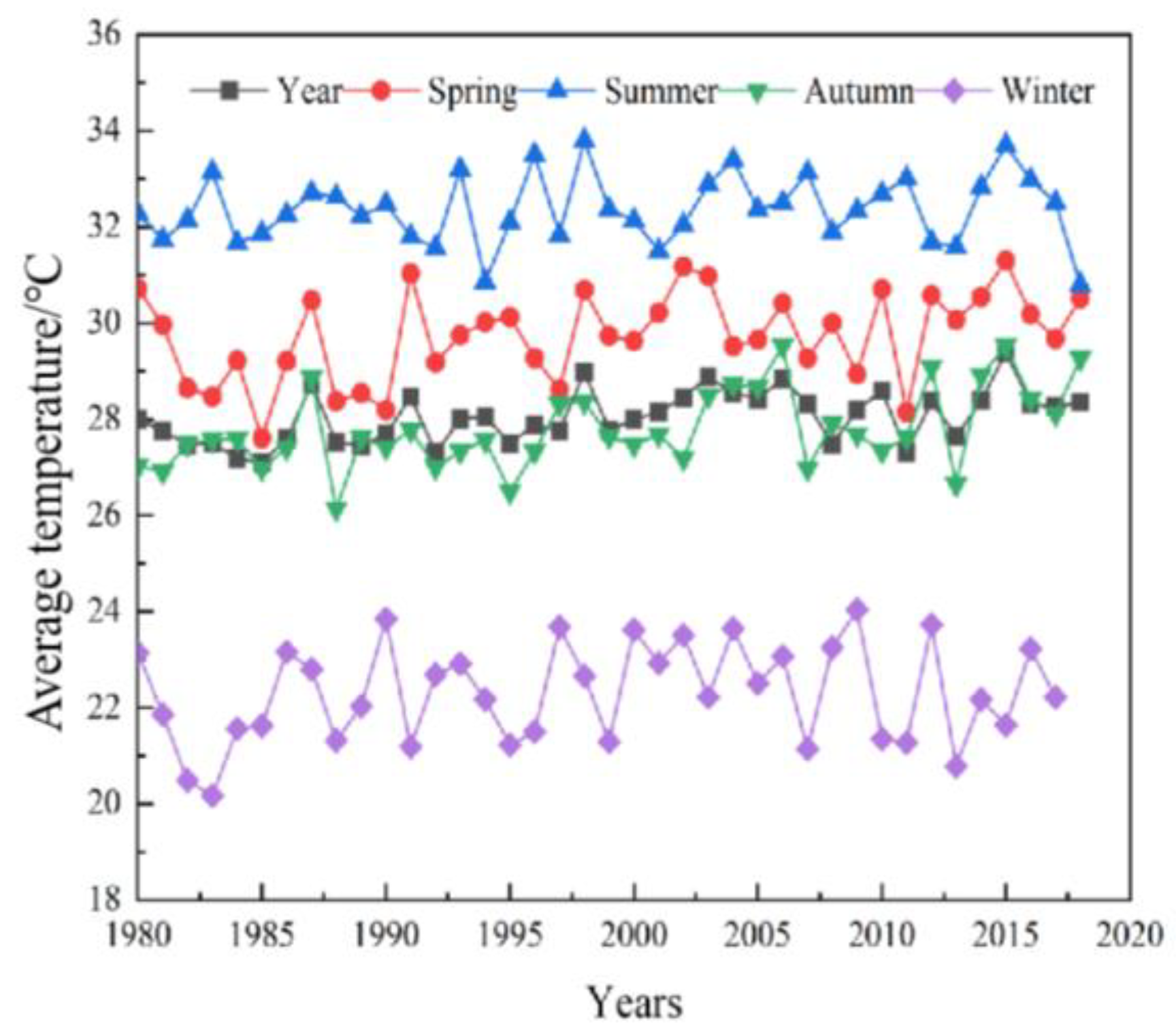
- Jia, C.; Zhang, Y. Spatial and temporal characteristics of satellite remote sensing sea surface temperature changes in the coral reef area of the South China Sea for 34 years. Acta Oceanol. Sin. 2018, 40, 112–120. [Google Scholar]
- Yang, Q.; Yi, X.; Shi, C. 0 cm ground temperature variation characteristics and related meteorological elements in Hainan Island. Meteorol. Sci. Technol. 2021, 49, 388–398. [Google Scholar]
- Chu, J.; Ivanov, V.M.; Stabnikov, V.; Li, B. Microbial method for construction of an aquaculture pond in sand. Geotechnique 2013, 10, 871–875. [Google Scholar] [CrossRef]
- Chu, J.; Stabnikov, V.; Ivanov, V. Microbially Induced Calcium Carbonate Precipitation on Surface or in the Bulk of Soil. Geomicrobiol. J. 2012, 29, 6. [Google Scholar] [CrossRef]
- Chen, H. Study on the Influence of Gradation on the Compression and Shear Properties of MICP Reinforced Sand. Master′s Thesis, Chongqing University, Chongqing, China, 2019. [Google Scholar]
- Xu, W.; Zheng, J.; Chu, J.; Zhang, R.; Cui, M.; Lai, H.; Zeng, C. New method for using N-(N-butyl)-thiophosphoric triamide to improve the effect of microbial induced carbonate precipitation. Constr. Build. Mater. 2021, 313, 125490. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, J.; Wu, Y.; Zeng, H.; Zeng, D.; Wang, Z. Experimental Study on the Preparation of a Highly Active Bacterial Suspension for MICP in the South China Sea. Sustainability 2022, 14, 9748. [Google Scholar] [CrossRef]
- Whiffin, V.S. Microbial CaCO3 Precipitation for the Production of Biocement. Ph.D Thesis, Murdoch University, Perth, Australia, 2004. [Google Scholar]
- Li, H.; Tang, D.; Yin, L.; Liu, B.; Lv, C.; Wang, D.; Pan, X.; Wang, H.; Shi, B. Experimental study on improving mechanical properties and erosion resistance of calcareous sand by MICP-FR synergistic effect. Chin. J. Geotech. Eng. 2021, 43, 1941–1949. [Google Scholar]
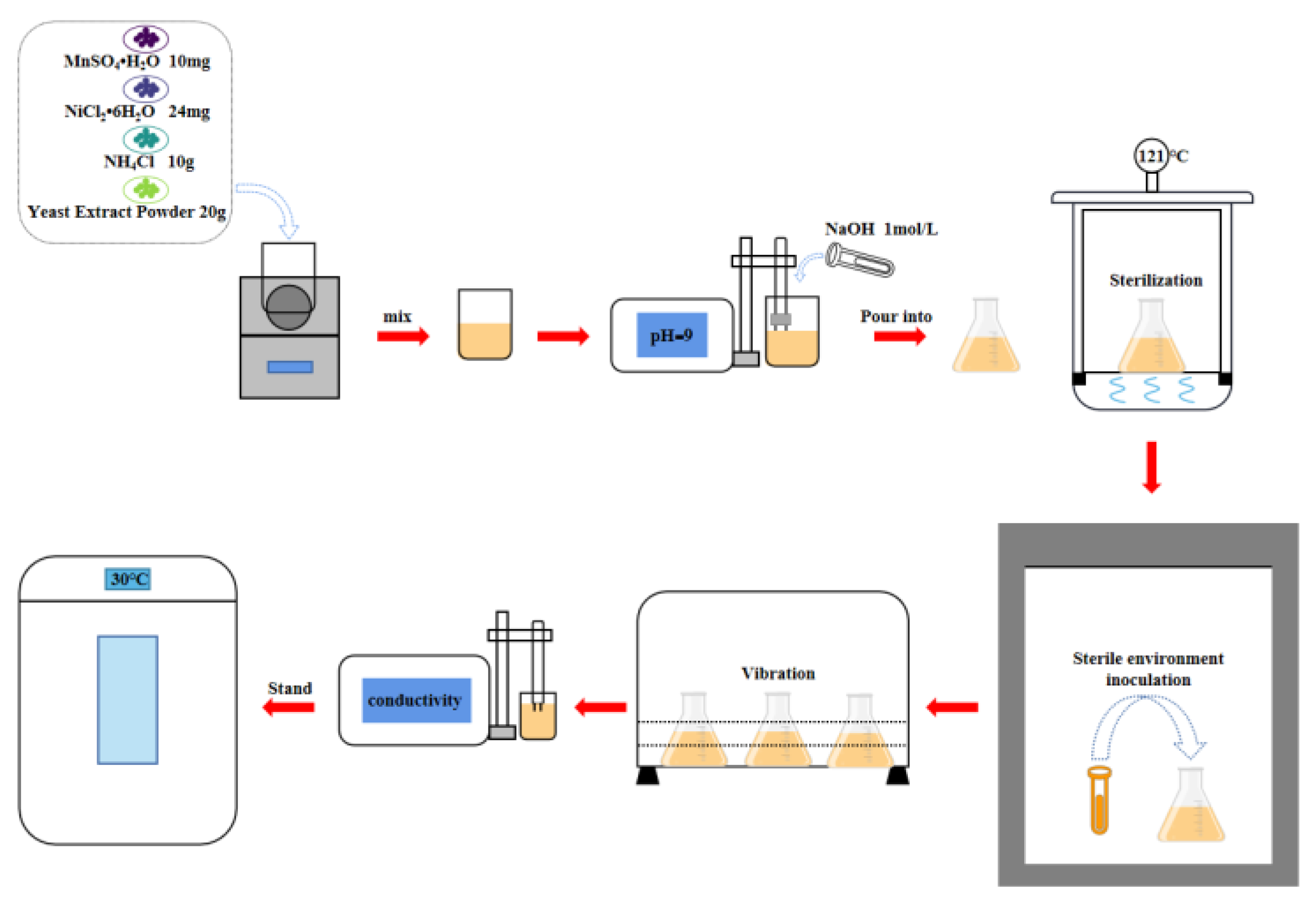




| Component | Content/L |
|---|---|
| Manganese sulphate | 10 mg |
| Nickel chloride | 24 mg |
| Ammonium chloride | 10 g |
| Yeast extract | 20 g |
| Agar powder | 15 g (added when configuring the solid medium) |
| Scheme | Soil Sample Temperature (°C) | Bacterial Liquid Temperature (°C) | Cementation Liquid Temperature (°C) |
|---|---|---|---|
| 20 | 20 | 20 | 20 |
| Room temperature | Room temperature | Room temperature | Room temperature |
| 30 | 30 | 30 | 30 |
| 40 | 40 | 40 | 40 |
| Temperature (°C) | 20 | Room Temperature | 30 | 40 |
|---|---|---|---|---|
| CaCO3 formation rate | 3.92 | 11.43 | 14.15 | 10.78 |
| Ca2+ conversion rate | 8.61 | 25.64 | 31.71 | 24.16 |
| Bacterial adhesion rate | 10.91 | 14.54 | 17.31 | 28.44 |
| Water absorption | 25.32 | 23.63 | 21.72 | 21.49 |
| UCS/kPa | 548 | 1522 | 2276.67 | 1598 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Yang, Y.; Zhou, Y.; Xiang, H.; Wei, K. Experimental Study of MICP-Solidified Calcareous Sand Based on Ambient Temperature Variation in the South China Sea. Sustainability 2023, 15, 8245. https://doi.org/10.3390/su15108245
Hu J, Yang Y, Zhou Y, Xiang H, Wei K. Experimental Study of MICP-Solidified Calcareous Sand Based on Ambient Temperature Variation in the South China Sea. Sustainability. 2023; 15(10):8245. https://doi.org/10.3390/su15108245
Chicago/Turabian StyleHu, Jun, Yufei Yang, Yuxuan Zhou, Hui Xiang, and Kai Wei. 2023. "Experimental Study of MICP-Solidified Calcareous Sand Based on Ambient Temperature Variation in the South China Sea" Sustainability 15, no. 10: 8245. https://doi.org/10.3390/su15108245





