Acute Effects of a Combat Sport Environment on Self-Control and Pain Perception Inhibition: A Preliminary Study in a New Ecological Framework
Abstract
1. Introduction
2. Materials and Methods
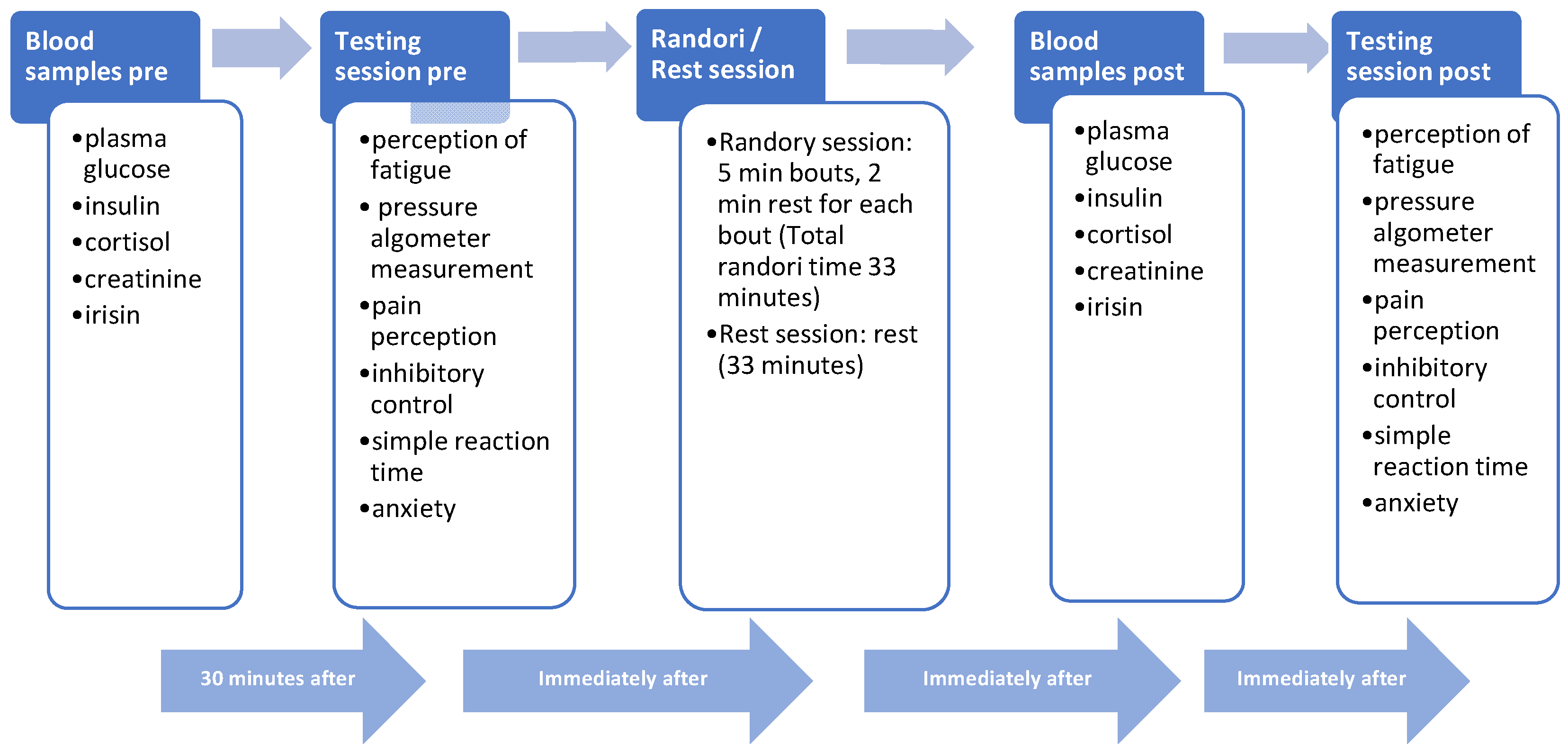
2.1. Study Protocol
2.2. Procedures
2.2.1. Cognitive Domains
2.2.2. Perceptual Domains
2.2.3. Affective Domain
2.2.4. Physiological Domain
2.3. Statistical Analysis
3. Results
4. Discussion
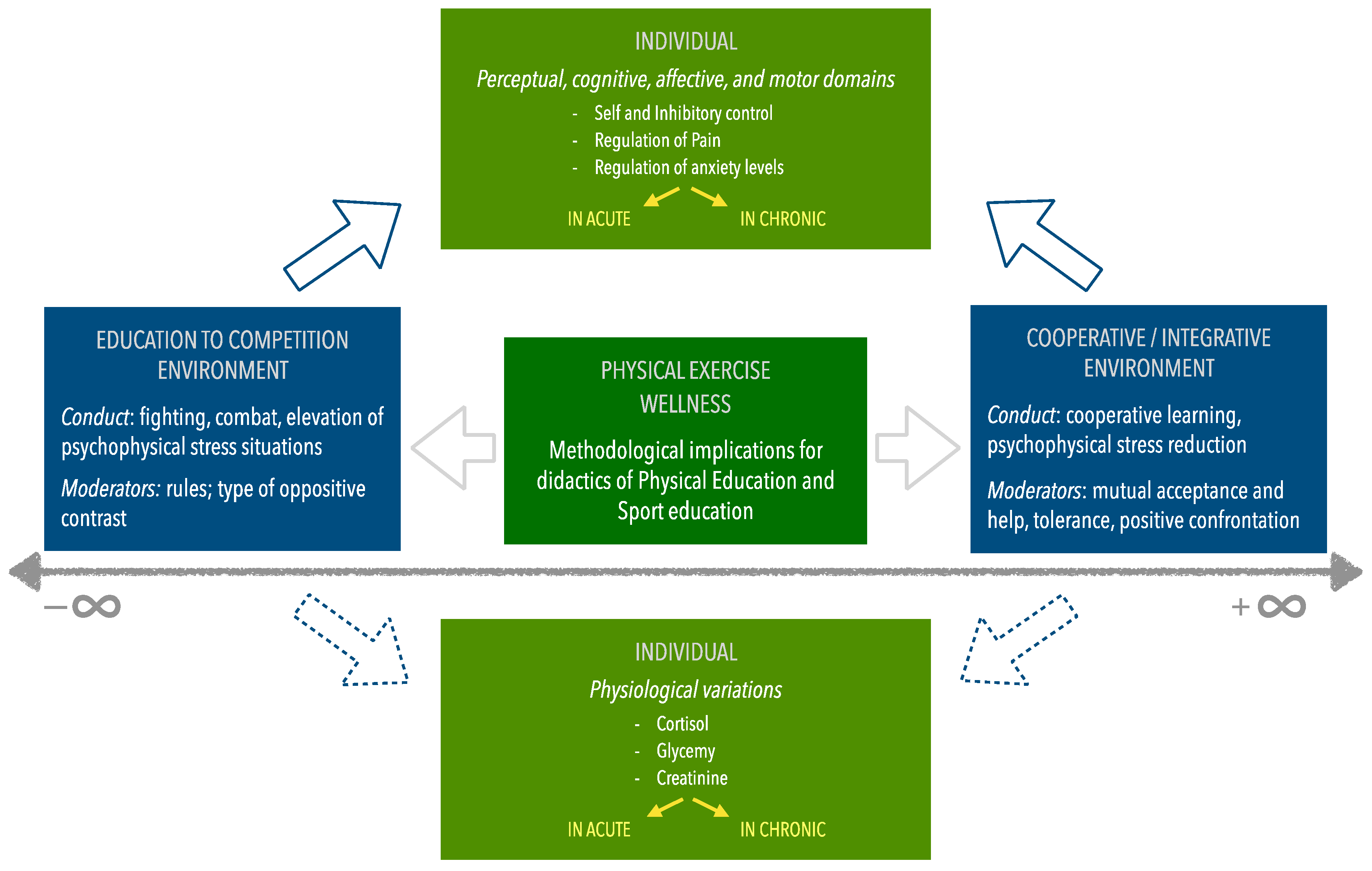
Contextualization into the Framework for a New Socio-Ecological Narrative
5. Conclusions
6. Practical Applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bronfenbrenner, U. Toward an Experimental Ecology of Human Development. Am. Psychol. 1977, 32, 513–531. [Google Scholar] [CrossRef]
- Faigenbaum, A.D.; Rebullido, T.R.; Chulvi-Medrano, I. Youth Physical Activity Is All About the “F-Words”. Strength Cond. J. 2020, 42, 2–6. [Google Scholar] [CrossRef]
- Schölmerich, V.L.; Kawachi, I. Translating the Socio-Ecological Perspective Into Multilevel Interventions: Gaps Between Theory and Practice. Health Educ. Behav. 2016, 43, 17–20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Batelaan, P.; Gundare, I. Intercultural education, co-operative learning and the changing society. Intercult. Educ. 2000, 11, 31–34. [Google Scholar] [CrossRef]
- Chen, M.A.; Cheesman, D.J. Mental toughness of mixed martial arts athletes at different levels of competition. Percept. Mot. Skills 2013, 116, 905–917. [Google Scholar] [CrossRef][Green Version]
- Torres-Luque, G.; Hernández-García, R.; Escobar-Molina, R.; Garatachea, N.; Nikolaidis, P.T. Physical and Physiological Characteristics of Judo Athletes: An Update. Sports 2016, 4, 20. [Google Scholar] [CrossRef][Green Version]
- Morales, J.; Franchini, E.; Garcia-Massó, X.; Solana-Tramunt, M.; Buscà, B.; González, L.-M. The Work Endurance Recovery Method for Quantifying Training Loads in Judo. Int. J. Sports Physiol. Perform. 2016, 11, 913–919. [Google Scholar] [CrossRef]
- Carmo, K.E.O.; Pérez, D.I.V.; Valido, C.N.; dos Santos, J.L.; Miarka, B.; Mendes-Netto, R.S.; Leite, M.M.R.; Antoniêtto, N.R.; Aedo-Muñoz, E.A.; Brito, C.J. Caffeine improves biochemical and specific performance after judo training: A double-blind crossover study in a real judo training situation. Nutr. Metab. 2021, 18, 15. [Google Scholar] [CrossRef]
- Johnstone, A.; Marí-Beffa, P. The Effects of Martial Arts Training on Attentional Networks in Typical Adults. Front. Psychol. 2018, 9, 80. [Google Scholar] [CrossRef]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain. Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef][Green Version]
- Formenti, D.; Cavaggioni, L.; Duca, M.; Trecroci, A.; Rapelli, M.; Alberti, G.; Komar, J.; Iodice, P. Acute Effect of Exercise on Cognitive Performance in Middle-Aged Adults: Aerobic Versus Balance. J. Phys. Act. Health 2020, 17, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Pontifex, M.B.; McGowan, A.L.; Chandler, M.C.; Gwizdala, K.L.; Parks, A.C.; Fenn, K.; Kamijo, K. A primer on investigating the after effects of acute bouts of physical activity on cognition. Psychol. Sport Exerc. 2019, 40, 1–22. [Google Scholar] [CrossRef]
- Pesce, C. Shifting the focus from quantitative to qualitative exercise characteristics in exercise and cognition research. J. Sport Exerc. Psychol. 2012, 34, 766–786. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pesce, C.; Crova, C.; Marchetti, R.; Struzzolino, I.; Masci, I.; Vannozzi, G.; Forte, R. Searching for cognitively optimal challenge point in physical activity for children with typical and atypical motor development. Ment. Health Phys. Act. 2013, 6, 172–180. [Google Scholar] [CrossRef]
- Kao, S.C.; Drollette, E.S.; Ritondale, J.P.; Khan, N.; Hillman, C.H. The acute effects of high-intensity interval training and moderate-intensity continuous exercise on declarative memory and inhibitory control. Psychol. Sport Exerc. 2018, 38, 90–99. [Google Scholar] [CrossRef]
- Moreau, D.; Chou, E. The Acute Effect of High-Intensity Exercise on Executive Function: A Meta-Analysis. Perspect. Psychol. Sci. 2019, 14, 734–764. [Google Scholar] [CrossRef][Green Version]
- Mann, D.T.; Williams, A.M.; Ward, P.; Janelle, C.M. Perceptual-cognitive expertise in sport: A meta-analysis. J. Sport Exerc. Psychol. 2007, 29, 457–478. [Google Scholar] [CrossRef] [PubMed]
- Vickers, J.N. Mind over muscle: The role of gaze control, spatial cognition, and the quiet eye in motor expertise. Cogn. Process. 2011, 12, 219–222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Logan, G.D.; Cowan, W.B.; Davis, K.A. On the ability to inhibit simple and choice reaction time responses: A model and a method. J. Exp. Psychol. Hum. Percept. Perform. 1984, 10, 276–291. [Google Scholar] [CrossRef]
- Posner, M.I. Chronometric Explorations of Mind; Lawrence Erlbaum: Oxford, UK, 1978. [Google Scholar]
- Cojocariu, A.; Abalasei, B. Does the reaction time to visual stimuli contribute to performance in judo? Arch. Budo 2014, 10, 73–78. [Google Scholar]
- Katz, N. The impact of pain management on quality of life. J. Pain Symptom. Manag. 2002, 24 (Suppl. S1), S38–S47. [Google Scholar] [CrossRef] [PubMed]
- Geneen, L.J.; Moore, R.A.; Clarke, C.; Martin, D.; Colvin, L.A.; Smith, B.H. Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 152, 440–446. [Google Scholar] [CrossRef][Green Version]
- Ray, C.A.; Carter, J.R. Central modulation of exercise-induced muscle pain in humans. J. Physiol. 2007, 585, 287–294. [Google Scholar] [CrossRef]
- Chayadi, E.; McConnell, B.L. Gaining insights on the influence of attention, anxiety, and anticipation on pain perception. J. Pain Res. 2019, 12, 851–864. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Ryckeghem, D.M.; Van Damme, S.; Eccleston, C.; Crombez, G. The efficacy of attentional distraction and sensory monitoring in chronic pain patients: A meta-analysis. Clin. Psychol. Rev. 2018, 59, 16–29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ströhle, A.; Feller, C.; Strasburger, C.J.; Heinz, A.; Dimeo, F. Anxiety modulation by the heart? Aerobic exercise and atrial natriuretic peptide. Psychoneuroendocrinology 2006, 31, 1127–1130. [Google Scholar] [CrossRef]
- Rossi, C.; Roklicer, R.; Tubic, T.; Bianco, A.; Gentile, A.; Manojlovic, M.; Maksimovic, N.; Trivic, T.; Drid, P. The Role of Psychological Factors in Judo: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 2093. [Google Scholar] [CrossRef] [PubMed]
- Urhausen, A.; Gabriel, H.; Kindermann, W. Blood Hormones as Markers of Training Stress and Overtraining. Sports Med. 1995, 20, 251–276. [Google Scholar] [CrossRef]
- Battezzati, A.; Benedini, S.; Fattorini, A.; Piceni Sereni, L.; Luzi, L. Effect of hypoglycemia on amino acid and protein metabolism in healthy humans. Diabetes 2000, 49, 1543–1551. [Google Scholar] [CrossRef][Green Version]
- Schulkin, J.; McEwen, B.S.; Gold, P.W. Allostasis, amygdala, and anticipatory angst. Neurosci. Biobehav. Rev. 1994, 18, 385–396. [Google Scholar] [CrossRef]
- Nonogaki, K.; Iguchi, A. Role of central neural mechanisms in the regulation of hepatic glucose metabolism. Life Sci. 1997, 60, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Li, Q.; Liu, J.; Jia, S. Irisin, an exercise-induced myokine as a metabolic regulator: An updated narrative review. Diabetes Metab. Res. Rev. 2016, 32, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef][Green Version]
- Benedini, S.; Dozio, E.; Invernizzi, P.L.; Vianello, E.; Banfi, G.; Terruzzi, I.; Luzi, L.; Corsi Romanelli, M.M. Irisin: A Potential Link between Physical Exercise and Metabolism—An Observational Study in Differently Trained Subjects, from Elite Athletes to Sedentary People. J. Diabetes Res. 2017, 2017, e1039161. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Joo, C.H. The effects of short term detraining and retraining on physical fitness in elite soccer players. PLoS ONE 2018, 13, e0196212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tomik, R.; Olex-Zarychta, D.; Mynarski, W. Social values of sport participation and their significance for youth attitudes towards physical education and sport. Stud. Phys. Cult. Tour. 2012, 19, 99–141. [Google Scholar]
- Formenti, D.; Trecroci, A.; Duca, M.; Cavaggioni, L.; D’Angelo, F.; Passi, A.; Longo, S.; Alberti, G. Differences in inhibitory control and motor fitness in children practicing open and closed skill sports. Sci. Rep. 2021, 11, 4033. [Google Scholar] [CrossRef]
- Formenti, D.; Trecroci, A.; Duca, M.; Vanoni, M.; Ciovati, M.; Rossi, A.; Alberti, G. Volleyball-Specific Skills and Cognitive Functions Can Discriminate Players of Different Competitive Levels. J. Strength. Cond. Res. 2022, 36, 813–819. [Google Scholar] [CrossRef]
- Eriksen, B.A.; Eriksen, C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974, 16, 143–149. [Google Scholar] [CrossRef][Green Version]
- Chesterton, L.S.; Sim, J.; Wright, C.C.; Foster, N.E. Interrater reliability of algometry in measuring pressure pain thresholds in healthy humans, using multiple raters. Clin. J. Pain 2007, 23, 760–766. [Google Scholar] [CrossRef]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Ilardi, C.R.; Gamboz, N.; Iavarone, A.; Chieffi, S.; Brandimonte, M.A. Psychometric properties of the STAI-Y scales and normative data in an Italian elderly population. Aging Clin. Exp. Res. 2021, 33, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Budde, H.; Voelcker-Rehage, C.; Pietrabyk-Kendziorra, S.; Ribeiro, P.; Tidow, G. Acute coordinative exercise improves attentional performance in adolescents. Neurosci. Lett. 2008, 441, 219–223. [Google Scholar] [CrossRef]
- Schmidt, M.; Jäger, K.; Egger, F.; Roebers, C.M.; Conzelmann, A. Cognitively Engaging Chronic Physical Activity, But Not Aerobic Exercise, Affects Executive Functions in Primary School Children: A Group-Randomized Controlled Trial. J. Sport Exerc. Psychol. 2015, 37, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Cowley, J.C.; Dingwell, J.B.; Gates, D.H. Effects of local and widespread muscle fatigue on movement timing. Exp. Brain. Res. 2014, 232, 3939–3948. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pavelka, R.; Třebický, V.; Třebická Fialová, J.; Zdobinský, A.; Coufalová, K.; Havlíček, J.; Tufano, J.J. Acute fatigue affects reaction times and reaction consistency in Mixed Martial Arts fighters. PLoS ONE 2020, 15, e0227675. [Google Scholar] [CrossRef]
- Lima, L.V.; Abner, T.S.S.; Sluka, K.A. Does exercise increase or decrease pain? Central mechanisms underlying these two phenomena. J. Physiol. 2017, 595, 4141–4150. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sluka, K.A.; Frey-Law, L.; Hoeger Bement, M. Exercise-induced pain and analgesia? Underlying mechanisms and clinical translation. Pain 2018, 159 (Suppl. S1), S91–S97. [Google Scholar] [CrossRef]
- Lesnak, J.B.; Sluka, K.A. Mechanism of exercise-induced analgesia: What we can learn from physically active animals. Pain Rep. 2020, 5, e850. [Google Scholar] [CrossRef]
- Kami, K.; Tajima, F.; Senba, E. Brain Mechanisms of Exercise-Induced Hypoalgesia: To Find a Way Out from “Fear-Avoidance Belief”. Int. J. Mol. Sci. 2022, 23, 2886. [Google Scholar] [CrossRef]
- Khani, S.; Tayek, J.A. Cortisol increases gluconeogenesis in humans: Its role in the metabolic syndrome. Clin. Sci. 2001, 101, 739–747. [Google Scholar] [CrossRef][Green Version]
- Fatouros, I.G. Is irisin the new player in exercise-induced adaptations or not? A 2017 update. Clin. Chem. Lab. Med. 2018, 56, 525–548. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Rioux, B.V.; Goulet, E.D.B.; Johanssen, N.M.; Swift, D.L.; Bouchard, D.R.; Loewen, H.; Sénéchal, M. Effect of an acute exercise bout on immediate post-exercise irisin concentration in adults: A meta-analysis. Scand. J. Med. Sci. Sports 2018, 28, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Sierra, A.P.R.; Oliveira, R.A.; Silva, E.D.; Lima, G.H.O.; Benetti, M.P.; Kiss, M.A.P.; Sierra, C.A.; Ghorayeb, N.; Seto, J.T.; Pesquero, J.B.; et al. Association Between Hematological Parameters and Iron Metabolism Response After Marathon Race and ACTN3 Genotype. Front. Physiol. 2019, 10, 697. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beunders, R.; Bongers, C.C.W.G.; Pickkers, P. The effects of physical exercise on the assessment of kidney function. J. Appl. Physiol. 2020, 128, 1459–1460. [Google Scholar] [CrossRef]
- Manning, M.L.; Lucking, R. The What, Why, and How of Cooperative Learning. Clear. House 1991, 64, 152–156. [Google Scholar] [CrossRef]
- Walter, M. Social Research Methods, 2nd ed.; Oxford University Press: South Melbourne, Australia, 2009. [Google Scholar]


| Randori Session | Resting Session | ||||||
|---|---|---|---|---|---|---|---|
| Domain | Variables | Pre | Post | Delta | Pre | Post | Delta |
| Cognitive | Flanker task | ||||||
| Congruent (n° of corrects) | 25.0 ± 2.4 | 25.2 ± 2.1 | 0.2 ± 3.6 # | 23.5 ± 1.6 | 21.0 ± 1.9 | −2.5 ± 2.6 | |
| Incongruent (n° of corrects) | 22.5 ± 3.3 | 23.4 ± 2.5 | 1.0 ± 4.3 # | 24.0 ± 2.3 | 20.2 ± 2.7 | −3.8 ± 3.7 | |
| Congruent (ms) | 482.0 ± 60.0 | 461.6 ± 46.0 | −13.7 ± 28.9 # | 440.1 ± 34.2 | 451.4 ± 40.1 | 11.3 ± 25.1 | |
| Incongruent (ms) | 581.5 ± 91.9 | 564.6 ± 78.9 | −12.5 ± 29.2 # | 533.7 ± 60.0 | 536.3 ± 58.2 | 11.3 ± 22.9 | |
| Clinical reaction time | |||||||
| Simple reaction time (ms) | 184.1 ± 18.2 | 190.6 ± 21.2 | 6.5 ± 24.3 | 175.5 ± 18.8 | 180.4 ± 29.7 | 4.9 ± 28.8 | |
| Perceptual | Pressure time (s) | 5.0 ± 2.3 | 5.8 ± 2.0 | 0.8 ± 1.3 | 5.3 ± 1.9 | 5.7 ± 2.1 | 0.5 ± 1.6 |
| Pressure (N cm−2) | 93.9 ± 28.9 | 96.7 ± 28.6 | 2.8 ± 9.0 | 90.0 ± 30.0 | 91.6 ± 32.0 | 1.6 ± 9.2 | |
| Pain (A.U.) | 5.1 ± 1.1 | 4.0 ± 0.6 | −1.1 ± 0.9 # | 4.9 ± 1.1 | 4.9 ± 0.7 | 0.0 ± 1.2 | |
| RPE (A.U.) | 0.9 ± 0.6 | 5.3 ± 2.1 | 4.4 ± 2.0 ### | 0.5 ± 0.4 | 0.6 ± 0.6 | 0.1 ± 0.8 | |
| Affective | State anxiety (A.U.) | 31.5 ± 5.4 | 29.6 ± 4.9 | −1.8 ± 1.7 | 32.3 ± 7.6 | 31.5 ± 5.8 | −0.7 ± 4.2 |
| Physiological | Glucose (mg dL−1) | 91.2 ± 17.0 | 106.9 ± 12.2 | 15.7 ± 14.4 ## | 82.4 ± 7.5 | 82.5 ± 6.7 | 0.1 ± 2.5 |
| Irisin (μg dL−1) | 12.5 ± 5.7 | 14.5 ± 5.4 | 2.0 ± 9.4 | 9.9 ± 4.5 | 11.1 ± 4.8 | 1.2 ± 6.2 | |
| Insulin (mg dL−1) | 11.6 ± 7.6 | 12.3 ± 6.4 | 0.7 ± 4.9 | 5.3 ± 2.5 | 6.8 ± 4.2 | 1.5 ± 4.3 | |
| Creatinine (mg dL−1) | 1.1 ± 0.1 | 1.2 ± 0.1 | 0.1 ± 0.1 ### | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.0 ± 0.1 | |
| Cortisol (μg dL−1) | 9.1 ± 4.3 | 11.4 ± 5.6 | 2.3 ± 2.5 ## | 9.1 ± 4.3 | 8.9 ± 4.2 | −0.2 ± 1.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Invernizzi, P.L.; Trecroci, A.; Scurati, R.; Signorini, G.; Formenti, D.; Bosio, A.; Rigon, M.; Benedini, S. Acute Effects of a Combat Sport Environment on Self-Control and Pain Perception Inhibition: A Preliminary Study in a New Ecological Framework. Sustainability 2023, 15, 8418. https://doi.org/10.3390/su15108418
Invernizzi PL, Trecroci A, Scurati R, Signorini G, Formenti D, Bosio A, Rigon M, Benedini S. Acute Effects of a Combat Sport Environment on Self-Control and Pain Perception Inhibition: A Preliminary Study in a New Ecological Framework. Sustainability. 2023; 15(10):8418. https://doi.org/10.3390/su15108418
Chicago/Turabian StyleInvernizzi, Pietro Luigi, Athos Trecroci, Raffaele Scurati, Gabriele Signorini, Damiano Formenti, Andrea Bosio, Marta Rigon, and Stefano Benedini. 2023. "Acute Effects of a Combat Sport Environment on Self-Control and Pain Perception Inhibition: A Preliminary Study in a New Ecological Framework" Sustainability 15, no. 10: 8418. https://doi.org/10.3390/su15108418
APA StyleInvernizzi, P. L., Trecroci, A., Scurati, R., Signorini, G., Formenti, D., Bosio, A., Rigon, M., & Benedini, S. (2023). Acute Effects of a Combat Sport Environment on Self-Control and Pain Perception Inhibition: A Preliminary Study in a New Ecological Framework. Sustainability, 15(10), 8418. https://doi.org/10.3390/su15108418









