Abstract
Exposure to asbestos fibres causes asbestosis, mesothelioma and several other cancers, which together are commonly referred to as asbestos-related diseases (ARDs). The use of asbestos increased rapidly in Australia and overseas throughout the 1900s, but knowledge about the health effects of exposure and subsequent controls came about more gradually. In Australia today, an estimated 4000 people still die annually from ARDs. While most of these deaths are due to past occupational exposures, there is ongoing concern about the many potential sources of asbestos exposure remaining in homes and the broader built environment as a legacy of past use. Current evidence indicates that Australians will continue to be exposed to legacy asbestos occupationally and non-occupationally, and continue to develop ARDs, without targeted action to prevent it. Evidence of ongoing exposure highlights the importance of better understanding how and why such exposures might still occur, and how they can be effectively prevented or controlled, with the aim of preventing the disease in the future. A better characterisation of this risk is also necessary to enable effective risk management and appropriate risk communication that is relevant to the current Australian context. This article explores the past, present and future of ARDs in Australia, considers the risk of a new wave of ARDs from legacy asbestos, and identifies where further study is required so that sustainable policies and practices can be developed to prevent a future wave of diseases.
1. Introduction
Exposure to asbestos fibres causes mesothelioma (cancer of the mesothelial cells which cover most internal organs), asbestosis (fibrosis of the lung because of exposure to asbestos dust), cancers of the lung, ovary and larynx, and benign pleural diseases such as pleural plaques. For the purposes of this paper, these diseases will be referred to collectively as asbestos-related diseases (ARDs). Other cancers, primarily of the abdominal organs, have also been linked with asbestos exposure [1].
Asbestos is estimated to be responsible for the largest number of deaths of all occupational carcinogens (63%) globally, in [2], and is estimated to account for 93% of all deaths in Australia due to occupational carcinogens [3]. Countries that had higher historical asbestos consumption rates per capita have recorded a higher rate of deaths from ARDs than countries with lower historical asbestos consumption [4]. Deaths from ARDs in Australia (and elsewhere) are understood to be mostly attributable to past occupational asbestos exposures that occurred before the introduction of asbestos bans and strict regulatory controls [3]. However, there is ongoing and increasing concern about the public health risk posed by remaining asbestos stocks and their potential impact on the incidence of ARDs in the future.
Asbestos-containing materials have essentially not been used in new applications in Australia since the comprehensive ban on asbestos products took effect in 2003. However, asbestos was used in over 3000 products and can be found in many buildings and structures built before 1990, creating a significant potential risk for further exposure in occupational and non-occupational settings. It is estimated that there are still approximately 6.2 million tonnes of asbestos-containing material (ACMs) in the Australian built environment as a legacy of past use [5]. Exposure to this legacy asbestos is not restricted to traditional occupational scenarios, since many potential sources of this asbestos remain in our built environment. This can include in homes, schools, hospitals and in public and commercial buildings.
The aim of this paper is to provide an insight into the history of asbestos use in Australia; the nature and extent of the resultant burden of ARDs in the past and the present; and understanding the potential for future ARDs and how these might be avoided.
2. Materials and Methods
A narrative review of the literature has been undertaken to provide an historical perspective and broad overview on the topic of ongoing asbestos exposure risk. The history of asbestos-related disease and asbestos risk awareness is reviewed, alongside the various practical and regulatory responses that have been implemented, and which were informed by the growing evidence base. Emerging theories about the continuing potential health risk posed by asbestos are considered, and areas for further study are recommended to inform sustainable policy and practice and eradicate ARDs.
Data from the Global Burden of Disease Study is reported. This identifies the estimated number of deaths from asbestos-related disease in Australia, and shows the change in the rates and proportions of men and women dying from ARDs. Data from the Australian Mesothelioma registry is also reported, enabling an assessment of the potential contribution from non-occupational asbestos exposures, and occupational asbestos exposures, to mesothelioma incidence in Australia today.
3. Results
3.1. Australia’s Regulatory Response to Asbestos
The history of asbestos-related disease in Australia began with the mining of asbestos from as early as 1880, before the risk associated with asbestos exposure had become known. Australia was one of the world’s highest consumers of asbestos per capita. Asbestos was mined in Australia for nearly 100 years, until 1983. It was also imported into Australia and was widely used in the manufacturing and construction industries, before being phased out by around 1990 [6].
In the 1930s, reports were mounting about respiratory disease among James Hardie factory workers in Western Australia. In around 1942 workers’ compensation laws that specifically addressed asbestos-related claims began to be enacted [7]. There were also growing concerns in the 1940s that workers and the surrounding community were being exposed to unsafe levels of asbestos dust from the asbestos mine at Wittenoom in Western Australia [8].
In the 1960s and 1970s, some of the first workplace laws were enacted to address asbestos safety [9]. From the 1970s to early 1980s, a series of asbestos exposure limits were adopted across Australia [10]. It was also in the 1980s that initial asbestos bans were implemented that limited the use of asbestos containing materials. In the 1990s, the first targeted asbestos regulations were introduced, and a comprehensive ban on asbestos use and importation took effect in 2003 [11].
3.2. The Established Link between Asbestos Exposure and ARDs
While the use of asbestos was rapidly increasing in Australia and overseas, knowledge about the risk from asbestos exposure, and the subsequent response to that risk developed more slowly from the 1920s onwards—asbestosis by the 1920s, lung cancer by the 1940s and 1950s and mesothelioma by the 1960s [12]. Between 1973 and 1977, the International Agency for Research on Cancer (IARC) carried out several extensive reviews of evidence that established associations between asbestos exposure and the development of lung cancer and mesothelioma in mining and manufacturing workers, and also in their families and those living in the vicinity of those workplaces [13,14]. Subsequent IARC reviews confirmed the causal link between exposure to all forms of asbestos and the development of mesothelioma, lung cancer, laryngeal cancer and ovarian cancer [1]. It is now well established that all forms of asbestos can cause cancer, and there is no level of exposure that is known to be safe [15,16]. Associations have also been observed between asbestos exposure and cancer of the pharynx, stomach and colorectum; however, the weight of evidence has not been sufficient to establish a causal relationship to these cancers [1,17]. The role of asbestos in causing these and other forms of cancer, such as renal cancer, continues to be investigated [18,19].
Asbestos is the only cause of asbestosis and is the predominant cause of mesothelioma [1]. Cancer of the lung, larynx and ovary are more commonly caused by other carcinogenic agents, in addition to asbestos [20]. Smoking has a synergistic, multiplicative effect on the lung cancer risk resulting from exposure to asbestos [21].
The primary risk arising from asbestos exposure is from inhalational of asbestos fibres. This increased cancer risk is directly proportional to the cumulative amount breathed in (the cumulative exposure), which in turn is directly proportional to the intensity (concentration), and duration, of exposure [22]. Concerns have been raised about the possible increased risk from the ingestion of asbestos, primarily as a result of drinking water supplied through asbestos pipes. However, there are limited epidemiological studies and, hence, evidence that the ingestion of fibres increases the risk of gastrointestinal cancers. There are also significant methodological problems with the relevant studies, and there is little evidence from animal studies of an increased risk [23].
Since the risk for all asbestos-related diseases is dose-dependent, increasing with increased cumulative exposure, many people with asbestos-related disease have had prolonged exposure to asbestos. However, meaningful increases in disease risk are also associated with a relatively low level or background level of exposure, particularly for mesothelioma [24].
The time between first exposure to asbestos and the occurrence of clinically detectable disease is many years, usually several decades, for all ARDs and particularly for asbestos-related cancers. For example, the median latency for malignant mesothelioma is about 30 years. Risk appears to initially increase continuously after first exposure but, at least for malignant mesothelioma, may later flatten due to the clearance of asbestos fibres. For example, a pooled analysis of Italian asbestos-exposed cohorts found that the rates of pleural mesothelioma flattened after about 40 years, and that a model that incorporated a fibre clearance term provided a better fit to the data than models that did not include such a term. However, this was not the case for the risk of peritoneal mesothelioma [20].
Having knowledge of a person’s history of asbestos exposure can assist in the early diagnosis of asbestosis and mesothelioma. In the case of lung cancer, ovarian cancer and laryngeal cancer, knowledge of past exposure can greatly assist in accurately attributing asbestos exposure as the cause of disease [20]. However, since several of the asbestos-associated cancers (lung cancer, laryngeal cancer and ovarian cancer) are commonly caused by other carcinogens apart from asbestos, and it is not possible to know the cause for an individual instance of one of these cancers, the burden of these cancers that is due to asbestos exposure can only be estimated through the application of broader epidemiological approaches (specifically, by estimating population attributable fractions).
3.3. Deaths from Asbestos-Related Diseases in Australia
Deaths from ARDs in Australia are reported through the Global Burden of Disease Study (the GBD Study). In December 2020, the GBD Study published its latest estimates of the global burden of 87 risk factors in 204 countries and territories between 1990 and 2019 (GBD 2019) [25].
According to GBD 2019, 4449 (95% Uncertainty Interval (95% UI) 3583–5260) Australians died from asbestos-related diseases in 2019. These deaths were attributed to past occupational asbestos exposure, although a small proportion of them probably arose from non-occupational exposure (non-occupational exposure was not separately reported) (Table 1). Australia’s death rate from ARDs was around 18 (95% UI 15–21) deaths per 100,000 in the population (30 (95% UI 24–35) deaths per 100,000 for men and 7 (95% UI 4–10) deaths per 100,000 for women). The results from the GBD study suggest this was the 12th highest death rate from ARDs among the 64 World Bank High Income Countries in 2019.

Table 1.
Number of deaths from ARDs among men and women, Australia in 2019.
Australia’s death rates are among the highest in the world for asbestos-related lung cancer (23 (95% UI 17–28) deaths per 100,000 for men and 5 (95% UI 3–7) deaths per 100,000 for women in 2019) and the third highest for mesothelioma (around 3 (95% UI 3–4) deaths per 100,000 of the population). The estimated proportion of deaths due to ARDs varied between 13% (95% UI 6–20) (for ovarian cancer) and 100% (for asbestosis) (Table 2).

Table 2.
Number of deaths from diseases and proportion attributable to asbestos exposure (2019).
The number of deaths due to ARDs in Australia increased for both men and women between 1990 and 2019 (the period currently covered by the GBD study). Although the vast majority of people who die from ARDs in Australia are male, the proportion of ARD deaths that were of women has increased over the last 30 years (Table 3).

Table 3.
Number and proportion of total deaths from ARDs in men and women between 1990 and 2019.
3.4. Australian Mesothelioma Registry
The AMR is a registry of all cases of mesothelioma diagnosed in Australia since 1 July 2010. The AMR captures information about mesothelioma incidence, mortality and asbestos exposure. It is the most up-to-date source of data on mesothelioma in Australia. The latest report from the AMR was published in 2019–2020 (March 2020) [26]. The results presented in this section are taken from that report.
The highest number of diagnosed cases of mesothelioma in Australia was in 2017 (819 cases), and the figure has dropped slightly since then. The number of deaths has remained relatively steady over the same period (approximately 700 per year). (Note that this is less than the GBD estimate shown in Table 1 and Table 2; this is because the GBD results involve modelled data to allow consistent methods to be used for all countries and time periods, whereas the AMR results are counts of cases reported to the AMR, based on the cause of death listed in death certificates of individuals).
In 2020, there were 642 cases of mesothelioma diagnosed, with the median age at diagnosis being 75 years. There were 696 deaths of people with mesothelioma recorded a mortality rate from mesothelioma of 2.1 deaths per 100,000 population. In Australia, most mesothelioma patients are male, almost certainly because men were more likely than women to have had past occupational exposures to asbestos.
Since about 2003, there has been a considerable decrease in the age-standardised rate of men diagnosed with the disease, while the age-standardised rate of women diagnosed has declined only slightly. As a result, the proportion of mesothelioma cases comprised by women has increased over this period (Figure 1 and Figure 2).
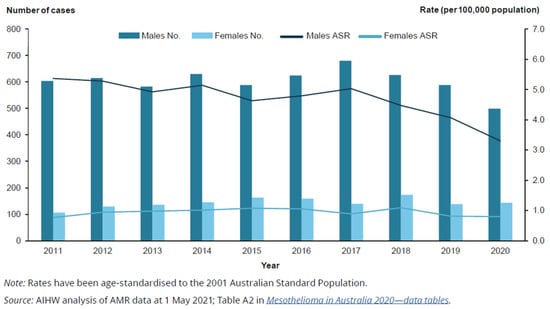
Figure 1.
Number and age-standardised rate (per 100,000 population) of people diagnosed with mesothelioma, by year and sex, 2011 to 2020. Rate (per 100,000 population) [26].
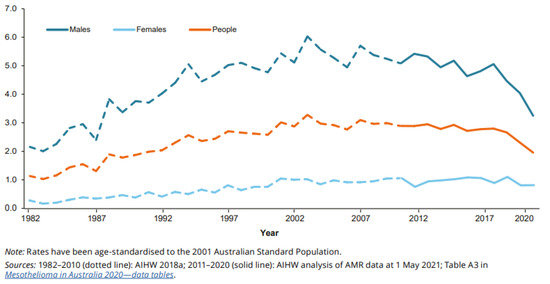
Figure 2.
Age-standardised rate (per 100,000 population) of people diagnosed with mesothelioma, by year and sex, 1982 to 2020 [26].
Evidence gathered from a detailed exposure assessment carried out by the AMR indicates that both occupational and non-occupational exposure to asbestos are potential contributing factors to the development of mesothelioma cases reported to the registry. However, occupational exposure is by far the most predominant relevant exposure for men.
Of the 1028 people for whom exposure was detected, 93% of women reported non-occupational exposure only, compared with 22% of men. Most men had occupational exposure (either alone or also with non-occupational exposure), whereas very few women had this (Table 4).

Table 4.
Occupational and non-occupational exposure assessment, by sex, 2010–2020.
3.5. What Is the “Third Wave” Risk Posed by Exposure to Legacy Asbestos, and How Might It Impact ARDs in Australia in the Future?
The IARC has identified that the nature of occupational exposures, once dominated by chronic exposures in the manufacturing industries, has changed: exposures are now more likely to occur in maintenance activities, or during remediation of buildings that contain asbestos [1]. This is consistent with the knowledge that the main potential sources of asbestos exposure in Australia today are from hazards such as asbestos-containing building products including roofs and fences, unsafe asbestos removal practices, asbestos in soils in land being remediated and illegal dumping, which are all a legacy of Australia’s past asbestos use [27]. The Australian Stocks and Flows Model for Asbestos provides a national snapshot of the status of legacy asbestos and its transition to asbestos waste. The model indicates that approximately 1 million tonnes of the estimated 6.2 million tonnes of legacy asbestos remaining in Australia’s built environment, will still be present and will not have transitioned to waste facilities by 2060.
There is, therefore, a considerable likelihood that Australians will continue to be at risk of exposure to asbestos due to one or more of these asbestos legacy hazards, and this could occur through their occupation, or non-occupationally, such as through background exposure to deteriorating ACM, or through do-it-yourself home renovations [28,29]. As described above, data from the AMR’s asbestos exposure assessments indicate that both occupational and non-occupational exposure to asbestos are potential contributing factors in the development of mesothelioma cases reported to the registry [26].
To help understand whether these hazards are contributing to current ARD mortality and to prevent future disease, several studies have investigated disease trends and sought to describe ARD mortality trends as “waves”, which are the result of different types of asbestos exposures that have occurred historically [30,31,32]. It was suggested by Landrigan in 1991 that industrialised countries (like Australia) were at the beginning of the “third wave” of asbestos-related disease, which would occur in people repairing, renovating or demolishing asbestos-containing buildings. As part of this thesis, the first wave occurred in those who mined, milled or transported raw asbestos, or worked in the manufacture of asbestos products, and the second wave occurred in workers who used those asbestos products [30].
A small number of subsequent studies have looked further at the potential risk from a third wave of exposures, such as that suggested by Landrigan. A focus of the research has been to determine whether the effect of the third wave is visible in current ARD mortality data, or whether it can still be prevented. These studies have found that there are gaps in evidence that need to be addressed in order to characterise the current risk posed by legacy asbestos and prevent future disease.
Armstrong and Driscoll synthesised much of the available evidence relating to the third wave in their 2016 paper, Mesothelioma in Australia: cresting the third wave. In this paper, the authors define third-wave exposure as both occupational and non-occupational exposure to asbestos as a consequence of the repair, renovation and demolition of buildings, and environmental exposure to asbestos (excluding ambient exposure and exposure to naturally occurring asbestos). They note that scientific evidence for the existence of the hypothesised third wave is limited, but that the third wave (as described) is likely to contribute to asbestos-related disease in Australia in the future without targeted action to prevent it [32]. They also identified knowledge gaps that, if addressed, can help to inform an appropriate response to the risk posed by legacy asbestos (that is, the risk associated with the third wave of ARDs)—the extent of deterioration of in situ asbestos and the risks arising from any resultant exposure, and the risk associated with so-called third wave exposures and with exposure to ambient levels of asbestos. Despite relevant work being undertaken since this paper was published, the key questions remain unanswered. In the Australian setting, the amount of in situ asbestos has been estimated [33]; the risk from in situ has been characterised quantitatively [8] and qualitatively [27,34]; the potential application of mobile phone applications has been explored [35,36]; and the challenges of communication have been considered [37]. Similar considerations have been undertaken in several countries—Colombia [38,39,40,41], the Czech Republic [42], Denmark [43], Iran [44,45], Italy [46,47,48,49], Korea [50,51,52,53,54,55,56], Peru [57], Poland [58,59], Serbia [60], South Africa [61], Turkey [62,63], the United States [64,65,66,67]—and more generally [68,69,70,71,72,73,74,75,76,77].
Evidence of ongoing exposure highlights the importance of building a better understanding of how and why such exposures might still occur and how they can be effectively prevented or controlled, with the aim of preventing disease in the future.
It is generally accepted that most exposure that occurs due to legacy asbestos is at lower levels than has occurred through past occupational exposure [24,78]. This is a key difference in the characterisation of risk from past occupational exposures and past exposure from non-occupational sources. Uncertainty remains about how to best describe or quantify the risk posed by these lower levels of exposure, including the effect of cumulative low level exposures on the development of ARDs [28]. Several studies have highlighted the potential for ARDs to develop as a result of lower levels of exposure, and there is still no threshold below which asbestos exposure is considered safe [15,16]. However, it is clear that risk is primarily directly proportional to cumulative exposure [78]. Low level exposure is typically a characteristic of environmental exposure to asbestos, where an associated increased risk of malignant mesothelioma has been identified in a number of studies [79,80,81,82,83,84,85].
4. Discussion
Much of what is known about the development of ARDs has come from studies about past occupational exposures. Given the changing nature of occupational asbestos exposures observed by IARC, and the potential contribution of occupational and non-occupational asbestos exposure to mesothelioma incidence in Australia today, there is a clear need to continue to measure and analyse the risks posed by the ongoing hazard of Australia’s legacy asbestos, to enable effective controls.
Indeed, surveys of mesothelioma patients in Australia conducted by the Australian Mesothelioma Registry indicate that both occupational and non-occupational exposures have been a factor in the development of mesothelioma cases recorded in Australia today.
Considering the many potential sources of asbestos exposure in the Australian built environment, and the health consequences of such exposure, clarification of the relationship between low level asbestos exposure and the development of ARDs is important in order to ensure an accurate characterisation of the risk posed by legacy asbestos.
5. Conclusions
Asbestos was used in Australia extensively before there was significant awareness that exposure to asbestos fibres cause serious disease. In Australia, and around the world, it is likely that many deaths could have been prevented if there had been a more comprehensive response to the science that was emerging, and which gradually demonstrated the devastating link between exposure to asbestos fibres and the development of life-threatening illness.
Australia now faces an ongoing potential risk to public health from asbestos-related diseases associated with exposure to legacy asbestos. This risk needs to be studied in order to enable effective risk management and appropriate risk communication that is relevant to the Australian context, and which can help to prevent a future wave of diseases.
This narrative review of the literature provides an opportunity to reflect upon the circumstances that have led to Australia’s current asbestos problem, and to consider areas of further study that are required to inform future actions and prevent disease.
Available evidence indicates that, without targeted action, asbestos exposure due to legacy asbestos will continue to cause severe illness in Australia. Studies have identified knowledge gaps (such as the risk arising from renovation of houses by members of the public; the risk arising from sources of environmental asbestos; identifying the most appropriate approach to managing asbestos in situ) that, if addressed, can help to inform an appropriate response to the risk posed by this legacy asbestos, and thereby help to minimise the risk of a large third wave of ARDs.
Author Contributions
Writing—original draft, K.M.; Writing—review & editing, K.M., T.D., J.C. and J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- International Agency for Research on Cancer (IARC). Arsenic, Metals, Fibres and Dusts. Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 100C: Asbestos (Chrysotile, Amosite, Crocidolite, Tremolite, Actinolite and Anthophyllite). 2012. Available online: http://publications.iarc.fr/120 (accessed on 28 February 2023).
- GBD 2016 Occupational Carcinogens Collaborators. Global and regional burden of cancer in 2016 arising from occupational exposure to selected carcinogens: A systematic analysis for the Global Burden of Disease Study 2016. Occup. Environ. Med. 2020, 77, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results. Institute for Health Metrics and Evaluation (IHME): Seattle, WA, USA. 2020. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 28 February 2023).
- Rath, E.M.; Yuen, M.L.; Odgerel, C.-O.; Lin, R.-T.; Soeberg, M.; Nowak, A.K.; Takahashi, K. The Ecological Association between Asbestos Consumption and Asbestos-Related Diseases 15 Years Later. Environ. Health Perspect. 2022, 130, 57703. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.; Hollins, I.; Pickin, J.; Donovan, S. Asbestos Stocks and Flows Legacy in Australia. Sustainability 2023, 15, 2282. [Google Scholar] [CrossRef]
- Australian Government, Asbestos Safety and Eradication Agency (ASEA). National Asbestos Profile for Australia. 2017. Available online: https://www.asbestossafety.gov.au/find-out-about-asbestos/asbestos-safety-information/brochures/national-asbestos-profile-australia (accessed on 28 February 2023).
- Workers’ Compensation (Dust Diseases) Act 1942 Workers’ Compensation (Dust Diseases) Act 1942 No 14–NSW Legislation. Available online: https://legislation.nsw.gov.au/view/html/inforce/current/act-1942-014 (accessed on 28 February 2023).
- Musk, A.W.; Reid, A.; Olsen, N.; Hobbs, M.; Armstrong, B.; Franklin, P.; Hui, J.; Layman, L.; Merler, E.; Brims, F.; et al. The Wittenoom legacy. Int. J. Epidemiol. 2020, 49, 467–476. [Google Scholar] [CrossRef]
- Queensland Government, Queensland Health. Queensland Health Report on the Investigation into Asbestos-Related Health Concerns Due to Former Asbestos Manufacturing Factories at Gaythorne and Newstead. 2015. Available online: https://www.publications.qld.gov.au/dataset/queensland-health-led-asbestos-investigations/resource/fcf53b47-41a8-471c-ab5e-587768741e92 (accessed on 28 February 2023).
- Australian Government, Safe Work Australia. Asbestos Exposure and Compliance Study of Construction and Maintenance Workers. 2009. Available online: https://www.safeworkaustralia.gov.au/system/files/documents/1702/asbestosexposureandcompliancestudyofconstructionandmaintenanceworkers_2010_pdf.pdf (accessed on 28 February 2023).
- Australian Government, Safe Work Australia. Mesothelioma in Australia: Incidence 1982 to 2008, Deaths 1997 to 2007. 2012. Available online: https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwww.safeworkaustralia.gov.au%2Fsystem%2Ffiles%2Fdocuments%2F1702%2Fmesotheliomainaustralia2012.docx&wdOrigin=BROWSELINK (accessed on 28 February 2023).
- Bartrip, P.W. History of asbestos related disease. Postgrad. Med. J. 2004, 80, 72–76. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man, Some Inorganic and Organometallic Compounds, Volume 2. 1973. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Some-Inorganic-And-Organometallic-Compounds-1973 (accessed on 28 February 2023).
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man, Volume 14. 1977. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Asbestos-1977 (accessed on 28 February 2023).
- World Health Organization. Asbestos: Elimination of Asbestos-Related Diseases. 2018. Available online: https://www.who.int/en/news-room/fact-sheets/detail/asbestos-elimination-of-asbestos-related-diseases (accessed on 28 February 2023).
- European Chemicals Agency. Committee for Risk Assessment RAC Opinion on Scientific Evaluation of Occupational Exposure Limits for Asbestos. 2021. Available online: https://echa.europa.eu/documents/10162/7937606/OEL_asbestos_Final_Opinion_en.pdf/cc917e63-e0e6-e9cd-86d2-f75c81514277?t=1626256168788 (accessed on 28 February 2023).
- Institute of Medicine. Asbestos: Selected Cancers; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar] [CrossRef]
- Fortunato, L.; Rushton, L. Stomach cancer and occupational exposure to asbestos: A meta-analysis of occupational cohort studies. Br. J. Cancer 2015, 26, 1805–1815. [Google Scholar] [CrossRef]
- Candura, S.M.; Boeri, R.; Teragni, C.; Chen, Y.; Scafa, F. Renal cell carcinoma and malignant peritoneal mesothelioma after occupational asbestos exposure: Case report. Med. Lavoro 2016, 107, 172–177. [Google Scholar]
- Wolff, H.; Vehmas, T.; Oksa, P.; Rantanen, J.; Vainio, H. Asbestos, asbestosis, and cancer, the Helsinki criteria for diagnosis and attribution 2014: Recommendations. Scand. J. Work Environ. Health 2015, 41, 5–15. [Google Scholar] [CrossRef]
- Klebe, S.; Leigh, J.; Henderson, D.W.; Nurminen, M. Asbestos, Smoking and Lung Cancer: An Update. Int. J. Environ. Res. Public Health 2020, 17, 258. [Google Scholar] [CrossRef]
- Carbone, M.; Ly, B.H.; Dodson, R.F.; Pagano, I.; Morris, P.T.; Dogan, U.A.; Gazdar, A.F.; Pass, H.I.; Yang, H. Malignant mesothelioma: Facts, myths, and hypotheses. J. Cell. Physiol. 2012, 227, 44–58. [Google Scholar] [CrossRef]
- Cheng, T.J.; More, S.L.; Maddaloni, M.A.; Fung, E.S. Evaluation of potential gastrointestinal carcinogenicity associated with the ingestion of asbestos. Rev. Environ. Health 2020, 36, 15–26. [Google Scholar] [CrossRef]
- Goldberg, M.; Luce, D. The health impact of nonoccupational exposure to asbestos: What do we know? Eur. J. Cancer Prev. 2009, 18, 489–503. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222, Erratum in Lancet 2020, 396, 1562. [Google Scholar] [CrossRef]
- Australian Government, Australian Institute of Health and Welfare. Mesothelioma in Australia 2020. 2021. Available online: https://www.aihw.gov.au/reports/cancer/mesothelioma-in-australia-2020/summary (accessed on 28 February 2023).
- Gray, C.; Carey, R.; Reid, A. Current and Future Risks of Asbestos Exposure in the Australian Community. Int. J. Occup. Environ. Health 2016, 22, 292–299. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5137554/ (accessed on 28 February 2023). [CrossRef]
- Australian Government, ASEA. Priority Areas Where ACMs May Present a Risk in the Australian Community. Australian Government, Canberra. 2018. Available online: https://www.asbestossafety.gov.au/sites/default/files/documents/2018-08/ASE-2259%20Priority%20Risk%20Factsheet.pdf (accessed on 28 February 2023).
- Bardsley, A. Asbestos Exposure in New Zealand: Review of the Scientific Evidence of Non-Occupational Risks. A Report on Behalf of the Royal Society of New Zealand and the Office of the Prime Minister’s Chief Science Advisor. 2015. Available online: https://royalsociety.org.nz/assets/documents/Asbestos-exposure-in-New-Zealand-April-2015.pdf (accessed on 28 February 2023).
- Landrigan, P.J. The third wave of asbestos disease: Exposure to asbestos in place. Public health control. Introduction. Ann. N. Y. Acad. Sci. 1991, 643, 15–16. Available online: https://nyaspubs.onlinelibrary.wiley.com/doi/10.1111/j.1749-6632.1991.tb24438.x (accessed on 28 February 2023).
- Olsen, N.J.; Franklin, P.J.; Reid, A.; Klerk, N.H.; Threlfall, T.J.; Shilkin, K.; Musk, B. Increasing incidence of malignant mesothelioma after exposure to asbestos during home maintenance and renovation. Med. J. Aust. 2011, 195, 271–274. [Google Scholar] [CrossRef]
- Armstrong, B.; Driscoll, T. Mesothelioma in Australia: Cresting the third wave. Public Health Res. Pract. 2016, 26, 2621614. [Google Scholar] [CrossRef]
- Donovan, S.; Pickin, J. An Australian stocks and flows model for asbestos. Waste Manag. Res. 2016, 34, 1081–1088. [Google Scholar] [CrossRef]
- Kottek, M.; Yuen, M.L. Public health risks from asbestos cement roofing. Am. J. Ind. Med. 2022, 65, 157–161. [Google Scholar] [CrossRef]
- Govorko, M.H.; Fritschi, L.; Reid, A. Accuracy of a mobile app to identify suspect asbestos-containing material in Australian residential settings. J. Occup. Environ. Hyg. 2018, 15, 598–606. [Google Scholar] [CrossRef]
- Govorko, M.; Fritschi, L.; Reid, A. Using a Mobile Phone App to Identify and Assess Remaining Stocks of In Situ Asbestos in Australian Residential Settings. Int. J. Environ. Res. Public Health 2019, 16, 4922. [Google Scholar] [CrossRef]
- Hooker, C.; Capon, A.; Hess, I.M. Communicating with the public about the risks of naturally occurring asbestos. Public Health Res. Pract. 2017, 27, 2751747. [Google Scholar] [CrossRef] [PubMed]
- Cely-García, M.F.; Lysaniuk, B.; Pasetto, R.; Ramos-Bonilla, J.P. The challenges of applying an Activity-Based Sampling methodology to estimate the cancer risk associated with asbestos contaminated landfilled zones. Environ. Res. 2020, 181, 108893. [Google Scholar] [CrossRef] [PubMed]
- Flórez Gutiérrez, P.; Cely-García, M.F.; Larrahondo, J.M. Environmental management criteria, aimed at public policymaking, for the removal and disposal of asbestos-containing building materials in Colombia. Integr. Environ. Assess. Manag. 2023. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lysaniuk, B.; Cely-García, M.F.; Giraldo, M.; Larrahondo, J.M.; Serrano-Calderón, L.M.; Guerrero-Bernal, J.C.; Briceno-Ayala, L.; Cruz Rodriguez, E.; Ramos-Bonilla, J.P. Using GIS to Estimate Population at Risk Because of Residence Proximity to Asbestos Processing Facilities in Colombia. Int. J. Environ. Res. Public Health 2021, 18, 13297. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Bonilla, J.P.; Cely-García, M.F.; Giraldo, M.; Comba, P.; Terracini, B.; Pasetto, R.; Marsili, D.; Ascoli, V.; Lysaniuk, B.; Rodríguez, M.C.; et al. An asbestos contaminated town in the vicinity of an asbestos-cement facility: The case study of Sibaté, Colombia. Environ. Res. 2019, 176, 108464. [Google Scholar] [CrossRef]
- Urban, M.; Pelclová, D.; Urban, P.; Vít, M.; Urban, P.; Fenclová, Z. Asbestos danger in central Europe is not yet over-the situation in the Czech Republic. Cent. Eur. J. Public Health 2022, 30, 67–73. [Google Scholar] [CrossRef]
- Panou, V.; Vyberg, M.; Meristoudis, C.; Hansen, J.; Bøgsted, M.; Omland, Ø.; Weinreich, U.M.; Røe, O.D. Non-occupational exposure to asbestos is the main cause of malignant mesothelioma in women in North Jutland, Denmark. Scand. J. Work Environ. Health 2019, 45, 82–89. [Google Scholar] [CrossRef]
- Taghizadeh, F.; Jafari, A.J.; Gholami, M.; Kermani, M.; Arfaeinia, H.; Mohammadi, S.; Dowlati, M.; Shahsavani, A. Monitoring of airborne asbestos fibers in an urban ambient air of Shahryar City, Iran: Levels, spatial distribution, seasonal variations, and health risk assessment. Environ. Sci. Pollut. Res. Int. 2019, 26, 6450–6459. [Google Scholar] [CrossRef]
- Nasirzadeh, N.; Rasoulzadeh, Y.; Mahmoudi, D.; Marioryad, H.; Abdollahi, M.H.; Najafian, H.; Khalili, M. Evaluating the airborne asbestos dispersion in enclosed parking lots in Iran. Arch. Environ. Occup. Health 2022, 77, 437–445. [Google Scholar] [CrossRef]
- Campopiano, A.; Cannizzaro, A.; Olori, A.; Angelosanto, F.; Ramires, D.; Basili, F.; Gargaro, G.; Massera, S.; Novembre, G.; Cavariani, F.; et al. Asbestos containing materials in schools of Rome and surrounding area (Italy). Ind. Health 2021, 59, 436–448. [Google Scholar] [CrossRef]
- Catelan, D.; Consonni, D.; Biggeri, A.; Dallari, B.; Pesatori, A.C.; Riboldi, L.; Mensi, C. Estimate of environmental and occupational components in the spatial distribution of malignant mesothelioma incidence in Lombardy (Italy). Environ. Res. 2020, 188, 109691. [Google Scholar] [CrossRef]
- Roccaro, P.; Vagliasindi, F.G.A. Indoor release of asbestiform fibers from naturally contaminated water and related health risk. Chemosphere 2018, 202, 76–84. [Google Scholar] [CrossRef]
- Vimercati, L.; Cavone, D.; Delfino, M.C.; Caputi, A.; De Maria, L.; Sponselli, S.; Corrado, V.; Ferri, G.M.; Serio, G. Asbestos Air Pollution: Description of a Mesothelioma Cluster Due to Residential Exposure from an Asbestos Cement Factory. Int. J. Environ. Res. Public Health 2020, 17, 2636. [Google Scholar] [CrossRef]
- Huh, D.A.; Kang, M.S.; Lee, J.; Choi, J.Y.; Moon, K.W.; Lee, Y.J. Occupational and environmental asbestos exposure and the risk of lung cancer in Korea: A case-control study in South Chungcheong Province of Korea. PLoS ONE 2021, 16, e0249790. [Google Scholar] [CrossRef]
- Kang, D.; Kim, Y.Y.; Shin, M.; Lee, M.S.; Bae, H.J.; Kim, S.Y.; Kim, Y.K. Relationships of Lower Lung Fibrosis, Pleural Disease, and Lung Mass with Occupational, Household, Neighborhood, and Slate Roof-Dense Area Residential Asbestos Exposure. Int. J. Environ. Res. Public Health 2018, 15, 1638. [Google Scholar] [CrossRef]
- Kang, D.; Hwang, Y.; Choi, Y.; Kim, S.Y.; Kim, Y.K. Monitoring and Simulating Environmental Asbestos Dispersion from a Textile Factory. Int. J. Environ. Res. Public Health 2018, 15, 1398. [Google Scholar] [CrossRef]
- Kim, Y.C.; Hong, W.H. Optimal management program for asbestos containing building materials to be available in the event of a disaster. Waste Manag. 2017, 64, 272–285. [Google Scholar] [CrossRef]
- Kwak, K.; Zoh, K.E.; Paek, D. Incidence of Cancer and Asbestos-Related Diseases among Residents Living near Abandoned Asbestos Mines in South Korea: A Retrospective Cohort Study Using National Health Insurance Database. Int. J. Environ. Res. Public Health 2021, 18, 875. [Google Scholar] [CrossRef]
- Lee, E.S.; Kim, Y.K. Asbestos Exposure Level and the Carcinogenic Risk Due to Corrugated Asbestos-Cement Slate Roofs in Korea. Int. J. Environ. Res. Public Health 2021, 18, 6925. [Google Scholar] [CrossRef]
- Lee, S.; Kang, D.; Kim, Y.; Kim, Y.J.; Kim, S.Y. Activity-Based Exposure Levels and Cancer Risk Assessment Due to Naturally Occurring Asbestos for the Residents Near Abandoned Asbestos Mines in South Korea. Int. J. Environ. Res. Public. Health 2021, 18, 5225. [Google Scholar] [CrossRef] [PubMed]
- Torres-Roman, J.S.; Gomez-Rubio, V.; Sanchez-Trujillo, L.; Delgado-Rosas, E.; Puche-Vergara, F.; Sanz-Anquela, J.M.; Ortega, M.A. Geographic study of mortality due to mesothelioma in Peru and its evolution. Cancer Epidemiol. 2020, 68, 101791. [Google Scholar] [CrossRef] [PubMed]
- Krówczyńska, M.; Wilk, E. Environmental and Occupational Exposure to Asbestos as a Result of Consumption and Use in Poland. Int. J. Environ. Res. Public Health 2019, 16, 2611. [Google Scholar] [CrossRef] [PubMed]
- Krówczyńska, M.; Wilk, E. Asbestos Exposure and the Mesothelioma Incidence in Poland. Int. J. Environ. Res. Public Health 2018, 15, 1741. [Google Scholar] [CrossRef]
- Zoraja, B.; Ubavin, D.; Stanisavljevic, N.; Vujovic, S.; Mucenski, V.; Hadzistevic, M.; Bjelica, M. Assessment of asbestos and asbestos waste quantity in the built environment of transition country. Waste Manag. Res. 2022, 40, 1285–1296. [Google Scholar] [CrossRef]
- Tlotleng, N.; Sidwell Wilson, K.; Naicker, N.; Koegelenberg, C.F.; Rees, D.; Phillips, J.I. The significance of non-occupational asbestos exposure in women with mesothelioma. Respirol. Case Rep. 2018, 7, e00386. [Google Scholar] [CrossRef]
- Metintas, S.; Ak, G.; Metintas, M. A review of the cohorts with environmental and occupational mineral fiber exposure. Arch. Environ. Occup. Health 2019, 74, 76–84. [Google Scholar] [CrossRef]
- Sandal, A.; Ecin, S.M.; Koyuncu, A.; Durhan, G.; Akpinar, M.G.; Demir, A.U.; Cöplü, L. Environmental asbestos exposure and nonmalignant pleural findings: A retrospective evaluation of a five-year chest CT repository. Arch. Environ. Occup. Health 2022, 77, 734–743, Erratum in Arch. Environ. Occup. Health 2022, 8, 1–2. [Google Scholar] [CrossRef]
- Glynn, M.E.; Keeton, K.A.; Gaffney, S.H.; Sahmel, J. Ambient Asbestos Fiber Concentrations and Long-Term Trends in Pleural Mesothelioma Incidence between Urban and Rural Areas in the United States (1973–2012). Risk Anal. 2018, 38, 454–471. [Google Scholar] [CrossRef]
- Hesari, R.Z.J.; Rasoulzadeh, Y.; Mohammadian, Y.; Nasirzadeh, N. Cancer risk assessment of exposure to asbestos during old building demolition. Work 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Neitzel, R.L.; Sayler, S.K.; Demond, A.H.; d’Arcy, H.; Garabrant, D.H.; Franzblau, A. Measurement of asbestos emissions associated with demolition of abandoned residential dwellings. Sci. Total Environ. 2020, 722, 137891. [Google Scholar] [CrossRef]
- Oberta, A.F.; Poye, L.; Compton, S.P. Releasability of asbestos fibers from weathered roof cement. J. Occup. Environ. Hyg. 2018, 15, 466–473. [Google Scholar] [CrossRef]
- Bolan, S.; Kempton, L.; McCarthy, T.; Wijesekara, H.; Piyathilake, U.; Jasemizad, T.; Padhye, L.P.; Zhang, T.; Rinklebe, J.; Wang, H.; et al. Sustainable management of hazardous asbestos-containing materials: Containment, stabilization and inertization. Sci. Total Environ. 2023, 14, 163456. [Google Scholar] [CrossRef]
- Douglas, T.; Van den Borre, L. Asbestos neglect: Why asbestos exposure deserves greater policy attention. Health Policy 2019, 123, 516–519. [Google Scholar] [CrossRef]
- Emmett, E.A. Asbestos in High-Risk Communities: Public Health Implications. Int. J. Environ. Res. Public Health 2021, 18, 1579. [Google Scholar] [CrossRef]
- Liu, B.; van Gerwen, M.; Bonassi, S.; Taioli, E.; International Association for the Study of Lung Cancer Mesothelioma Task Force. Epidemiology of Environmental Exposure and Malignant Mesothelioma. J. Thorac. Oncol. 2017, 12, 1031–1045. [Google Scholar] [CrossRef]
- Marsh, G.M.; Riordan, A.S.; Keeton, K.A.; Benson, S.M. Non-occupational exposure to asbestos and risk of pleural mesothelioma: Review and meta-analysis. Occup. Environ. Med. 2017, 74, 838–846. [Google Scholar] [CrossRef]
- Noonan, C.W. Environmental asbestos exposure and risk of mesothelioma. Ann. Transl. Med. 2017, 5, 234. [Google Scholar] [CrossRef]
- Obmiński, A. Asbestos cement products and their impact on soil contamination in relation to various sources of anthropogenic and natural asbestos pollution. Sci. Total. Environ. 2022, 848, 157275. [Google Scholar] [CrossRef]
- Wolfe, C.; Buck, B.; Miller, A.; Lockey, J.; Weis, C.; Weissman, D.; Jonesi, A.; Ryan, P. Exposure to naturally occurring mineral fibers due to off-road vehicle use: A review. Int. J. Hyg. Environ. Health 2017, 220, 1230–1241. [Google Scholar] [CrossRef]
- Xu, R.; Barg, F.K.; Emmett, E.A.; Wiebe, D.J.; Hwang, W.T. Association between mesothelioma and non-occupational asbestos exposure: Systematic review and meta-analysis. Environ. Health 2018, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, A.T.; Ha, H. People, planet and profit: Unintended consequences of legacy building materials. J. Environ. Manag. 2017, 204, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Luberto, F.; Ferrante, D.; Silvestri, S.; Angelini, A.; Cuccaro, F.; Nannavecchia, A.M.; Oddone, E.; Vicentini, M.; Barone-Adesi, F.; Cena, T.; et al. Cumulative asbestos exposure and mortality from asbestos related diseases in a pooled analysis of 21 asbestos cement cohorts in Italy. Environ. Health 2019, 18, 71. [Google Scholar] [CrossRef] [PubMed]
- Airoldi, C.; Magnani, C.; Lazzarato, F.; Mirabelli, D.; Tunesi, S.; Ferrante, D. Environmental asbestos exposure and clustering of malignant mesothelioma in community: A spatial analysis in a population-based case-control study. Environ. Health 2021, 20, 103. [Google Scholar] [CrossRef]
- Dalsgaard, S.B.; Würtz, E.T.; Hansen, J.; Røe, O.D.; Omland, Ø. Cancer Incidence and Risk of Multiple Cancers after Environmental Asbestos Exposure in Childhood-A Long-Term Register-Based Cohort Study. Int. J. Environ. Res. Public Health 2021, 19, 268. [Google Scholar] [CrossRef]
- Dalsgaard, S.B.; Würtz, E.T.; Hansen, J.; Røe, O.D.; Omland, Ø. Environmental asbestos exposure in childhood and risk of mesothelioma later in life: A long-term follow-up register-based cohort study. Occup. Environ. Med. 2019, 76, 407–413. [Google Scholar] [CrossRef]
- Dalsgaard, S.B.; Würtz, E.T.; Hansen, J.; Røe, O.D.; Omland, Ø. A Cohort Study on Cancer Incidence among Women Exposed to Environmental Asbestos in Childhood with a Focus on Female Cancers, including Breast Cancer. Int. J. Environ. Res. Public Health 2022, 19, 2086. [Google Scholar] [CrossRef]
- Fazzo, L.; Minelli, G.; Bruno, C.; Comba, P.; Conti, S.; De Santis, M.; Zona, A.; Binazzi, A.; Magnani, C.; Marinaccio, A.; et al. Early mortality from malignant mesothelioma in Italy as a proxy of environmental exposure to asbestos in children. Ann. Ist. Super. Sanita 2020, 56, 478–486. [Google Scholar] [CrossRef]
- Ferrante, D.; Mirabelli, D.; Tunesi, S.; Terracini, B.; Magnani, C. Pleural mesothelioma and occupational and non-occupational asbestos exposure: A case-control study with quantitative risk assessment. Occup. Environ. Med. 2016, 73, 147–153. [Google Scholar] [CrossRef]
- Kurumatani, N.; Kumagai, S. Mapping the risk of mesothelioma due to neighborhood asbestos exposure. Am. J. Respir. Crit. Care Med. 2008, 178, 624–629. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).