First Report of Giant African Snail (Lissachatina fulica) in a Protected Area of the Cottian Alps, Northwest Italy
Abstract
1. Introduction
2. Materials and Methods
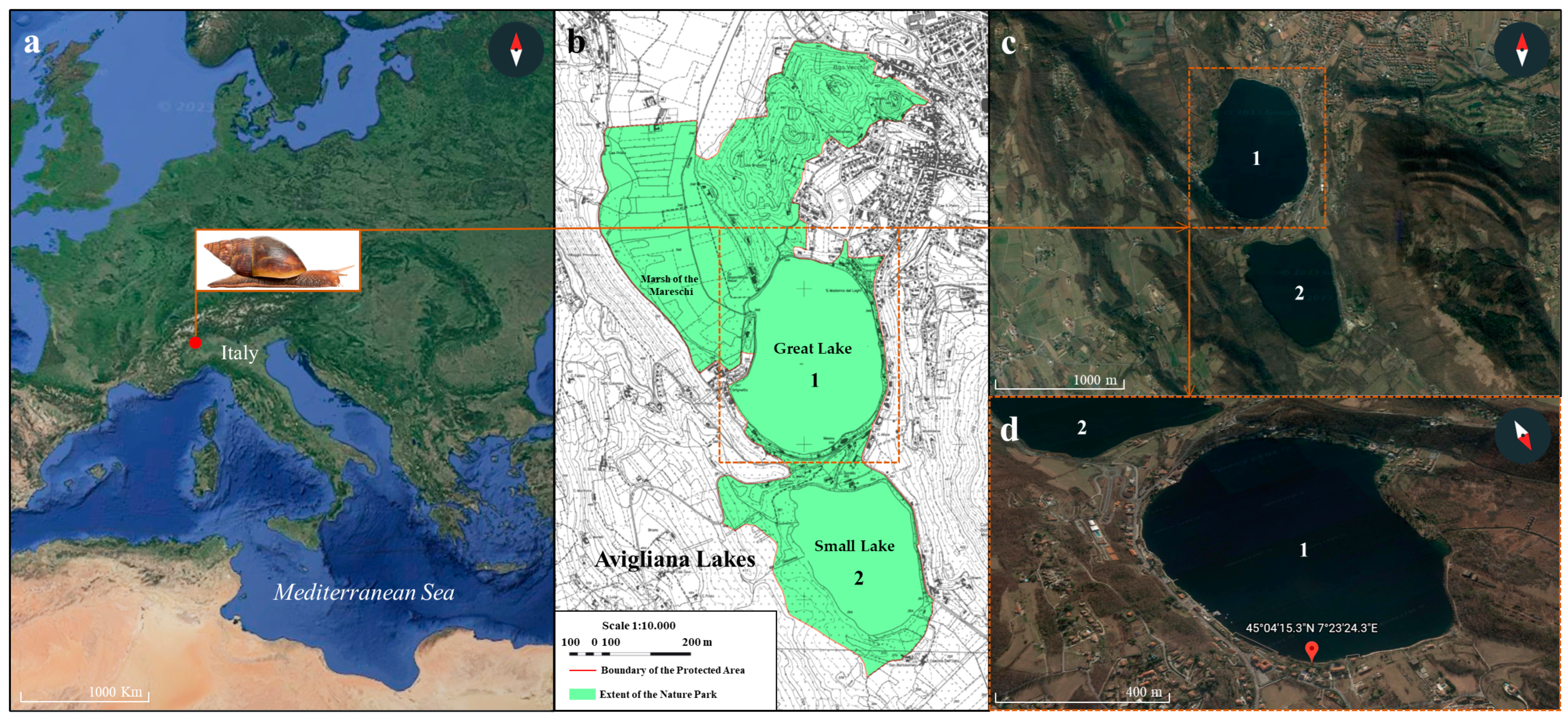
2.1. Study Site
2.2. Species Detection
2.3. Molecular Analysis
3. Results and Discussion
| References | Present Study | [30] 1 | [27] | [29] 2 | [28] |
|---|---|---|---|---|---|
| Country | Italy | Thailand; Malaysia | Brazil | Nigeria | Colombia |
| Specimens | 1 | 215 | 1460 | 100 | 106 |
| Weight | 98.82 | - | - | 59.58 ± 2.33 | - |
| SL | 102.5 | 78.34 ± 9.37 | 42.18 ± 69.08 | 67.86 ± 0.13 | 71.70 ± 17.90 |
| SW | 42.30 | 38.57 ± 3.71 | - | 26.61 ± 0.05 | - |
| AL | 53.00 | 38.15 ± 3.69 | - | 32.43 ± 0.08 | - |
| AW | 36.00 | 22.52 ± 1.86 | - | 10.61 ± 0.04 | - |
| BWL | 63.50 | - | - | - | - |
| PWL | 16.00 | - | - | - | - |
| PWW | 24.50 | - | - | - | - |
| SpL | 39.00 | 42.96 ± 6.42 | - | - | - |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species; The Invasive Species Specialist Group (ISSG): Auckland, New Zealand, 2000. [Google Scholar]
- Pastorino, P.; Polazzo, F.; Bertoli, M.; Santi, M.; Righetti, M.; Pizzul, E.; Prearo, M. Consequences of fish introduction in fishless Alpine lakes: Preliminary notes from a sanitary point of view. Turk. J. Fish. Aquat. Sci. 2019, 20, 1–8. [Google Scholar] [CrossRef]
- Piras, P.; Esposito, G.; Meloni, D. On the occurrence of the blue crab Callinectes sapidus (Rathbun, 1896) in Sardinian coastal habitats (Italy): A present threat or a future resource for the regional fishery sector? BioInvasions Rec. 2019, 8, 134–141. [Google Scholar] [CrossRef]
- Celis-Ramírez, M.; Quintero-Angel, M.; Varela, M.R.E. Control of invasive alien species: The Giant African snail (Lissachatina fulica) a difficult urban public management challenge. J. Environ. Manag. 2022, 322, 116159. [Google Scholar] [CrossRef]
- Patiño-Montoya, A.; Giraldo, A.; Tidon, R. Effect of the invasion history of the giant African snail (Lissachatina fulica) on its realized climatic niche. Invertebr. Biol. 2022, 141, e12385. [Google Scholar] [CrossRef]
- Vázquez, A.A.; Sánchez, J. First record of the invasive land snail Achatina (Lissachatina) fulica (Bowdich, 1822) (Gastropoda: Achatinidae), vector of Angiostrongylus cantonensis (Nematoda: Angiostrongylidae), in Havana, Cuba. Molluscan Res. 2015, 35, 139–142. [Google Scholar] [CrossRef]
- Bohatá, L.; Patoka, J. Invasion Potential of Ornamental Terrestrial Gastropods in Europe Based on Climate Matching. Diversity 2023, 15, 272. [Google Scholar] [CrossRef]
- Rekha Sarma, R.; Munsi, M.; Neelavara Ananthram, A. Effect of climate change on invasion risk of giant African snail (Achatina fulica Férussac, 1821: Achatinidae) in India. PLoS ONE 2015, 10, e0143724. [Google Scholar] [CrossRef]
- Silva, G.M.; Thiengo, S.C.; Sierpe Jeraldo, V.L.; Rego, M.I.F.; Silva, A.B.P.; Rodrigues, P.S.; Gomes, S.R. The invasive giant African land snail, Achatina fulica (Gastropoda: Pulmonata): Global geographical distribution of this species as host of nematodes of medical and veterinary importance. J. Helminthol. 2022, 96, e86. [Google Scholar] [CrossRef]
- Penagos-Tabares, F.; Lange, M.K.; Vélez, J.; Hirzmann, J.; Gutiérrez-Arboleda, J.; Taubert, A.; Hermosilla, C.; Chaparro Gutiérrez, J.J. The invasive giant African snail Lissachatina fulica as natural intermediate host of Aelurostrongylus abstrusus, Angiostrongylus vasorum, Troglostrongylus brevior, and Crenosoma vulpis in Colombia. PLoS Negl. Trop. Dis. 2019, 13, e0007277. [Google Scholar] [CrossRef]
- Fontanilla, I.K.C. Achatina (Lissachatina) fulica Bowdich: Its Molecular Phylogeny, Genetic Variation in Global Populations, and its Possible Role in the Spread of the Rat Lungworm Angiostrongylus cantonensis (Chen). Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2010. [Google Scholar]
- De La Ossa-Lacayo, A.; De La Ossa, J.; Lasso, C. Registro del caracol africano gigante Achatina fulica (Bowdich 1822) (Mollusca: Gastropoda-Achatinidae) en Sincelejo, costa Caribe de Colombia. Biota Colomb. 2012, 13, 299–304. [Google Scholar]
- Šteffek, J.; Kušík, P. Giant African Land Snail (Achatina fulica): New Entry on a List of Allochtonous Mollusc Species in Slovakia. In The invasive Species Bulletin; Newsletter of the IUCN/SSC Invasive Species Specialist Group; Institute for Environmental Protection and Research (ISPRA): Rome, Italy, 2010. [Google Scholar]
- EPPO. Global Database of the European and Mediterranean Plant Protection Organization (EPPO). Available online: https://gd.eppo.int/taxon/ACHAFU (accessed on 7 March 2023).
- Ministerio Para La Transición Ecológica Y El Reto Demográfico Website. Available online: https://www.miteco.gob.es/es/biodiversidad/temas/conservacion-de-especies/especies-exoticas-invasoras/ce_eei_invertebrados_na.aspx (accessed on 7 March 2023).
- Dickens, K.L.; Capinera, J.L.; Smith, T.R. Laboratory assessment of growth and reproduction of Lissachatina fulica (Gastropoda: Achatinidae). J. Molluscan Stud. 2018, 84, 46–53. [Google Scholar] [CrossRef]
- Roda, A.; Nachman, G.; Weihman, S.; Yong Cong, M.; Zimmerman, F. Reproductive ecology of the giant African snail in south Florida: Implications for eradication programs. PLoS ONE 2016, 11, e0165408. [Google Scholar] [CrossRef]
- Roda, A.; Cong, M.Y.; Donner, B.; Dickens, K.; Howe, A.; Sharma, S.; Smith, T. Designing a trapping strategy to aid Giant African Snail (Lissachatina fulica) eradication programs. PLoS ONE 2018, 13, e0203572. [Google Scholar] [CrossRef] [PubMed]
- d’Ovidio, D.; Nermut, J.; Adami, C.; Santoro, M. Occurrence of Rhabditid Nematodes in the Pet Giant African Land Snails (Achatina fulica). Front. Vet. Sci. 2019, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Council of the European Communities. Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora; Council of the European Communities: Brussels, Belgium, 1992. [Google Scholar]
- European Commission. Natura 2000. Available online: http://ec.europa.eu/environment/nature/natura2000/ (accessed on 14 March 2023).
- Council of the European Communities. Directive 2009/147/EC of the European Parliament and of the Council of 30 November 2009 on the Conservation of Wild Birds; Council of the European Communities: Brussels, Belgium, 2009. [Google Scholar]
- O’Loughlin, L.S.; Green, P.T. The secondary invasion of giant African land snail has little impact on litter or seedling dynamics in rainforest. Austral Ecol. 2017, 42, 819–830. [Google Scholar] [CrossRef]
- Afiademanyo, K.M.; Awaga, K.L.; Ouro-Sama, K.; Solitoke, H.D. Morphometric Differentiation between Two Closely Related Achatinid Snails (Gastropoda: Achatinidae) of West Africa and Implications for the Conservation of Achatina togoensis (Bequaert & Clench, 1934). Open J. Anim. Sci. 2021, 11, 559–579. [Google Scholar] [CrossRef]
- Dawnay, N.; Ogden, R.; McEwing, R.; Carvalho, G.R.; Thorpe, R.S. Validation of the barcoding gene COI for use in forensic genetic species identification. Forensic Sci. Int. 2007, 173, 1–6. [Google Scholar] [CrossRef]
- Venkatesan, V.; Mohamed, K.S. Gastropod classification and taxonomy. Recent Adv. Mar. Biodivers. Conserv. Manag. 2015, 38, 38–41. [Google Scholar]
- Albuquerque, F.S.D.; Peso-Aguiar, M.C.; Assunção-Albuquerque, M.J.T.; Gálvez, L. Do climate variables and human density affect Achatina fulica (Bowditch) (Gastropoda: Pulmonata) shell length, total weight and condition factor? Braz. J. Biol. 2009, 69, 879–885. [Google Scholar] [CrossRef]
- Patiño-Montoya, A.; Murillo-García, O.; Giraldo, A. Allometry and geographic variation of the morphology of Achatina fulica (Achatinidae) in Colombia. Molluscan Res. 2021, 41, 57–63. [Google Scholar] [CrossRef]
- Okon, B.; Ibom, L.A.; Halilu, A.; Onwuka, P.O. Comparative morphometric traits differentiation and phenotypic correlations between Achatina achatina (Linne, 1758) and Achatina fulica (Bowdich, 1822) snails with four and five whorls. J. Anim. Sci. Vet. Med. 2017, 2, 102–108. [Google Scholar] [CrossRef]
- Pattamarnon, T. Shell Morphological Differences and Genetic Variation of the Giant African Snail Achatina fulica (Bowdich, 1822) in Thailand. Ph.D. Thesis, Suranaree University of Technology, Nakhon Ratchasima, Thailand, 2004. [Google Scholar]
- Raut, S.K.; Ghose, K.C. Pestiferous Land Snails of India; Bani Press: Calcutta, India, 1984. [Google Scholar]
- Sharma, S.; Dickens, K. Effect of temperature and egg laying depths on giant African land snail (Gastropoda: Achatinidae) viability. Fla. Entomol. 2018, 101, 150–151. [Google Scholar] [CrossRef]
- Gutiérrez-Gregoric, D.E.G.; Núñez, V.; Vogler, R.; Rumi, A. Invasion of the Argentinean Paranense rainforest by the giant African snail Achatina fulica. Am. Malacol. Bull. 2011, 29, 135–137. [Google Scholar] [CrossRef]
- Rasal, V.; Dhakad, M.; Khandal, D. Ecological invasion of the giant African snail Lissachatina fulica (Bowdich, 1822) in a semi-arid forest of western India. Biodivers. Obs. 2022, 12, 60–64. [Google Scholar] [CrossRef]
- Gerlach, J.; Barker, G.M.; Bick, C.S.; Bouchet, P.; Brodie, G.; Christensen, C.C.; Collins, T.; Coote, T.; Cowie, R.H.; Fiedler, G.C.; et al. Negative impacts of invasive predators used as biological control agents against the pest snail Lissachatina fulica: The snail Euglandina ‘rosea’ and the flatworm Platydemus manokwari. Biol. Invasions 2021, 23, 997–1031. [Google Scholar] [CrossRef]
- Silva, G.M.; Santos, M.B.; Melo, C.M.; Jeraldo, V.L.S. Achatina fulica (Gastropoda: Pulmonata): Occurrence, environmental aspects and presence of nematodes in Sergipe, Brazil. Braz. J. Biol. 2020, 80, 245–254. [Google Scholar] [CrossRef]
- Morocoima, A.; Rodríguez, V.; Rivas, R.; Coriano, H.; Rivero, S.; Errante, R.; Mitchell, M.; Herrera, L.; Urdaneta-Morales, S. Achatina fulica Bowdich, 1822 (Mollusca, Gastropoda, Achatinidae) carrier of Helminthes, Protozoa and Bacteria in northeast Venezuela. Bol. Malariol. Salud Ambient. 2014, 54, 174–185. [Google Scholar]
- European Union (EU). Regulation No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the prevention and management of the introduction and spread of invasive alien species. Off. J. Eur. Union 2014, 57, 35–55. [Google Scholar]
- Monaco, A.; Carnevali, L.; Cerri, J.; Tricarico, E.; Genovesi, P. Risultati dell’horizon-Scanning e Proposta per un Elenco di Specie Esotiche Invasive di Rilevanza Nazionale. Rapporto Tecnico Life ASAP 2020, (Life15 GIE/IT/001039 “Alien Species Awareness Program”). Available online: https://www.lifeasap.eu/images/prodotti/6.1.11.1%20-%20Black%20list.pdf (accessed on 10 March 2023).
- Nielsen, A.; Hatteland, B.A.; Malmstrøm, M.; von Proschwitz, T.; Velle, G.; de Boer, H.; Gjershaug, J.O.; Kirkendall, L.R.; Rueness, E.K.; Vandvik, V. Assessment of Risks to Norwegian Biodiversity from the Import and Keeping of Terrestrial Gastropods in Terraria. Opinion of the Panel on Alien Organisms and Trade in Endangered Species of the Norwegian Scientific Committee for Food and Environment; VKM Report; Norwegian Scientific Committee for Food and Environment (VKM): Oslo, Norway, 2017; ISBN 978-82-8259-290-1. [Google Scholar]
- Wittenberg, R.; Cock, M.J.W. Invasive Alien Species: A Toolkit of Best Prevention and Management Practices; Centre for Agriculture and Bioscience International: Wallingford, UK, 2001. [Google Scholar]
- Dumidae, A.; Janthu, P.; Subkrasae, C.; Dekumyoy, P.; Thanwisai, A.; Vitta, A. Genetic characterization of Angiostrongylus larvae and their intermediate host, Achatina fulica, in Thailand. PLoS ONE 2019, 14, e0223257. [Google Scholar] [CrossRef]
- Igbinosa, I.B.; Isaac, C.; Adamu, H.O.; Adeleke, G. Parasites of edible land snails in Edo State, Nigeria. Helminthologia 2016, 53, 331–335. [Google Scholar] [CrossRef]
- Liboria, M.; Morales, G.; Carmen, S.; Isbelia, S.; Luz, A.P. First finding in Venezuela of Schistosoma mansoni eggs and other helminths of interest in public health found in faeces and mucous secretion of the mollusc Achatina fulica (Bowdich, 1822). Zootec. Trop. 2010, 28, 383–394. [Google Scholar]
- Constantino-Santos, D.M.A.; Basiao, Z.U.; Wade, C.M.; Santos, B.S.; Fontanilla, I.K.C. Identification of Angiostrongylus cantonensis and other nematodes using the SSU rDNA in Achatina fulica populations of Metro Manila. Trop. Biomed. 2014, 31, 327–335. [Google Scholar] [PubMed]
- Orico, L.D.; Barbosa, C.M.; Luca, L.R.; Soares, R.M.; Gregori, F. Angistrongylus cantonensis and Ancylostoma caninum detection in snails of São Paulo city (2016–2017), Brazil. Ars Vet. 2019, 35, 115–121. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabetti, A.; Maganza, A.; Prearo, M.; Riina, M.V.; Nodari, S.; Rizzioli, B.; Mangini, V.; Di Tizio, L.; Acutis, P.; Dondo, A.; et al. First Report of Giant African Snail (Lissachatina fulica) in a Protected Area of the Cottian Alps, Northwest Italy. Sustainability 2023, 15, 8633. https://doi.org/10.3390/su15118633
Gabetti A, Maganza A, Prearo M, Riina MV, Nodari S, Rizzioli B, Mangini V, Di Tizio L, Acutis P, Dondo A, et al. First Report of Giant African Snail (Lissachatina fulica) in a Protected Area of the Cottian Alps, Northwest Italy. Sustainability. 2023; 15(11):8633. https://doi.org/10.3390/su15118633
Chicago/Turabian StyleGabetti, Alice, Alessandra Maganza, Marino Prearo, Maria Vittoria Riina, Sabrina Nodari, Barbara Rizzioli, Valentina Mangini, Luciano Di Tizio, Pierluigi Acutis, Alessandro Dondo, and et al. 2023. "First Report of Giant African Snail (Lissachatina fulica) in a Protected Area of the Cottian Alps, Northwest Italy" Sustainability 15, no. 11: 8633. https://doi.org/10.3390/su15118633
APA StyleGabetti, A., Maganza, A., Prearo, M., Riina, M. V., Nodari, S., Rizzioli, B., Mangini, V., Di Tizio, L., Acutis, P., Dondo, A., Esposito, G., & Pastorino, P. (2023). First Report of Giant African Snail (Lissachatina fulica) in a Protected Area of the Cottian Alps, Northwest Italy. Sustainability, 15(11), 8633. https://doi.org/10.3390/su15118633











