Studies on the Accumulation, Translocation, and Enrichment Capacity of Soils and the Plant Species Phragmites Australis (Common Reed) with Heavy Metals
Abstract
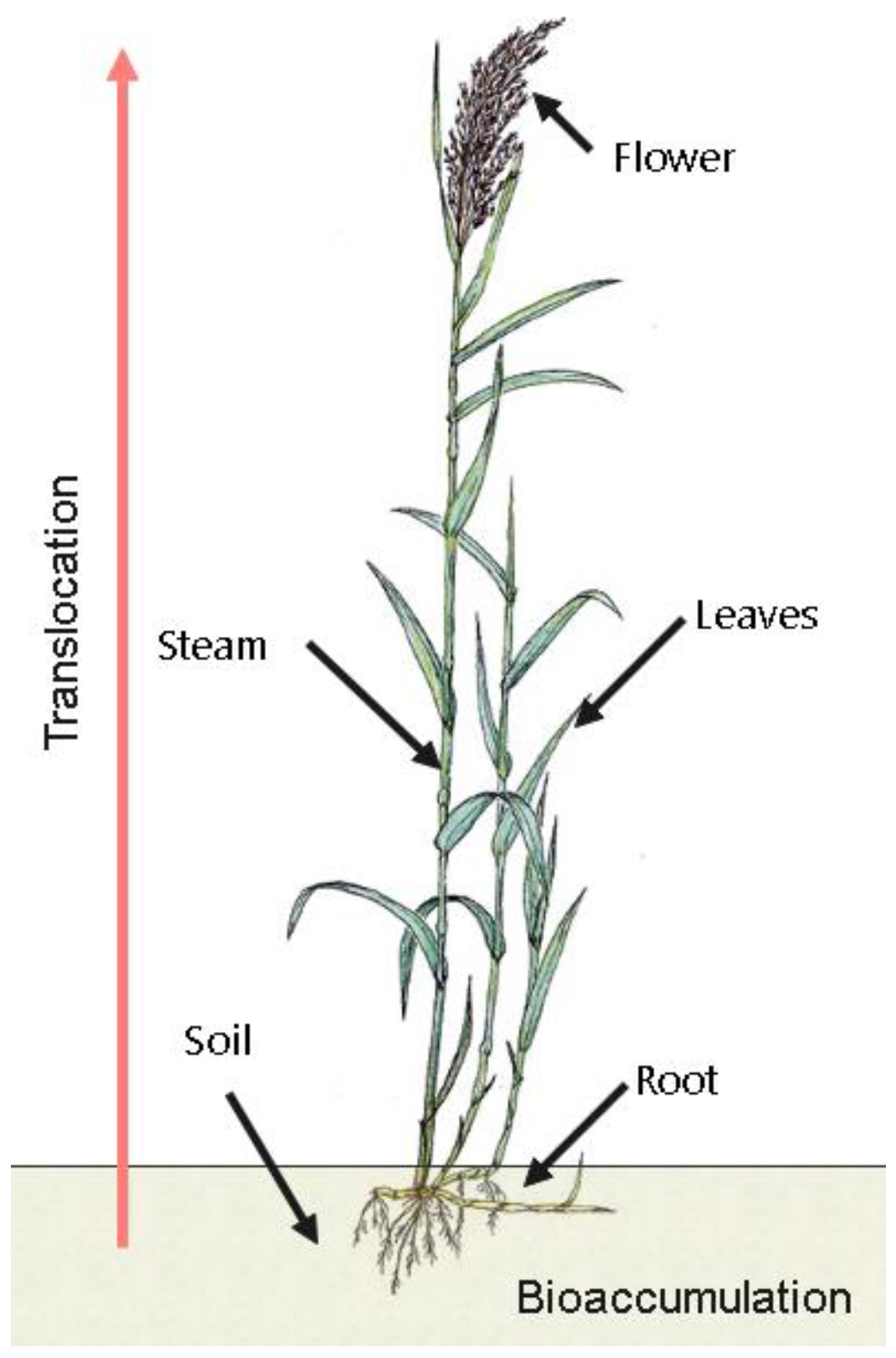
1. Introduction
- -
- The process of uptake by the roots of the surface plants;
- -
- The flow of water that circulates as a carrier through cormophytic plants.
2. Materials and Methods
2.1. Experimental Part
- -
- Sample point 1: 0 cm of soil–water interface;
- -
- Sample point 2: 50 cm of soil–water interface on the river bank;
- -
- Sample point 3: 100 cm of soil–water interface on the river bank.
2.2. Data Analysis
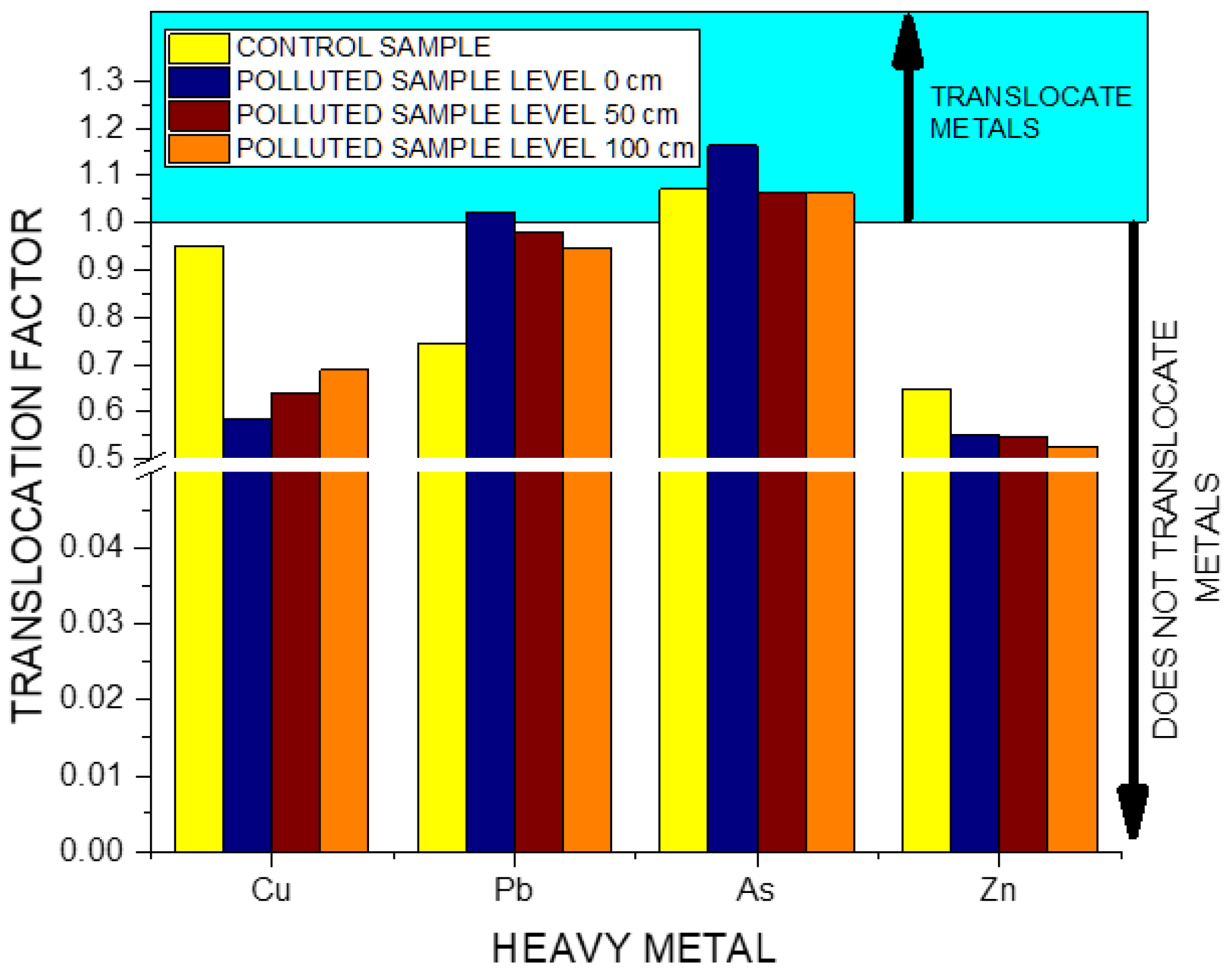
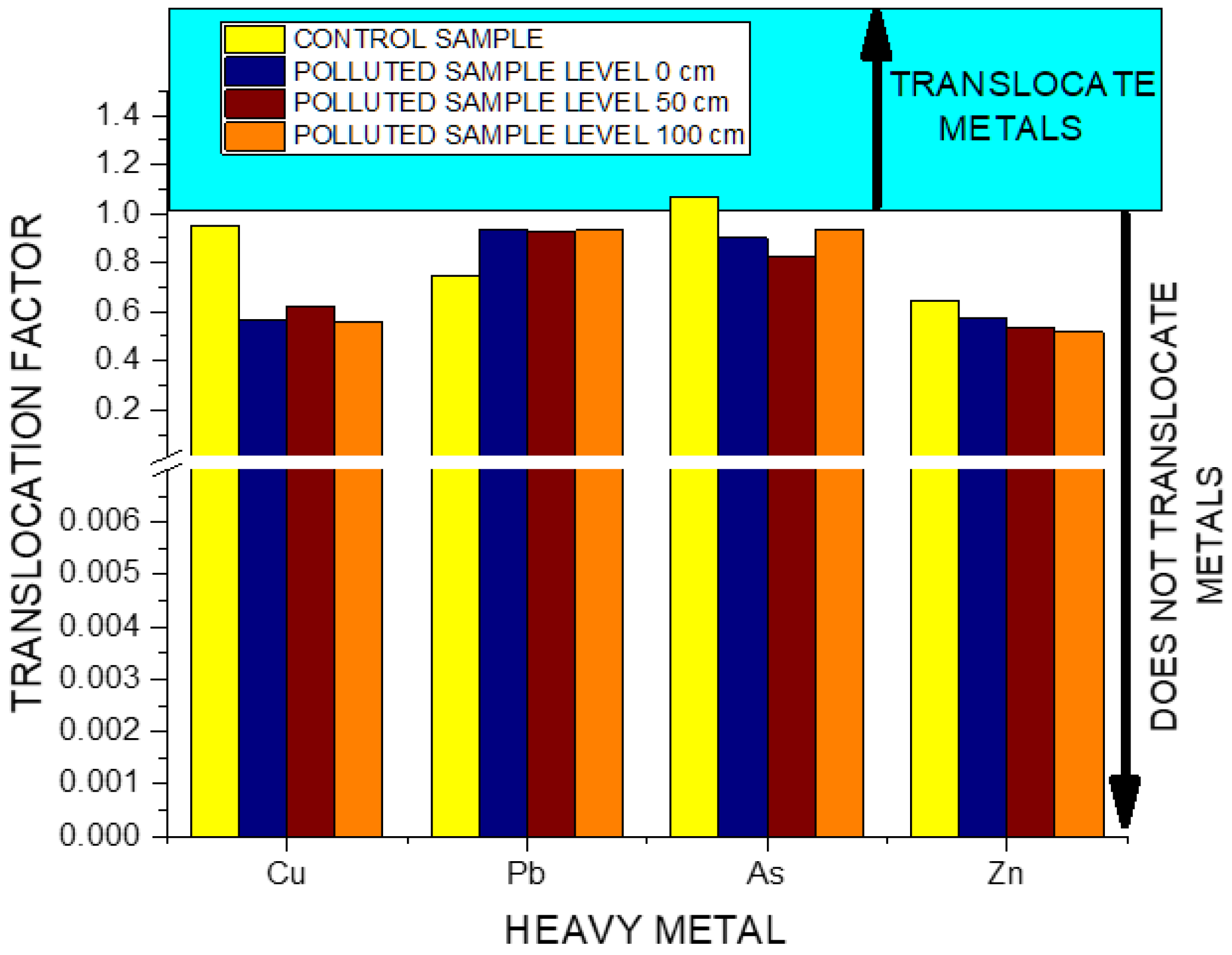
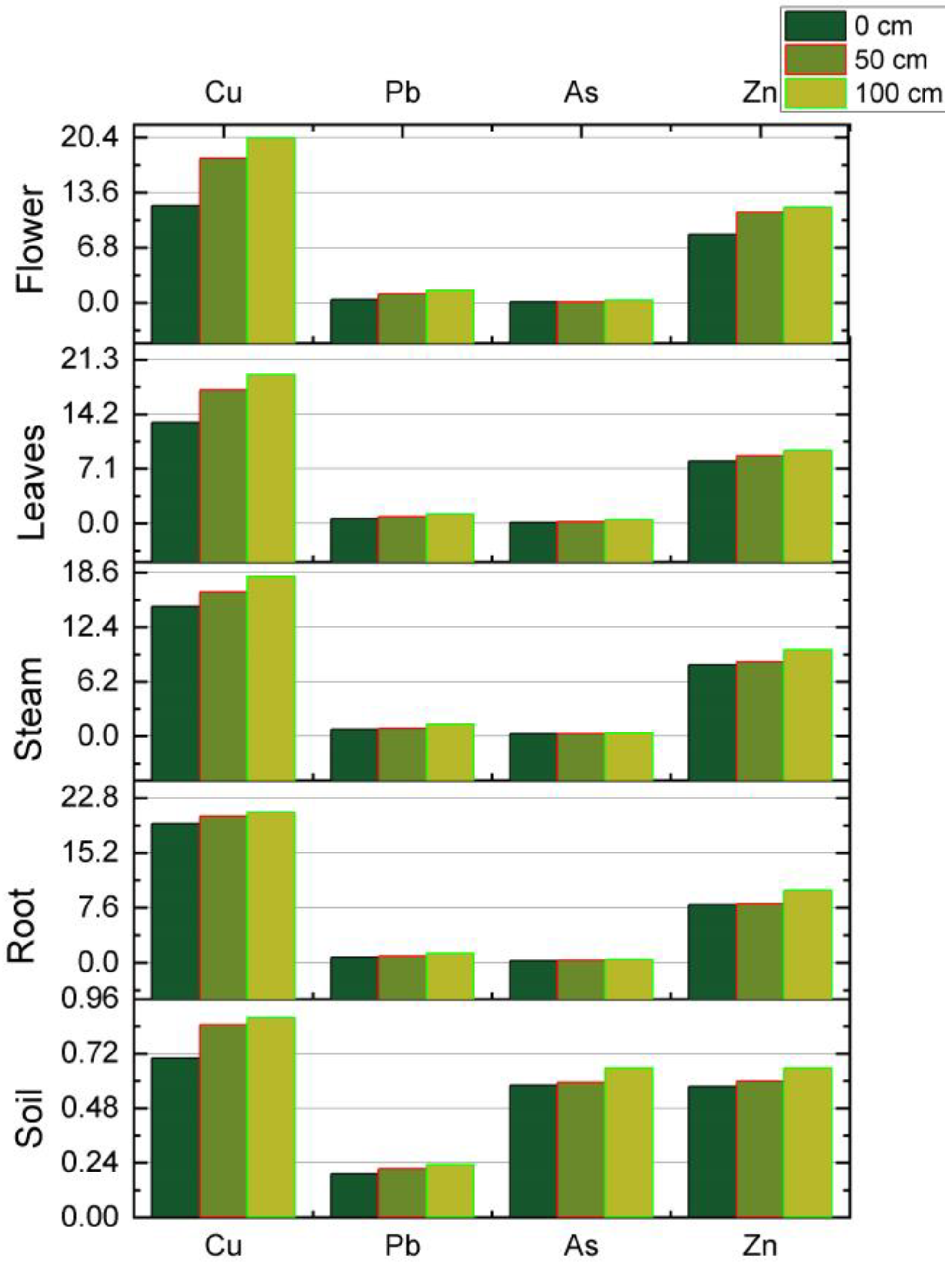
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohammad, M.U.; Mohamed, C.M.Z.; Junaida, S.Z.; Faiz, M.M.T.M.; Israt, J. Heavy metal accumulation in rice and aquatic plants used as human food: A general review. Toxics 2021, 9, 360. [Google Scholar]
- Xu, D.M.; Fu, R.B.; Tong, Y.H.; Shen, D.L.; Guo, X.P. The potential environmental risk implications of heavy metals based on their geochemical and mineralogical characteristics in the size-segregated zinc smelting slags. J. Clean. Prod. 2021, 315, 128199. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Atabaki, N.; Shaharuddin, N.A.; Ahmad, S.A.; Nulit, R.; Abiri, R. Assessment of water mimosa (neptunia oleracea lour.) morphological, physiological, and removal efficiency for phytoremediation of arsenic-polluted water. Plants 2020, 9, 1500. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, X.; Wang, L. Spatial distribution and source analysis of heavy metals in soils influenced by industrial enterprise distribution: Case study in Jiangsu Province. Sci. Total Environ. 2020, 710, 134953. [Google Scholar] [CrossRef]
- Proshad, R.; Kormoker, T.; Mursheed, N.; Islam, M.M.; Bhuyan, M.I.; Islam, M.S.; Mithu, T.N. Heavy metal toxicity in agricultural soil due to rapid industrialization in Bangladesh: A review. Int. J. Adv. Geosci. 2018, 6, 83–88. [Google Scholar] [CrossRef]
- DalCorso, G.; Fasani, E.; Manara, A.; Visioli, G.; Furini, A. Heavy metal pollutions: State of the art and innovation in phytoremediation. Int. J. Mol. Sci. 2019, 20, 3412. [Google Scholar] [CrossRef]
- Luo, Y.; Zhao, X.; Xu, T.; Liu, H.; Li, X.; Johnson, D.; Huang, Y. Bioaccumulation of heavy metals in the lotus root of rural ponds in the middle reaches of the Yangtze River. J. Soils Sediments 2017, 17, 2557–2565. [Google Scholar] [CrossRef]
- Young, G.; Chen, Y.; Yang, M. Concentrations, distribution, and risk assessment of heavy metals in the iron tailings of Yeshan National Mine Park in Nanjing, China. Chemosphere 2021, 271, 129546. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Hasan, M.M.; Uddin, M.N.; Ara-Sharmeen, F.I.; Alharby, H.; Alzahrani, Y.; Hakeem, K.R.; Li, Z. Assisting phytoremediation of heavy metals using chemical amendments. Plants 2019, 8, 295. [Google Scholar] [PubMed]
- Parihar, J.K.; Parihar, P.K.; Pakade, Y.B.; Katnoria, J.K. Bioaccumulation potential of indigenous plants for heavy metal phytoremediation in rural areas of shaheed bhagat Singh Nagar, Punjab (India). Environ. Sci. Pollut. Res. 2020, 8, 2426–2442. [Google Scholar] [CrossRef] [PubMed]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment? Front Plant Sci. 2018, 9, 1476. [Google Scholar]
- Zuo, S.; Dai, S.; Li, Y.; Tang, J.; Ren, Y. Analysis of heavy metal sources in the soil of riverbanks across an urbanization gradient. Int. J. Environ. Res. Public Health 2018, 15, 2175. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, H.Q.; Gong, Y.; Wei, Y.; Miao, A.J.; Yang, L.Y.; Zhong, H. Risk assessment of metals in urban soils from a typical industrial city, Suzhou, Eastern China. Int. J. Environ. Res. Public Health 2017, 14, 1025. [Google Scholar] [CrossRef] [PubMed]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation–a review. Earth-Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, L.; Wahid, Z.A.; Siddiqui, M.F.; Atnaw, S.M.; Din, M.F.M. Plant-driven removal of heavy metals from soil: Uptake, translocation, tolerance mechanism, challenges, and future perspectives. Environ. Monit. Assess. 2016, 188, 206. [Google Scholar]
- Bhatia, A.; Singh, S.; Kumar, A. Heavy metal contamination of soil, irrigation water and vegetables in peri-urban agricultural areas and markets of Delhi. Water Environ. Res. 2015, 87, 2027–2034. [Google Scholar] [CrossRef]
- Vymazal, J. Concentration is not enough to evaluate accumulation of heavy metals and nutrients in plants. Sci. Total Environ. 2016, 544, 495–498. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar]
- Cao, Y.; Tan, Q.; Zhang, F.; Ma, C.; Xiao, J.; Chen, G. Phytoremediation potential evaluation of multiple Salix clones for heavy metals (Cd, Zn and Pb) in flooded soils. Sci. Total Environ. 2022, 813, 152482. [Google Scholar] [CrossRef]
- Masindi, V.; Muedi, K.L. Environmental contamination by heavy metals. Heavy Met. 2018, 10, 115–132. [Google Scholar]
- Song, T.; An, Y.; Cui, G.; Tong, S.; He, J. Bioconcentrations and health risk assessment of heavy metals in crops in the Naoli River Basin agricultural area, Sanjiang Plain, China. Environ. Earth Sci. 2021, 80, 452. [Google Scholar] [CrossRef]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef]
- Luo, X.; Ren, B.Z.; Hursthouse, A.S.; Jiang, F.; Deng, R.J. Potentially toxic elements (PTEs) in crops, soil, and water near Xiangtan manganese mine, China: Potential risk to health in the foodchain. Environ. Geochem. Health 2020, 42, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Qu, L.; Wang, T.; Luo, L.; Chen, H.; Dahlgren, R.A.; Zhang, M.; Mei, K.; Huang, H. Distribution and source analysis of heavy metal pollutants in sediments of a rapid developing urban river system. Chemosphere 2018, 207, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Christakos, G.; Guo, M.; Xiao, L.; Huang, W. Space-time quantitative source apportionment of soil heavy metal concentration increments. Environ. Pollut. 2017, 223, 560–566. [Google Scholar] [CrossRef]
- Chintapenta, L.K.; Ommanney, K.I.; Ozbay, G. Presence and plant uptake of heavy metals in tidal marsh wetland soils. Front. Public Health 2022, 10, 821892. [Google Scholar] [CrossRef]
- Pérez-Sirvent, C.; Hernández-Pérez, C.; Martínez-Sánchez, M.J.; García-Lorenzo, M.L.; Bech, J. Metal uptake by wetland plants: Implications for phytoremediation and restoration. J. Soils Sediments 2017, 17, 1384–1393. [Google Scholar]
- Yang, J.; Zheng, G.; Yang, J.; Wan, X.; Song, B.; Cai, W.; Guo, J. Phytoaccumulation of heavy metals (Pb, Zn, and Cd) by 10 wetland plant species under different hydrological regimes. Ecol. Eng. 2017, 107, 56–64. [Google Scholar] [CrossRef]
- Yang, Y.; Yue, L.; Yunzhao, L.; Zhenbo, L.; Zhaohong, D.; Bo, G.; Zhikang, W.; Xuehong, W.; Jisong, Y.; Junbao, Y. Distribution and Influencing Factors of Metals in Surface Soil from the Yellow River Delta, China. Land 2022, 11, 523. [Google Scholar]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Qian, W.; Jin, Z.; Zhou, Z.; Wang, X.; Yang, X. Evaluation of soil heavy metals pollution and the phytoremediation potential of copper-nickel mine tailings ponds. PLoS ONE 2023, 3, e0277159. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Lam, Y.M. Assessment of heavy metal and metalloid levels and screening potential of tropical plant species for phytoremediation in Singapore. Environ. Pollut. 2022, 295, 118681. [Google Scholar] [CrossRef]
- de Oliveira Mesquita, F.; Pedrosa, T.D.; Batista, R.O.; de Andrade, E.M. Translocation factor of heavy metals by elephant grass grown with varying concentrations of landfill leachate. Environ. Sci. Pollut. Res. 2021, 28, 43831–43841. [Google Scholar] [CrossRef]
- Al-Homaidan, A.A.; Al-Otaibi, T.G.; El-Sheikh, M.A.; Al-Ghanayem, A.A.; Ameen, F. Accumulation of heavy metals in a macrophyte Phragmites Australis: Implications to phytoremediation in the Arabian Peninsula wadis. Environ. Monit. Assess. 2020, 192, 202. [Google Scholar] [CrossRef]
- Szymańska-Pulikowska, A.; Wdowczyk, A. Changes of a landfill leachate toxicity as a result of treatment with Phragmites australis and Ceratophyllum demersum—A case study. Front. Environ. Sci. 2021, 9, 739562. [Google Scholar] [CrossRef]
- Cristaldi, A.; Conti, G.O.; Jho, E.H.; Zuccarello, P.; Grasso, A.; Copat, C.; Ferrante, M. Phytoremediation of contaminated soils by heavy metals and PAHs. A brief review. Environ. Technol. Innov. 2017, 8, 309–326. [Google Scholar] [CrossRef]
- Capozzi, F.; Sorrentino, M.C.; Caporale, A.G.; Fiorentino, N.; Giordano, S.; Spagnuolo, V. Exploring the phytoremediation potential of Cynara cardunculus: A trial on an industrial soil highly contaminated by heavy metals. Environ. Sci. Pollut. Res. 2020, 27, 9075–9084. [Google Scholar] [CrossRef]
- Elshamy, M.M.; Heikal, Y.M.; Bonanomi, G. Phytoremediation efficiency of Portulaca oleracea L. naturally growing in some industrial sites, Dakahlia District, Egypt. Chemosphere 2019, 225, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.K.; Gupta, N.; Kumar, A.; Reece, L.M.; Singh, N.; Rezania, S.; Ahmad, K.S. Mechanistic understanding and holistic approach of phytoremediation: A review on application and future prospects. Ecol. Eng. 2018, 120, 274–298. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Minkina, T.; Fedorenko, G.; Nevidomskaya, D.; Fedorenko, A.; Chaplygin, V.; Mandzhieva, S. Morphological and anatomical changes of Phragmites australis Cav. due to the uptake and accumulation of heavy metals from polluted soils. Sci. Total. Environ. 2018, 636, 392–401. [Google Scholar] [CrossRef]
- Cicero-Fernández, D.; Peña-Fernández, M.; Expósito-Camargo, J.A.; Antizar-Ladislao, B. Longterm (two annual cycles) phytoremediation of heavy metal-contaminated estuarine sediments by Phragmites australis. New Biotechnol. 2017, 38, 56–64. [Google Scholar] [CrossRef]
- Dvořáková, T.B.; Vymazal, J. Distribution of heavy metals in Phragmites australis growing in constructed treatment wetlands and comparison with natural unpolluted sites. Ecol. Eng. 2022, 175, 106505. [Google Scholar]
- Klink, A. A comparison of trace metal bioaccumulation and distribution in Typha latifolia and Phragmites australis: Implication for phytoremediation. Environ. Sci. Pollut. Res. 2017, 24, 3843–3852. [Google Scholar] [CrossRef]
- Google Earth. Available online: https://earth.google.com/web/@46.52162417,26.96078664,153.71082858a,17872.55466484d,35y,60.06918231h,54.89536481t,0r (accessed on 4 April 2023).
- MO, Order no. 161 of February 16, 2006 Approving the Norms Regarding Surface Water Quality Classification in Order to Determine the Ecological Status of Water Bodies, Minister of Environ of 13 June 2006. Available online: https://legislatie.just.ro/Public/DetaliiDocumentAfis/72574 (accessed on 4 April 2023).






| Uncontaminated Location | Cu | Pb | As | Zn |
|---|---|---|---|---|
| (mg/kg d.m.) | ||||
| Soil | 35 | 80 | 26 | 124 |
| Phragmites Australis (common reed) steam + leaves + flower | 1.14 | 2.88 | 3.17 | 2.55 |
| Root | 1.2 | 3.86 | 2.96 | 3.94 |
| Steam | 0.85 | 3.65 | 2.81 | 3.32 |
| Leaves | 0.41 | 2.03 | 1.73 | 2.89 |
| Flower | 0.06 | 0.95 | 0.99 | 1.75 |
| Level | Cu | Pb | As | Zn |
|---|---|---|---|---|
| (mg/kg d.m.) | ||||
| Siret River—Holt Bridge—Pod Holt WP0019 Lat. 46.59010° N/Long. 26.97448° E + 0 m | ||||
| minimum level (0 cm) water–soil interface | 54.31 | 151.4 | 15.65 | 440.4 |
| medium level (50 cm) water–soil interface | 37.63 | 77.5 | 12.5 | 256.4 |
| maximum level (100 cm) water–soil interface | 26.55 | 24.93 | 14.54 | 108.5 |
| Siret River—canal UHE—WP0025 Lat. 46.50682° N/Long. 26.97929° E + 176 m | ||||
| minimum level (0 cm) water–soil interface | 24.52 | 15.28 | 15.13 | 71.3 |
| medium level (50 cm) water–soil interface | 29.65 | 17.25 | 15.44 | 74.18 |
| maximum level (100 cm) water–soil interface | 30.73 | 18.6 | 17.07 | 81.35 |
| Siret River—downstream confluence Bistrita/Siret—WP0026 Lat. 46.46347° N/Long. 26.96729° E + 157 m | ||||
| minimum level (0 cm) water–soil interface | 36.03 | 20.5 | 18.21 | 90.3 |
| medium level (50 cm) water–soil interface | 38.52 | 28.14 | 14.2 | 120.5 |
| maximum level (100 cm) water–soil interface | 30.25 | 27.2 | 15.68 | 102.5 |
| Level | Cu | Pb | As | Zn |
|---|---|---|---|---|
| (mg/kg d.m.) | ||||
| Siret River—Holt Bridge—Pod Holt WP0019 Lat. 46.59010° N/Long. 26.97448° E + 0 m | ||||
| minimum level (0 cm) water–soil interface | 29.7 | 40.15 | 1.23 | 92.28 |
| medium level (50 cm) water–soil interface | 20.9 | 18.894 | 0.674 | 60.88 |
| maximum level (100 cm) water–soil interface | 15.751 | 4.712 | 1.124 | 26.55 |
| Siret River—canal UHE—WP0025 Lat. 46.50682° N/Long. 26.97929° E + 176 m | ||||
| minimum level (0 cm) water–soil interface | 13.55 | 3.156 | 0.978 | 17.54 |
| medium level (50 cm) water–soil interface | 15.557 | 3.658 | 1.127 | 17.58 |
| maximum level (100 cm) water–soil interface | 17.222 | 4.887 | 1.371 | 20.89 |
| Siret River—downstream confluence Bistrita/Siret—WP0026 Lat. 46.46347° N/Long. 26.96729° E + 157 m | ||||
| minimum level (0 cm) water–soil interface | 20.2 | 5.22 | 1.404 | 22.889 |
| medium level (50 cm) water–soil interface | 22.54 | 7.32 | 0.905 | 25.22 |
| maximum level (100 cm) water–soil interface | 14.2 | 6.92 | 1.021 | 24.25 |
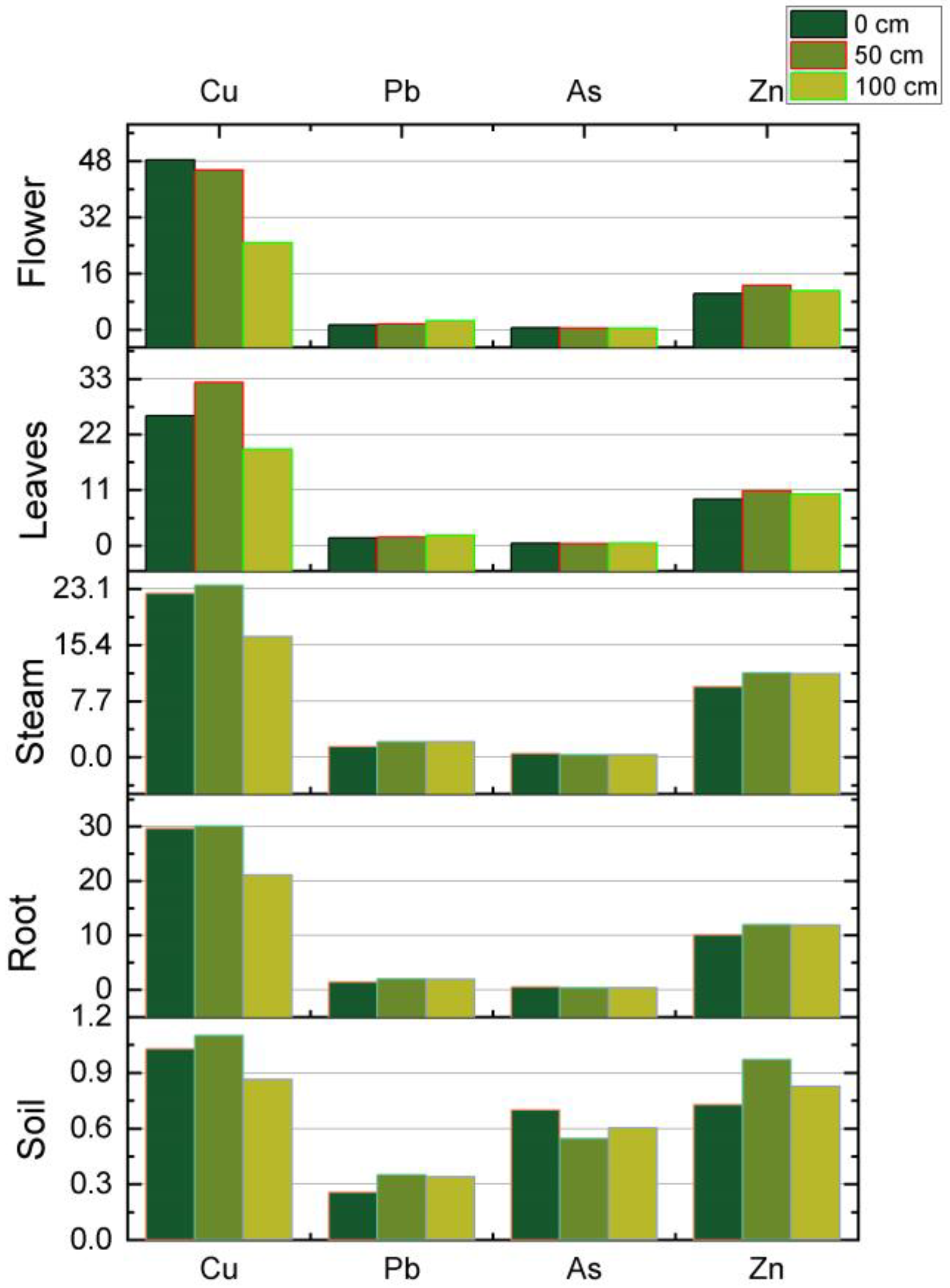
| Siret River—Holt Bridge—Pod Holt WP0019 Lat. 46.59010° N/Long. 26.97448° E + 0 m | ||||||||
|---|---|---|---|---|---|---|---|---|
| Level | Root | Steam | Leaves | Flower | Root | Steam | Leaves | Flower |
| Cu (mg/kg d.m.) | Pb (mg/kg d.m.) | |||||||
| 0 cm | 42.53 | 26.85 | 11.95 | 2.48 | 42.85 | 38.61 | 21 | 10.7 |
| 50 cm | 38.51 | 20.73 | 8.43 | 0.95 | 19.23 | 16.46 | 8.9 | 3.41 |
| 100 cm | 27.6 | 12.28 | 6.9 | 0.77 | 4.34 | 3.1 | 1.53 | 0.44 |
| As (mg/kg d.m.) | Zn (mg/kg d.m.) | |||||||
| 0 cm | 11.02 | 0.86 | 0.61 | 0.25 | 135.94 | 100.59 | 88.76 | 50.14 |
| 50 cm | 0.59 | 0.46 | 0.39 | 0.14 | 73.69 | 65.11 | 47.39 | 30.61 |
| 100 cm | 0.91 | 0.63 | 0.27 | 0.09 | 30.4 | 25.68 | 17.13 | 10.84 |
| Siret River—canal UHE—WP0025 Lat. 46.50682° N/Long. 26.97929° E + 176 m | ||||||||
| Cu (mg/kg d.m.) | Pb (mg/kg d.m.) | |||||||
| 0 cm | 23.1 | 12.56 | 5.39 | 0.72 | 3.09 | 2.84 | 1.3 | 0.39 |
| 50 cm | 24.32 | 13.95 | 7.12 | 1.07 | 3.74 | 3.25 | 1.81 | 1.03 |
| 100 cm | 24.98 | 15.43 | 7.94 | 1.22 | 5.16 | 4.98 | 2.46 | 1.48 |
| As (mg/kg d.m.) | Zn (mg/kg d.m.) | |||||||
| 0 cm | 0.84 | 0.75 | 0.24 | 0.08 | 31.85 | 26.99 | 23.45 | 14.78 |
| 50 cm | 1.06 | 0.82 | 0.36 | 0.09 | 32.1 | 28.15 | 25.39 | 19.56 |
| 100 cm | 1.29 | 1.1 | 0.85 | 0.31 | 39.62 | 32.7 | 27.56 | 20.63 |
| Siret River—downstream confluence Bistrita/Siret—WP0026 Lat. 46.46347° N/Long. 26.96729° E + 157 m | ||||||||
| Cu (mg/kg d.m.) | Pb (mg/kg d.m.) | |||||||
| 0 cm | 35.62 | 19.16 | 10.54 | 2.9 | 5.56 | 5.37 | 3.14 | 1.36 |
| 50 cm | 36.15 | 20.07 | 13.25 | 2.73 | 7.89 | 7.77 | 3.45 | 1.59 |
| 100 cm | 25.38 | 14.1 | 7.82 | 1.49 | 7.43 | 7.4 | 4.26 | 2.44 |
| As (mg/kg d.m.) | Zn (mg/kg d.m.) | |||||||
| 0 cm | 1.56 | 1.48 | 0.83 | 0.57 | 39.68 | 32.19 | 26.4 | 18.16 |
| 50 cm | 1.1 | 1.05 | 0.71 | 0.43 | 47.15 | 38.65 | 31.34 | 22.19 |
| 100 cm | 1.089 | 0.94 | 0.77 | 0.5 | 46.9 | 38.1 | 29.59 | 19.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chitimus, D.; Nedeff, V.; Mosnegutu, E.; Barsan, N.; Irimia, O.; Nedeff, F. Studies on the Accumulation, Translocation, and Enrichment Capacity of Soils and the Plant Species Phragmites Australis (Common Reed) with Heavy Metals. Sustainability 2023, 15, 8729. https://doi.org/10.3390/su15118729
Chitimus D, Nedeff V, Mosnegutu E, Barsan N, Irimia O, Nedeff F. Studies on the Accumulation, Translocation, and Enrichment Capacity of Soils and the Plant Species Phragmites Australis (Common Reed) with Heavy Metals. Sustainability. 2023; 15(11):8729. https://doi.org/10.3390/su15118729
Chicago/Turabian StyleChitimus, Dana, Valentin Nedeff, Emilian Mosnegutu, Narcis Barsan, Oana Irimia, and Florin Nedeff. 2023. "Studies on the Accumulation, Translocation, and Enrichment Capacity of Soils and the Plant Species Phragmites Australis (Common Reed) with Heavy Metals" Sustainability 15, no. 11: 8729. https://doi.org/10.3390/su15118729
APA StyleChitimus, D., Nedeff, V., Mosnegutu, E., Barsan, N., Irimia, O., & Nedeff, F. (2023). Studies on the Accumulation, Translocation, and Enrichment Capacity of Soils and the Plant Species Phragmites Australis (Common Reed) with Heavy Metals. Sustainability, 15(11), 8729. https://doi.org/10.3390/su15118729









