Abstract
Chloride is a necessary micronutrient for plant growth, and with long-term application of chlorine-containing fertilizer, significant amounts of Cl− are introduced into farmland ecosystems. Many recent studies have focused on chlorinated fertilizers’ effects on crop yield and quality, while few studies have examined their effects on soil properties. To determine the effects of the long-term application of chlorinated fertilizer on soil Cl− ions and soil acidification, we conducted a 35 year long-term study of chlorine-containing fertilizer in a peanut–corn rotation (since 1984). We chose three of eight experimental treatments: (1) no fertilization (CK), (2) urea + monoammonium phosphate + potassium chloride (lower Cl), and (3) urea + ammonium chlorophosphite + potassium chloride (higher Cl). We measured the Cl− concentrations, pH, cation exchange capacity (CEC), exchangeable H+ and Al3+, and exchangeable alkali ions (K+, Na+, Ca2+, and Mg2+) at different soil depths (0–20, 20–40, and 40–60 cm). Compared to CK, chlorine-containing fertilizer application significantly increased the content of Cl− in the soil profile. Compared to the control, the Cl− content of lower Cl treatment of 0–20, 20–40, and 40–60 cm soil layers increased 11.08, 9.01, 15.21 mg kg−1 respectively, and the higher Cl treatment increased 38.71, 34.71, 32.05 mg kg−1 respectively. Compared to CK, chlorine-containing fertilizer application significantly reduced the soil pH by 0.41, 0.17, and 0.25 and 1.25, 0.91, and 0.88, respectively, in the 0–20, 20–40, and 40–60 cm soil layers. The higher chlorine treatment significantly increased the exchangeable Al3+ content in the 0–20, 20–40, and 40–60 cm soil layers by 2.79, 1.64, and 0.94 mg kg−1, respectively, significantly increasing the risk of aluminum toxicity. Furthermore, the soil exchangeable Ca2+ and Mg2+ contents and soil base saturation were significantly reduced. Although the Cl− content in the high-chlorine-treated soil was far from endangering crop growth, it accelerates soil acidification and the loss of base ions and increases the risk of Al3+ toxicity, which will not only affect the topsoil, but also the subsoil. Therefore, the long-term application of high content chloride fertilizers should be avoided in agricultural production.
1. Introduction
Chloride (Cl) is an essential micronutrient for plant growth and development. Most Cl in soil occurs in the soil solution as Cl− and comes from rainfall, marine aerosols, volcanic emissions, irrigation water, and fertilizers. There is usually no lack of soil chlorine, and it can thus meet crop needs through rainfall or irrigation. However, in recent years, with the application of Cl-containing fertilizers such as ammonium chloride and potassium chloride, a large amount of Cl− enters the farmland ecosystem as companion ions and participates in soil and plant nutrient cycling. However, most researchers have mainly focused on the impacts of Cl− on crop yield and quality [1,2], while ignoring its impacts on the soil’s physical and chemical properties. Although Cl− is easily leached and will not accumulate in high rainfall areas, the long-term application of chlorinated fertilizer will significantly increase the soil Cl− content [3]. Chloride is a strong acid anion which can directly cause soil acidification; thus, it will also remove a large number of base ions and further aggravate soil acidification. The long-term application of chlorine-containing chemical fertilizers, especially at a high rate, could decrease the nutrient supply of some soils because Cl− can induce nutrient leaching, thus making them unavailable for uptake in rice crops [3]. Zhou et al. [4] reported that the lowest pH (5.81) was found after long-term fertilization with NH4Cl and KCl treatments. Soil acidification is an important aspect of soil quality, and can cause numerous negative ecosystem effects. For example, it can decrease the number of plant fine roots [5,6], lower crop yields [7], and reduce biological diversity [8,9,10].
Therefore, under the current situation of the high-cost performance and common supply of chlorinated fertilizer, it is especially important for scientific and rational fertilizer use to determine the impact of the long-term application of chlorinated fertilizer on soil properties. Chinese scholars have focused their research on the effects of chlorine application on paddy soil in south China, but less is known on the impacts on upland fields and especially the impacts in deep soil. Therefore, based on a Shenyang Agricultural University long-term (35 years) experiment in Northeast China, we studied the effects of long-term application of low chloride and high chloride fertilizer on Cl− content, pH, exchangeable acidity, and exchangeable base ions in soil. We hypothesized that the long-term application of high chlorine could increase the Cl− content in the soil profile and accelerate soil acidification. By studying soil properties in areas of long-term varying chlorine fertilizer application, we can deeply understand its long-term effects and provide scientific guidance for local agricultural production based on achieving a balance between farmers’ input and sustainable development of the soil environment.
2. Materials and Methods
2.1. Experimental Site
The long-term fertilization experimental site is located in Shenyang Agricultural University, Northeast China (40°48′ N, 123°33′ E) and was established in 1984. The area has a temperate, semi-humid climate, with a mean annual temperature of 7.0–8.1 °C, mean annual precipitation of 574–684 mm, and potential evaporation of 1435.6 mm per year. The soil type is a typical brown earth loam containing 48% sand, 29% silt, and 23% clay at 0–20 cm depth [11]. The soil properties at 0–20 cm depth in 1984 were as follows: 6.10 pH in water (1:2.5), 99.1 mg kg−1 alkali-hydrolyzed N, 10.5 mg kg−1 Olsen-P, 717.5 mg kg−1 slow available K, 87.3 mg kg−1 available K, 16.4 cmol kg−1 cation exchangeable capacity, and 26.0 mg kg−1 Cl−.
2.2. Soil Sampling and Analysis
Soil samples were collected at the maize seedling and harvest stages in 2018, with sampling depths of 0–20 cm, 20–40 cm, and 40–60 cm. Three soil samples from each micro plot were mixed evenly and air-dried for Cl− content determination. In addition, other indicators of harvest stage soil samples, such as pH, exchangeable H+, exchangeable Al3+, cation exchange capacity (CEC), and exchangeable base ions (K+, Na+, Ca2+, and Mg2+), were determined.
The soil pH was measured with a glass electrode under a soil/water ratio of 1:2.5 [12]. Measurements of soil Cl− concentrations were taken using the Mohr silver nitrate titration method [13]. Soil exchangeable H+ and exchangeable Al3+ were determined by the potassium chloride exchangeable neutralization titration method [13]. The exchangeable basic cation concentrations (K+, Na+, Ca2+, and Mg2+) and cation exchange capacity (CEC) were determined using the ammonium acetate (1 M and pH 7.0) method [12]. Exchangeable K+ and Na+ were measured with a flame photometer (Sherwood 410, Sherwood Scientific Ltd., Cambridge, UK). Exchangeable Ca2+ and Mg2+ were determined by the atomic absorption method (Varian AA-240FS, Varian Corporation, Palo Alto, US) [12].
2.3. Statistical Analyses
Statistical data analyses were performed using the SPSS 19.0 software package. The data were compared using the means of Duncan’s SSR test between the treatments with significant differences evaluated at the p = 0.05 level.
2.4. Experimental Design
The experiment was conducted in micro plots, each covering 2 m2 (2 m × 1 m). Low and high chlorine treatments with equal amounts of N, P, and K were established. We selected three treatments: (1) Control (CK); (2) urea + monoammonium phosphate + potassium chloride (ClL); and (3) urea + ammonium chlorophosphate + potassium chloride (ClH) (corn planting year) or urea + calcium superphosphate + potassium chloride (ClH) (peanut planting year). Among them, (1) was treated without fertilizer, (2) was treated with low amounts of chlorine, and (3) was treated with high amounts of chlorine. There were three replicates for each treatment, and these were distributed randomly. The spring corn–spring soybean rotation began in 1984, and changed to a spring peanut and spring corn rotation from 2009. In the corn planting year, base fertilizer was applied at rates of 120 kg N ha−1, 60 kg P2O5 ha−1, and 60 kg K2O ha−1 before sowing, with the details as follows: ClL treatment with urea 234.5 kg ha−1, monoammonium phosphate 120 kg ha−1, potassium chloride 100 kg ha−1, ClH treatment with urea 74 kg ha−1, ammonium chlorophosphate 428.5 kg ha−1, and potassium chloride 100 kg ha−1. In the peanut planting year, base fertilizer was applied at rates of 37.5 kg N ha−1, 112.5 kg P2O5 ha−1, and 37.5 kg K2O ha−1 before sowing, with the details as follows: ClL treatment with urea 32.5 kg ha−1, monoammonium phosphate phosphate 225 kg ha−1, potassium chloride 62.5 kg ha−1, ClH treatment with ammonium chlorophosphate 187.5 kg ha−1, calcium superphosphate 719 kg ha−1, and potassium chloride 62.5 kg ha−1.
The amount of chlorine (Cl−) input in spring corn–spring peanut of the ClL was 45 kg ha−1 and 28 kg ha−1, respectively, every year, and the ClH was 233 kg ha−1 and 110 kg ha−1, respectively, every year. During the whole process, fertilizer was applied as base fertilizer once. In our study, the crop tested in 2018 was maize, which was manually harvested; in addition, ground crop residues were removed from the field annually.
3. Results
3.1. Effects of Different Fertilization Treatments on Soil Profile Cl− Content
Samples collected during seedling and harvesting of maize in 2018 showed that the Cl− content in 0-20, 20-40, 40-60cm soil layers under high chlorine and low chlorine treatments increased compared with the control, with a trend of ClH > ClL > CK, and the differences among all treatments were significant (Figure 1a,b). At the maize seedling stage, the Cl− content in the 0–20 cm soil layer was 222.4 mg kg−1 in the ClH treatment, 44.5 mg kg−1 in the ClL treatment, and 11.8 mg kg−1 in the CK treatment. The Cl− contents in the 20–40 cm soil layer treated with ClH, ClL, and CK were 47.6, 18.0, and 12.7 mg kg−1, and in the 40–60 cm soil layer were 69.7, 25.7, and 14.8 mg kg−1, respectively. At the maize harvesting stage, the Cl− content in the 0–20 cm soil layer in the ClH treatment was 52.7 mg kg−1. In the ClL treatment, it was 25.7 mg kg−1, and in the CK treatment, it was 14.7 mg kg−1. In the 0–60 cm profile, the Cl− content of all treatments decreased with increasing soil depth.
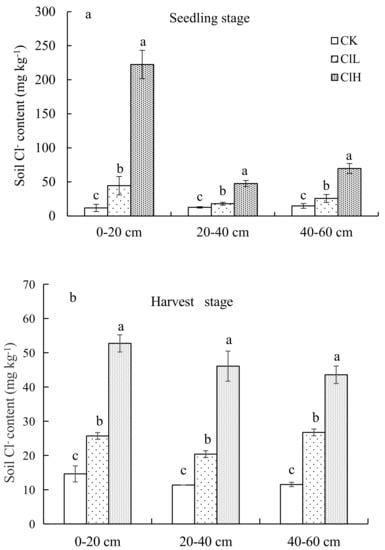
Figure 1.
Cl− content in soil profile of different fertilization treatments at the maize seedling stage (a) and harvest stage (b). Error bars represent standard error of the mean. Means followed by the same letter were not signifificantly difffferent at the 0.05 probability level based on analyses by one-way ANOVAs followed by Duncan’s multiple range test.
3.2. Effects of Different Fertilization Treatments on Soil Profile pH, Exchangeable H+ and Exchangeable Al3+
Compared to CK, after 35 years of continuous fertilization, the pH of the 0–20, 20–40, and 40–60 cm layers under ClL and ClH treatments declined significantly, with the pH of the ClH treatment being significantly lower than the ClL treatment (Figure 2). The pH of CK in the soil profile was between 5.50 and 6.36, that of the ClL treatment was 5.95–6.00, and for the ClH treatment was 5.11–5.38. Compared to CK, the soil pH of the 0–20, 20–40, and 40–60 cm layers treated with ClH decreased by 1.25, 0.91, and 0.87, respectively, and for ClL decreased by 0.41, 0.17, and 0.25 units, respectively. Compared to the initial pH (6.10) at the beginning of the experiment in 1984, the pH of topsoil treated with ClL and ClH decreased by 0.16 and 1, respectively.
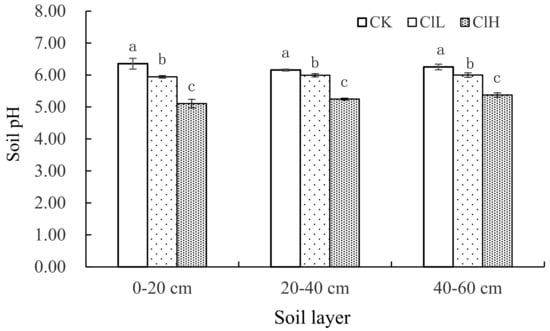
Figure 2.
pH of soil profile under different fertilization treatments. Error bars represent standard error of the mean. Means followed by the same letter were not signifificantly difffferent at the 0.05 probability level based on analyses by one-way ANOVAs followed by Duncan’s multiple range test.
The exchangeable H+ and Al3+ contents in the 0–20, 20–40, and 40–60 cm soil layers reduced in the order of ClH > ClL > CK, and there were significant differences among treatments (except exchangeable H+ in the topsoil of CK and ClL, Figure 3).
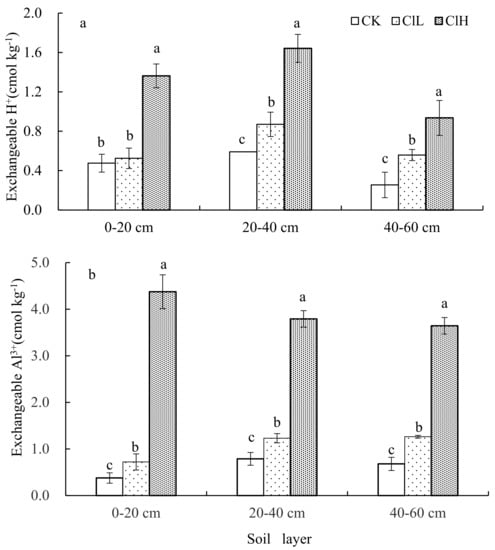
Figure 3.
Contents of exchangeable H+ (a) and Al3+ (b) in soil profiles of different treatments. Error bars represent standard error of the mean. Means followed by the same letter were not signifificantly difffferent at the 0.05 probability level based on analyses by one-way ANOVAs followed by Duncan’s multiple range test.
3.3. Effects of Different Fertilization Treatments on Soil Profile Exchangeable Base Ions and Topsoil CEC
In the 0–20 cm soil layer, there was no significant difference in the exchangeable K+ content between ClL and ClH, but both were significantly lower than the CK treatment (Table 1). In the 20–40 cm soil layer, the exchangeable K+ of CK was significantly higher than ClL, but significantly lower than the ClH treatment. In the 40–60 cm soil layer, the exchangeable K+ concentration in ClH was significantly higher than in the CK and ClL treatments. For exchangeable Na+, in the 0–20 and 20–40 cm soil layers, there were no significant differences between CK and ClL, but both were significantly higher than the ClH treatment. In the 40–60 cm soil layer, there was no significant difference in Na+ content between ClL and ClH, but it was significantly lower than the CK treatment. Compared with CK, the ClH and ClL treatments significantly reduced the exchangeable Ca2+ content at 0–20 cm, and the effect of ClH treatment was greater. In the 20–40 and 40–60 cm soil layers, the exchangeable Ca2+ content between ClL treatment and CK was not significantly different, but the above two treatments were significantly higher than ClH treatment. The exchangeable Mg2+ content in the 0–20 and 20–40 cm soil layers decrease in the order CK > ClL > ClH, and there were significant differences between treatments. In the 40–60 cm soil layer, there was no significant difference in exchangeable Mg2+ between ClL and ClH, but both of them were significantly lower than the CK treatment.

Table 1.
Exchangeable base ion content of soil profile under different fertilization treatments.
Compared with CK, the total exchangeable base cations (K+ + Na+ + Ca2+ + Mg2+) of different soil layers were reduced both in the ClH and ClL treatments, and the decrease caused by ClH was greater. Compared with CK, the ClL and ClH treatments both increased the topsoil CEC, and there was a significant difference between ClH and CK (Table 2). The ratio of base ions (K+, Na+, Ca2+, and Mg2+) to CEC in the ClL and ClH treatments decreased (except K+ and Na+ in the ClL treatment). Therefore, the base saturations of ClL and ClH all decreased, and the ClH treatment was significantly different from CK and ClL treatments.

Table 2.
Cation exchange capacity, base saturation, and percentages of K+, Na+, Ca2+, and Mg2+ in topsoil (0–20 cm).
3.4. Effects of Different Fertilization Treatments on Exchangeable Ca/Al and Mg/Al in Soil Profile
Compared to CK, in the 0–20, 20–40, and 40–60 cm soil layers, the exchangeable Ca/Al and Mg/Al ratios of the ClL and ClH treatments decreased significantly, especially in the ClH treatment (Table 3).

Table 3.
Exchangeable Ca/Al and Mg/Al in soil profiles under different fertilization treatments.
3.5. All Index Relationships
The Cl− content had a highly significant negative correlation with soil pH and exchangeable base ions (p < 0.05) (Table 4) and had a very significant positive correlation with exchangeable H+ and Al3+ (p < 0.05). pH had a very significant negative correlation with exchangeable H+ and Al3+ (p < 0.05) and had a very significant positive correlation with exchangeable base ions (p < 0.05).

Table 4.
Correlation among soil pH, Cl−, and exchangeable base ions.
4. Discussion
4.1. Soil Profile Cl− Content
During the 2018 harvest period, after 35 years of continuous planting, the Cl− removed through crop harvesting and rainwater leaching was not fully supplemented, so the topsoil Cl− content in the chlorine-free treatment was significantly lower than the initial value in 1984 (26 mg kg−1). The topsoil Cl− content in the ClL treatment did not change significantly, at only 1.2% lower than the initial 1984 value. This may be due to the limited chloride ions applied annually to the soil and absorption by crop roots which maintain the Cl− content balance in topsoil. The Cl− content in the ClH-treated soil was significantly higher than the initial value in 1984. Notably, the Cl− content of ClH-treated topsoil was 102.8% higher than the initial value. This is because Cl− was brought into the soil by the ClH treatment and far exceeded the required crop demand, leading to a large accumulation of Cl− in the soil. At the maize seedling and harvest stages, the Cl− content of different profiles in different treatments varied from 11.3 to 70.0 mg kg−1 (except the topsoil layer at the seedling stage), which is far lower than the critical value of chlorine tolerance of maize [14]. There were no negative and even some positive effects of chloride salinity on maize grain quality when the concentration of Cl− in soil exceeds 800 mg kg−1 [15]. Although the topsoil Cl− content reached 222.4 mg kg−1 after ClH treatment at the maize seedling stage, it did not harm corn growth.
4.2. Soil pH Change
Compared to CK, both the ClL and ClH treatments significantly reduced the soil pH. Both fertilization treatments contain nitrogen fertilizer, and many studies have proven that the long-term application of N fertilizer accelerates soil acidification [16,17,18,19], as soil acidification caused by nitrogen input is mainly driven by the conversion of ammonium to nitrate and subsequent leaching [20]. Nitrogen type will affect the degree of soil acidification [21] because nitrification of NH4+-N produces 2 moles of H+, while nitrification of urea produces 1 mole of H+ [22], so ammonium nitrogen fertilizer generally acidifies soil faster than urea [23,24,25]. In our study, with ClH treatment, the pH decreased more not only in the topsoil, but also in the subsoil (Figure 2). It may be that the nitrogen in ClH treatment is mainly ammonium nitrogen fertilizer, while the nitrogen in ClL treatment is mainly urea. Alternatively, it may be due to the increase in the Cl− content in the ClH-treated soil profile (Figure 1), leading to more base ions filtered out of the soil along with Cl−. There was a significant negative correlation between Cl− content and soil exchangeable Ca2+ and Mg2+, indicating that increasing Cl− content accelerates the leaching of Ca2+ and Mg2+ (Table 4). This is consistent with the results of Zou et al. [3]. The leaching of base ions is an important factor leading to the acceleration of soil acidification [3,26], which is supported by our correlation between pH and soluble base ions. In the ClH treatment, the pH of the soil was between 5.11 and 5.38. Studies have shown that a low pH inhibits nitrification in soil [16], so more soil nitrogen exists in the form of NH4+-N, a finding confirmed by our previous research [27]. In addition, NH4+-N is absorbed by crops and H+ will be released from roots, which will also acidify the soil. Simultaneously, NH4+ retained in the soil will exchange with base ions, making them enter the soil solution, resulting in their further loss. Thus, the pH of the ClH-treated soils decreased more.
4.3. Exchangeable H+, Al3+, and Base Ions, CEC
Exchangeable H+ and Al3+ are two important ions in soil acidity. Our study showed that both low and high chlorine treatment significantly increased the content of exchangeable H+ and Al3+ in the soil profile, and the high chlorine treatment increased them more. The proportion of exchangeable Al3+ in total exchangeable acid increased significantly in the three soil layers, from 69.8 to 79.6%. Exchangeable Al3+, therefore, clearly played a leading role in driving soil acidification.
The base saturation of the ClH and ClL treatments decreased, with ClH decreasing more (Table 2). This may be because the high chlorine treatment increases crop yields, and the salt-based ions in the soil are removed more when the crops are harvested [28], resulting in a decrease in salt-based ions in the soil. In this study, the amount of salt-based ions removed in crops was not measured, but we will account for it in future studies. It may also be due to the leaching of base ions, resulting in a reduction in the base ion effectiveness (including Ca2+, Mg2+, and K+), which will lead to soil fertility degradation and a reduction in crop yield [29].
Ca/Al and Mg/Al ratios in soil and plant tissues have been widely used as indicators to evaluate the response of terrestrial ecosystems to acid deposition or other processes [30,31]. Cronan and Grigal (1995) considered that the risk of adverse effects of aluminum stress on plant growth increased with the decrease in the Ca/Al ratio, as the risk was 50% when the soil Ca/Al ratio was as low as 1.0. When the ratio was as low as 0.5, the risk was 75%, and when the ratio was as low as 0.2, close to 100% risk is observed [32]. In our study, the available Ca/Al in each soil layer decreased significantly with increasing chlorine applications. The available Ca/Al in 0–20 cm soil treated with CK, ClL, and ClH was 21.6, 10.6, and 1.4, respectively; in 20–40 cm soil it was 9.6, 6.0, and 1.9, respectively; and in 40–60 cm soil it was 12.0, 6.2, and 2.0, respectively (Table 3). Therefore, with increasing chlorine application, the risk of aluminum stress on plants increases.
Compared with CK, the application of Cl-containing fertilizer led to an increase in soil CEC, which may be because the soil lacks sufficient leaching, so exogenous Cl− leads to an increase in soil CEC. Compared to CK, the CEC of high chlorine treatment reached a significant level because more Cl− was applied.
4.4. Relationship between Soil pH, Cl−, Exchangeable Acid, and Exchangeable Base Ions
There was a significant positive correlation between soil pH and exchangeable base ions (p < 0.05) (Table 4), and a significant negative correlation between soil pH and exchangeable Al3+. When soil base ions are depleted, Al might play a major role in the process of buffer acidification [9,17]. Cl− was negatively correlated with soil pH and base ions (p < 0.05) and positively correlated with exchangeable Al3+ (p < 0.05). Thus, with increasing Cl− content in soil, the soil acidification increases, base ions decrease, and the Al3+ increase accelerates.
5. Conclusions
Through a study on the long-term application of chlorine-containing fertilizer to a peanut and corn rotation in brown soil, we found that the long-term application of chlorine-containing fertilizer caused the contents of K+, Ca2+, Mg2+, and Na+ to decrease both in the topsoil and deeper soil, and caused the content of exchangeable Al3+ to increase, thus causing the soil to acidify. This phenomenon was particularly obvious when applying high-chloride content fertilizer. Moreover, the long-term application of high chlorine content fertilizer has a greater impact than low chlorine content fertilizer on deep soil. Therefore, in the brown soil of northeast China, chlorine-containing fertilizer should be appropriately selected during crop planting to help to reduce the cost of agricultural production, but it is necessary to avoid using multiple or excessive doses of chlorine-containing fertilizers. This will help to achieve an optimal balance between reducing production input costs and delaying soil acidification.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Y.W., X.L., L.W., H.L. and S.Z. The first draft of the manuscript was written by Y.W. and all authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (no. 41602363) and the China Agriculture Research System (CARS-13).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all individual participants included in the study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The study is supported by National Natural Science Foundation of China (no. 41602363) and the China Agriculture Research System (CARS-13).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Xu, G.H.; Magen, H.; Tarchitzky, J.; Kafkafi, V. Advances in chloride nutrition. Adv. Agron. 2000, 68, 96–150. [Google Scholar]
- Mengel, D.; Lamond, R.; Martin, V.; Duncan, S.; Whitney, D.; Gordon, B. Chloride Fertilization and Soil Testing Update for Major Crops in Kansas. Better Crops 2009, 93, 20–22. [Google Scholar]
- Zou, C.M.; Gao, J.S. Effects of long-term application of chlorine-containing chemical fertilizers on chloride accum ulation and nutrient balance in paddy fields. Acta Ecol. Sin. 2004, 24, 2557–2563. (In Chinese) [Google Scholar]
- Zhou, Z.F.; Shi, X.J.; Zheng, Y.; Qin, Z.X.; Xie, D.T.; Li, Z.L.; Guo, T. Abundance and community structure of ammonia-oxidizing bacteria and archaea in purple soil under long-term fertilization. Eur. J. Soil Biol. 2014, 60, 24–33. [Google Scholar] [CrossRef]
- Braun, S.; Cantaluppi, L.; Flückiger, W. Fine roots in stands of Fagus sylvatica and Picea abiesalong a gradient of soil acidification. Environ. Pollut. 2005, 137, 574–579. [Google Scholar] [CrossRef]
- Nadelhoffer, K.J. The potential effects of nitrogen deposition on fine-root production in forest ecosystems. New Phytol. 2000, 147, 131–139. [Google Scholar] [CrossRef]
- Zhao, B.Q.; Li, X.Y.; Li, X.P.; Shi, X.J.; Huang, S.M.; Wang, B.R.; Zhu, P.; Yang, X.Y.; Liu, H.; Chen, Y.; et al. Long-term fertilizer experiment network in China: Crop yields and soil nutrient trends. Agron. J. 2010, 102, 216–230. [Google Scholar] [CrossRef]
- Zhang, X.M.; Liu, W.; Zhang, G.M.; Jiang, L.; Han, X.G. Mechanisms of soil acidification reducing bacterial diversity. Soil Biol. Biochem. 2015, 81, 275–281. [Google Scholar] [CrossRef]
- Schroder, J.L.; Zhang, H.; Girma, K.; Raun, W.R.; Penn, C.J.; Payton, M.E. Soil acidification from long-term use of nitrogen fertilizers on winter wheat. Soil Sci. Soc. Am. J. 2011, 75, 957–964. [Google Scholar] [CrossRef]
- Bobbink, R.; Hicks, K.; Galloway, J.; Spranger, T.; Alkemade, R.; Ashmore, M.; Bustamante, M.; Cinderby, S.; Davidson, E.; Dentener, F.; et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecol. Appl. 2010, 20, 30–59. [Google Scholar] [CrossRef]
- Yan, W.T.; Jiang, W.T.; Han, X.R.; Hua, W.; Yang, J.F.; Luo, P.Y. Simulating and predicting crop yield and soil fertility under climate change with fertilizer management in Northeast China based on the decision support system for agrotechnology transfer model. Sustainability 2020, 12, 2194. [Google Scholar] [CrossRef]
- Lu, R.K. Methods of Soil and Agrochemical Analysis; China Agricultural Science and Technology Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemical Analysis; China Agriculture Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Parker, M.B.; Gascho, G.J.; Gaines, T.P. Chloride toxicity of soybean grown on Atlantic coast flatwoods soils. Agron. J. 1983, 75, 439–443. [Google Scholar] [CrossRef]
- Jin, A.S.; Guo, P.C.; Zhang, X.Y. Chloride tolerance and its effects on yield and quality of crops. Chin. J. Soil Sci. 1992, 33, 257–259. (In Chinese) [Google Scholar]
- Zhou, J.; Xia, F.; Liu, X.M.; He, Y.; Xu, J.M.; Brookes, P.C. Effects of nitrogen fertilizer on the acidification of two typical acid soils in South China. J. Soils Sediments 2014, 14, 415–422. [Google Scholar] [CrossRef]
- Tian, D.S.; Niu, S.L. A global analysis of soil acidifification caused by nitrogen addition. Environ. Res. Lett. 2015, 10, 024019. [Google Scholar] [CrossRef]
- Mao, Q.G.; Lu, X.K.; Zhou, K.J.; Chen, H.; Zhu, X.M.; Mori, T.; Mo, J.M. Effects of long-term nitrogen and phosphorus additions on soil acidification in an N-rich tropical forest. Geoderma 2017, 285, 57–63. [Google Scholar] [CrossRef]
- Yang, X.D.; Ni, K.; Shi, Y.Z.; Yi, X.Y.; Zhang, Q.F.; Fang, L.; Ma, L.F.; Ruan, J. Effects of long-term nitrogen application on soil acidification and solution chemistry of a tea plantation in China. Agric. Ecosyst. Environ. 2018, 252, 74–82. [Google Scholar] [CrossRef]
- Gundersen, P.; Rasmussen, L. Nitrification in forest soils-effects from nitrogen deposition on soil acidification and aluminum release. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 1990; Volume 113, pp. 1–45. [Google Scholar]
- Hao, T.X.; Zhu, Q.C.; Zeng, M.F.; Shen, J.B.; Shi, X.J.; Liu, X.J.; Zhang, F.S. Impacts ofnitrogen fertilizer type and application rate on soil acidifcation rate under a wheat-maize double cropping system. J. Environ. Manag. 2020, 270, 110888. [Google Scholar] [CrossRef]
- Barak, P.; Jobe, B.O.; Krueger, A.R.; Peterson, L.A.; Laird, D.A. Effects of long-term soil acidification due to nitrogen fertilizer inputs in Wisconsin. Plant Soil 1997, 197, 61–69. [Google Scholar] [CrossRef]
- Bouman, O.; Curtin, D.; Campbell, C.; Biederbeck, V.; Ukrainetz, H. Soil acidification fromlong-tenm use ofanhydrous ammonia and urea. Soil Sci. Soc. Am. J. 1995, 59, 1488–1494. [Google Scholar] [CrossRef]
- Chien, S.H.; Gearhart, M.M.; Collamer, D.J. The effect of different ammonical nitrogen sources on soil acidification. Soil Sci. 2008, 173, 544–551. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Vries, W.D.; Thomas, B.W.; Hao, X.Y.; Shi, X.J. Impacts of long-term nitrogen fertilization on acid buffering rates and mechanisms of a slightly calcareous clay soil. Geoderma 2017, 305, 92–99. [Google Scholar] [CrossRef]
- Tarkalson, D.D.; Payero, J.O.; Hergert, G.W.; Cassman, K.G. Acidification of soil in a dry land winter wheat-sorghum/corn-fallow rotation in the semiarid U.S. Great Plains. Plant Soil 2006, 283, 367–379. [Google Scholar] [CrossRef]
- Ma, L.Y.; Wang, Y.; CAI, F.F.; Zhang, S.Y.; Luo, P.Y.; Yang, J.F.; Han, X.R.J. Effects of long-term application of chlorinated fertilizer on nitrification and ammonia oxidizing microorganisms in brown soil. Plant Nutr. Fertil. 2019, 25, 824–831. (In Chinese) [Google Scholar]
- Hao, T.X.; Zhu, Q.C.; Zeng, M.F.; Shen, J.B.; Shi, X.J.; Liu, X.J.; de Vries, W. Quantification of the contribution ofnitrogen fertilization and crop harvesting to soil acidification in a wheat-maize double cropping system. Plant Soil 2019, 434, 167–184. [Google Scholar] [CrossRef]
- Zhang, Y.T.; He, X.H.; Liang, H.; Zhao, J.; Zhang, Y.; Xu, C.; Shi, X.J. Long-term tobacco plan tation induces soil acidifification and soil base cation loss. Environ. Sci. Pollut. Res. 2016, 23, 5442–5450. [Google Scholar] [CrossRef]
- Levia, D.F.; Shiklomanov, A.N.; Stan, J.T.V.; Scheick, C.E.; Inamdar, S.P.; Mitchell, M.J.; Mchale, P.J. Calcium and aluminum cycling in a temperate broadleaved deciduous forest of the eastern USA: Relative impacts of tree species, canopy state, and flux type. Environ. Monit. Assess. 2015, 187, 458. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, W.R.; Meng, M.J.; Fu, Z.Y.; Xu, L.H.; Zha, Y.Y.; Yue, J.M.; Zhang, S.F.; Zhang, J.C. Comparative effects of simulated acid rain of different ratios of SO42− to NO3− on fine root in subtropical plantation of China. Sci. Total Environ. 2018, 618, 336–346. [Google Scholar] [CrossRef]
- Cronan, C.S.; Grigal, D.F. Use of calcium/alumiunum ratios as indicators of stress in forest ecosystems. J. Environ. Qual. 1995, 24, 209–226. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).