Anti-Scale Performance and Mechanism of Valonia Tannin Extract for Calcium Carbonate in Circulating Cooling Water System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
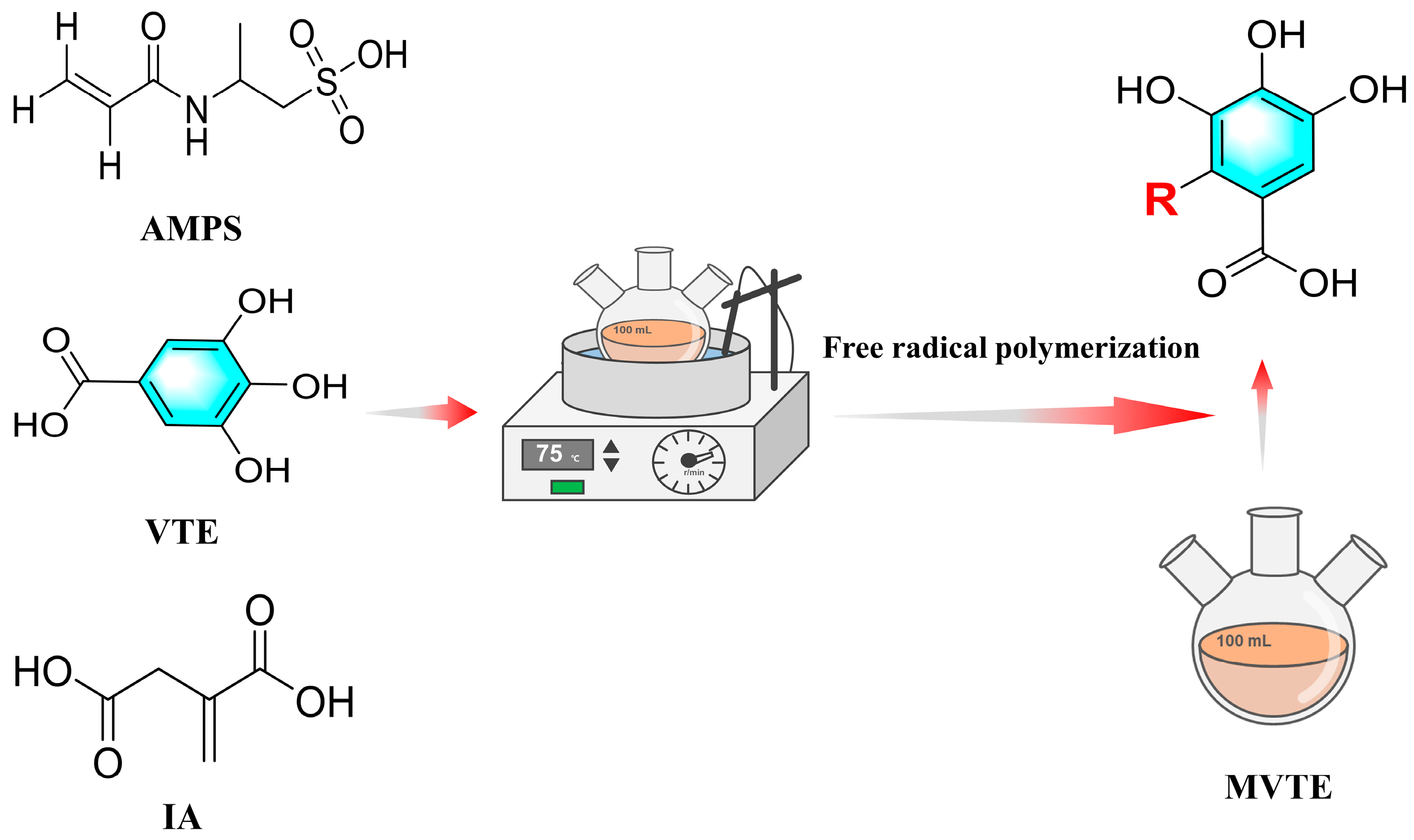
2.2. The Preparation Method of MVTE
2.3. Characterization
2.4. The Static Scale Inhibition Tests
2.5. The Single Factor Method
2.6. Molecular Dynamics Simulation
2.6.1. Simulation Parameters and Force Field
2.6.2. Model Construction
3. Results and Discussions
3.1. Determination of Optimum Synthesis Conditions of MVTE Antiscalant
3.1.1. Selection of the Suitable Tannin Extract and Initiator
3.1.2. The Effect of the Dosage of Initiator
3.1.3. The Effect of the Dosage of Valonia
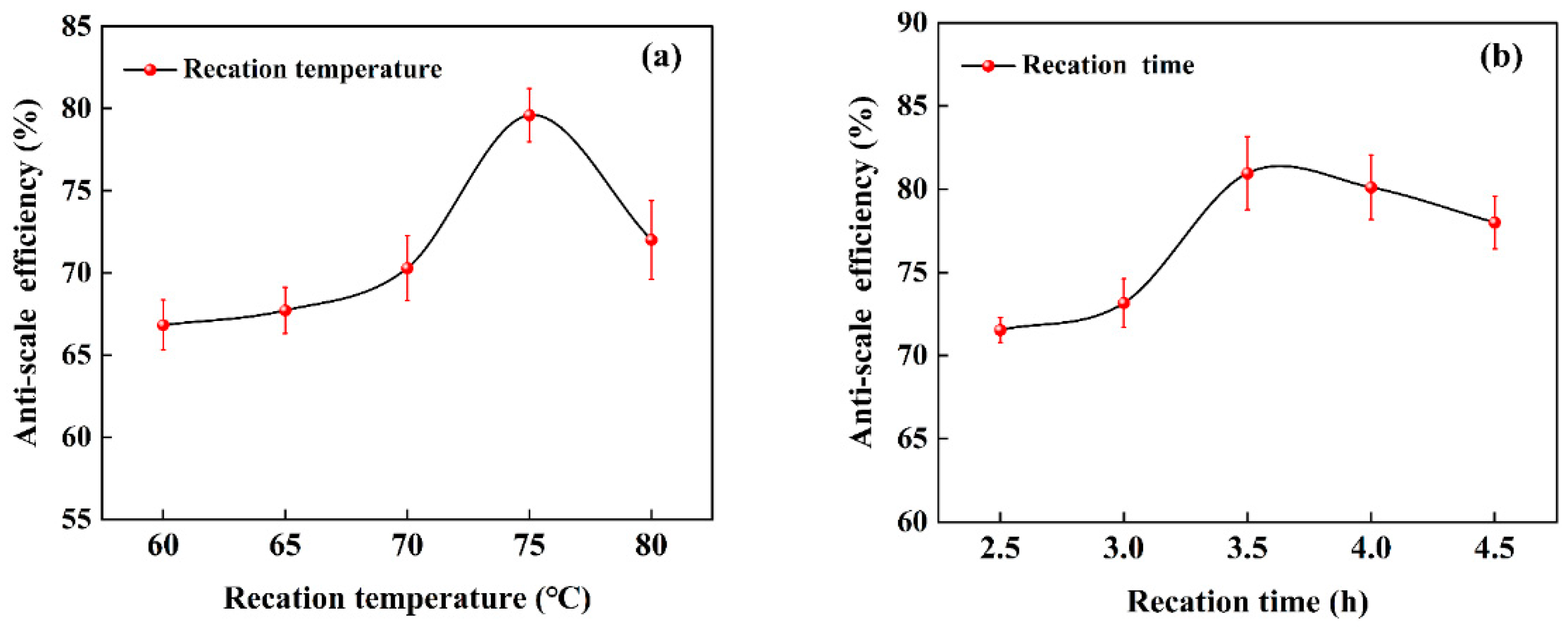
3.1.4. The Effect of the Reaction Temperature
3.1.5. The Effect of the Reaction Time
3.2. The Characterizations of MVTE
3.3. Analysis of Anti-Scale Performance of MVTE
3.3.1. Effect of Different MVTE Concentrations against CaCO3
3.3.2. Effect of pH on the Anti-Scale Performance
3.3.3. Effect of Temperature on the Anti-Scale Performance
3.3.4. Effect of the Concentration of Ca2+ on the Anti-Scale Performance
3.4. Anti-Scale Mechanisms
3.4.1. SEM Analysis of Scale Crystal Morphology
3.4.2. XRD Analysis of Scale Crystal Structure
3.4.3. XPS Analysis of Scale Crystal
3.5. Analysis of MD Simulation
3.5.1. The Equilibrium Criteria of Interaction Systems
3.5.2. Binding Energy of Antiscalant on the Surface of Calcite
3.5.3. Deformation Energy of Antiscalant on the Surface of Calcite
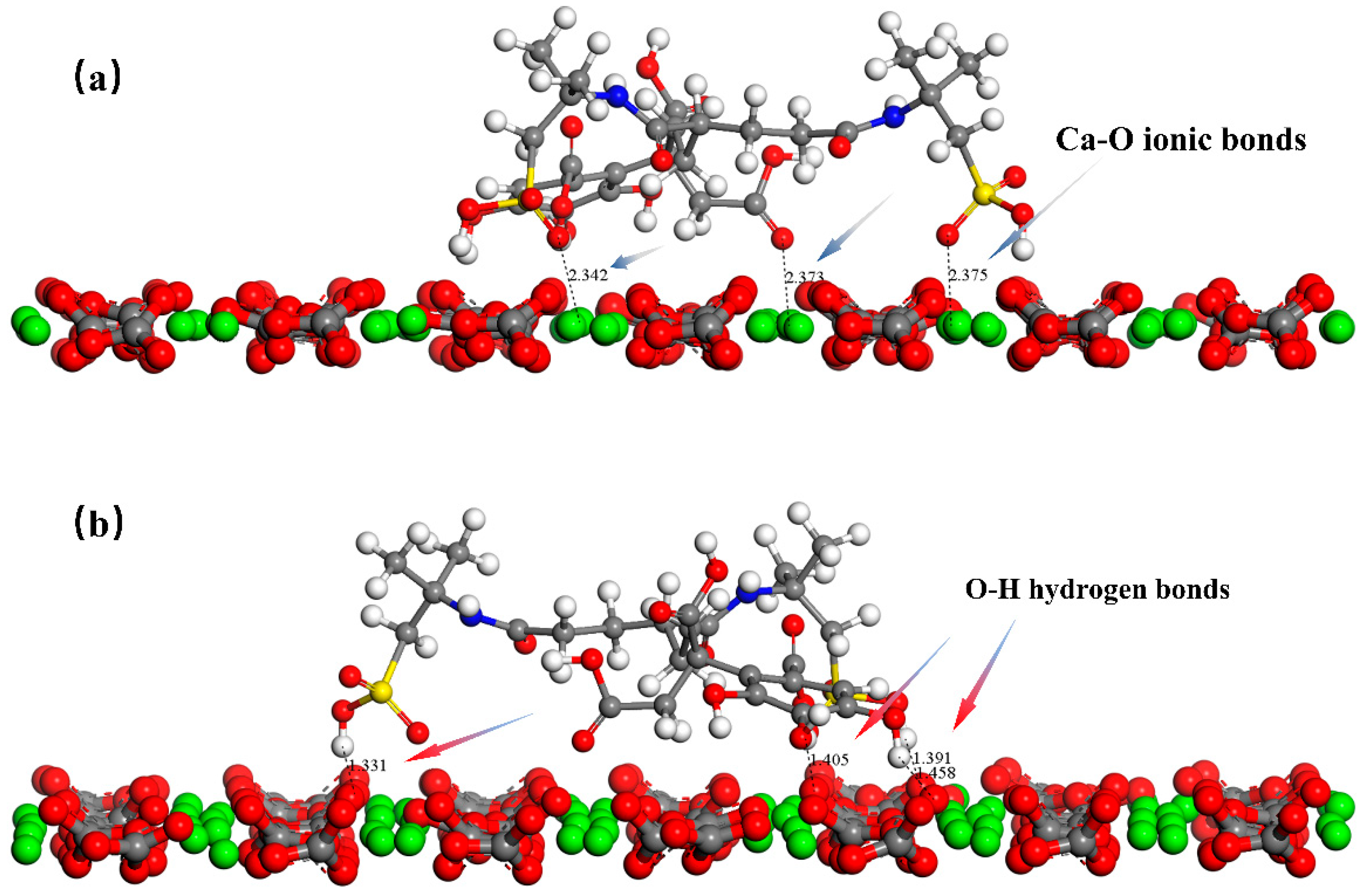
3.5.4. Radial Distribution Function of Antiscalant with Calcite Surface
3.6. The Anti-Scale Performance of MVTE on Calcium Phosphate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Yin, H.Q.; Zhang, Q.S.; Li, Y.Z.; Yao, P.J. Synthesis and characterization of novel polyaspartic acid/urea graft copolymer with acylamino group and its scale inhibition performance. Desalination 2016, 395, 92–98. [Google Scholar] [CrossRef]
- Haghtalab, S.; Jadidian, R.; Rahmani, K. Evaluation of inhibitors and biocides on the corrosion, scaling and biofouling control of carbon steel and copper-nickel alloys in a power plant cooling water system. Desalin. Int. J. Sci. Technol. Desalt. Water Purif. 2016, 393, 174–185. [Google Scholar]
- Zhu, T.; Wang, L.; Sun, W.; Wang, M.; Tian, J.; Yang, Z.; Wang, S.; Xia, L.; He, S.; Zhou, Y.; et al. The role of corrosion inhibition in the mitigation of CaCO3 scaling on steel surface. Corros. Sci. 2018, 140, 182–195. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Jia, L.L.; Liu, K.Y.; Gao, P.; Ge, H.H.; Fu, L.J. Inhibition of calcium sulfate scale by poly (citric acid). Desalination 2016, 392, 1–7. [Google Scholar] [CrossRef]
- Can, H.K.; Ner, G. Water-soluble anhydride containing alternating copolymers as scale inhibitors. Desalination 2015, 355, 225–232. [Google Scholar] [CrossRef]
- Wang, C.; Shen, T.; Li, S.; Wang, X. Investigation of influence of low phosphorous co-polymer antiscalant on calcium sulfate dihydrate crystal morphologies. Desalination 2014, 348, 89–93. [Google Scholar] [CrossRef]
- Alroomi, Y.M.; Hussain, K.F. Potential kinetic model for scaling and scale inhibition mechanism. Desalination 2016, 393, 186–195. [Google Scholar] [CrossRef]
- Amjad, Z. Mineral Scales in Biological and Industrial Systems; CRC Press: Boca Raton, FL, USA, 2013; Volume 26, pp. 77–102. [Google Scholar]
- Rahman, F. Calcium sulfate precipitation studies with scale inhibitors for reverse osmosis desalination. Desalination 2013, 319, 79–84. [Google Scholar] [CrossRef]
- Dobberschütz, S.; Nielsen, M.R.; Sand, K.K.; Civioc, R.; Andersson, M.P. The mechanisms of crystal growth inhibition by organic and inorganic inhibitors. Nat. Commun. 2018, 9, 1578. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Z.; Yang, W.; Zhang, K.; Chen, Y.; Li, M.; Zuo, Y.; Yin, X.; Liu, Y. Effect of scale inhibitors on the structure and morphology of CaCO3 crystal electrochemically deposited on TA1 alloy. J. Colloid Interface Sci. 2020, 562, 558–566. [Google Scholar] [CrossRef]
- Zuo, Y.W.; Sun, Y.; Yang, W.Z.; Zhang, K.G.; Chen, Y.; Yin, X.S.; Liu, Y. Performance and mechanism of 1-hydroxy ethylidene-1,1-diphosphonic acid and 2-phosphonobutane-1,2,4-tricarboxylic acid in the inhibition of calcium carbonate scale. J. Mol. Liq. 2021, 334, 116093. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Gao, Y.H.; Fan, L.H.; Roddick, F.A.; Liu, Z.F. Antiscaling effect of polyaspartic acid and its derivative for RO membranes used for saline wastewater and brackish water desalination. Desalination 2017, 404, 224–229. [Google Scholar] [CrossRef]
- Yuan, X.J.; Dong, S.Y.; Zheng, Q.; Yang, W.K.; Huang, T.L. Novel and efficient curcumin based fluorescent polymer for scale and corrosion inhibition. Chem. Eng. J. 2020, 389, 124296. [Google Scholar] [CrossRef]
- Quan, Z.H.; Chen, Y.C.; Wang, X.R.; Shi, C.; Liu, Y.J.; Chongfang, M.A. Experimental study on scale inhibition performance of a green scale inhibitor polyaspartic acid. Sci. China Ser. B Chem. 2008, 51, 695–699. [Google Scholar] [CrossRef]
- Wu, X.; Bo, S.; Jing, W.; Shi, C. Evaluation on Biodegradability of a Novel Copolymer. Acta Sci. Nat. Univ. Nankaiensis 2009, 21, 202–228. [Google Scholar]
- Touir, R.; Cenoui, M.; Bakri, M.E.; Touhami, M.E. Sodium gluconate as corrosion and scale inhibitor of ordinary steel in simulated cooling water. Corros. Sci. 2008, 50, 1530–1537. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, D.; Lv, X.; Xu, Y.; Cui, Y. Synthesis of polyaspartic acid/3-amino-1H-1,2,4-triazole-5-carboxylic acid hydrate graft copolymer and evaluation of its corrosion inhibition and scale inhibition performance. Desalination. 2013, 327, 32–38. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Han, J.; Su, M.; Wu, Q. Synthesis of modified polyaspartic acid and evaluation of its scale inhibition and dispersion capacity. Desalination 2015, 358, 42–48. [Google Scholar] [CrossRef]
- Wang, H.; Hu, J.; Yang, Z.; Yin, Z.; Xiong, Q.; Zhong, X. The study of a highly efficient and environment-friendly scale inhibitor for calcium carbonate scale in oil fields. Petroleum 2021, 7, 325–334. [Google Scholar] [CrossRef]
- Cui, K.; Li, C.; Yao, B.; Yang, F.; Sun, G. Synthesis and evaluation of an environment-friendly terpolymer CaCO3 scale inhibitor for oilfield produced water with better salt and temperature resistance. J. Appl. Polym. Sci. 2020, 137, 48460. [Google Scholar] [CrossRef]
- Liu, Z.F.; Wang, S.S.; Zhang, L.H.; Liu, Z. Dynamic synergistic scale inhibition performance of IA/SAS/SHP copolymer with magnetic field and electrostatic field. Desalination 2015, 362, 26–33. [Google Scholar] [CrossRef]
- Zhao, L.N.; Zhou, Y.M.; Yao, Q.Z.; Wang, Y.Y.; Ge, S.J.; Liu, X.L. Calcium Scale Inhibition of Stimulated Oilfield Produced Water Using Polyaspartic Acid/Aminomethanesulfonic Acid. Chemistryselect 2021, 6, 3692–3701. [Google Scholar] [CrossRef]
- Liu, D.; Dong, W.; Li, F.; Hui, F.; Lédion, J. Comparative performance of polyepoxysuccinic acid and polyaspartic acid on scaling inhibition by static and rapid controlled precipitation methods. Desalination 2012, 304, 1–10. [Google Scholar] [CrossRef]
- Yan, M.; Tan, Q.; Liu, Z.; Li, H.; Zheng, Y.; Zhang, L.; Liu, Z. Synthesis and Application of a Phosphorous-Free and Non-Nitrogen Polymer as an Environmentally Friendly Scale Inhibition and Dispersion Agent in Simulated Cooling Water Systems. ACS Omega 2020, 5, 15487–15494. [Google Scholar] [CrossRef]
- Wang, L.C.; Zhu, C.G.; Liu, H.B.; Zhao, W.D.; Che, Y.; Zhang, Q.L.; Wang, L.B. Evaluation of maleic acid-based copolymers containing polyoxyethylene ether as inhibitors for CaCO3 scale. J. Appl. Polym. Sci. 2019, 136, 47470. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Yao, Q.; Bu, Y.; Wang, H.; Wu, W.; Sun, W. Preparation of a low-phosphorous terpolymer as a scale, corrosion inhibitor, and dispersant for ferric oxide. J. Appl. Polym. Sci. 2015, 132, 1–10. [Google Scholar] [CrossRef]
- Zhang, H.P.; Luo, X.G.; Lin, X.Y.; Tang, P.P.; Lu, X.; Yang, M.J.; Tang, Y. Biodegradable carboxymethyl inulin as a scale inhibitor for calcite crystal growth: Molecular level understanding. Desalination 2016, 381, 1–7. [Google Scholar] [CrossRef]
- Yu, W.; Song, D.; Li, A.; Yang, H. Control of gypsum-dominated scaling in reverse osmosis system using carboxymethyl cellulose. J. Memb. Sci. 2019, 577, 20–30. [Google Scholar] [CrossRef]
- Guo, X.; Qiu, F.; Dong, K.; Zhou, X.; Qi, J.; Zhou, Y.; Yang, D. Preparation, characterization and scale performance of scale inhibitor copolymer modification with chitosan. J. Ind. Eng. Chem. 2012, 18, 2177–2183. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Amaral-Labat, G.; Boss, A.F.N.; Lacoste, C.; Pizzi, A. Tannin gels and their carbon derivatives: A review. Biomolecules 2019, 9, 587. [Google Scholar] [CrossRef] [Green Version]
- Khademian, E.; Salehi, E.; Sanaeepur, H.; Galiano, F.; Figoli, A. A systematic review on carbohydrate biopolymers for adsorptive remediation of copper ions from aqueous environments-part A: Classification and modification strategies. Sci. Total Environ. 2020, 738, 139829. [Google Scholar] [CrossRef]
- Özacar, M.; Soykan, C.; Şengil, I.A. Studies on synthesis, characterization, and metal adsorption of mimosa and valonia tannin resins. J. Appl. Polym. Sci. 2006, 102, 786–797. [Google Scholar] [CrossRef]
- Abdalla, S.; Pizzi, A.; Bahabri, F.; Ganash, A. Analysis of valonia oak (Quercus aegylops) acorn tannin and wood adhesives application. BioResources 2015, 10, 7165–7177. [Google Scholar] [CrossRef] [Green Version]
- Resins, T.; Li, J.; Li, C.; Wang, W.; Zhang, W.; Li, J. Reactivity of Larch and Valonia Tannins in Synthesis of Tannin-Formaldehyde Resins. Bioresources 2016, 11, 2256–2268. [Google Scholar]
- Garro Galvez, J.M.; Fechtal, M.; Riedl, B. Gallic acid a model of tannins in condensation with formaldehyde. Thermochim. Acta 1996, 274, 149–163. [Google Scholar] [CrossRef]
- Can, M.; Bulut, E.; Örnek, A.; Özacar, M. Synthesis and characterization of valonea tannin resin and its interaction with palladium (II), rhodium (III) chloro complexes. Chem. Eng. J. 2013, 221, 146–158. [Google Scholar] [CrossRef]
- Pasch, H.; Pizzi, A. Considerations on the macromolecular structure of chestnut ellagitannins by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry. J. Appl. Polym. Sci. 2002, 85, 429–437. [Google Scholar] [CrossRef]
- Haslam, E. Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef]
- Jankun, J.; Selman, S.H.; Swiercz, R.; Skrzypczak-Jankun, E. Why drinking green tea could prevent cancer. Nature 1997, 387, 561. [Google Scholar] [CrossRef]
- Guo, Q.; Shi, Z.; Ying, J. Natural products condensed tan- nins as both co-initiator and crosslinker to prepare highly stretch- able polyacrylamide hydrogel. J. Funct. Polym. 2017, 30, 34–35. [Google Scholar]
- Shi, W.Y.; Ding, C.; Yan, J.L.; Han, X.Y.; Lv, Z.M.; Wu, L.; Xia, M.Z.; Wang, F.Y. Molecular dynamics simulation for interaction of PESA and acrylic copolymers with calcite crystal surfaces. Desalination 2012, 291, 8–14. [Google Scholar] [CrossRef]
- Agbabiaka, O.G.; Adegun, M.H.; Chan, K.-Y.; Zhang, H.; Shen, X.; Kim, J.-K. BN-PVDF/rGO-PVDF Laminate Nanocomposites for Energy Storage Applications. Nanomaterials 2022, 12, 4492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.F.; Chen, F.J.; Han, J.; Tian, T.; Jin, Y.H.; Zhang, Z.X.; Chen, J.X. Evaluation of Arginine-Modified Polyepoxysuccinic Acid as Anti-scaling and Anti-corrosion Agent. Chem. Eng. Technol. 2021, 44, 1131–1140. [Google Scholar] [CrossRef]
- Wan, C.; Wang, L.T.; Sha, J.Y.; Ge, H.H. Effect of Carbon Nanoparticles on the Crystallization of Calcium Carbonate in Aqueous Solution. Nanomaterials 2019, 9, 179. [Google Scholar] [CrossRef] [Green Version]
- Ling, L.; Zhou, Y.; Huang, J.; Yao, Q.; Liu, G.; Zhang, P.; Sun, W.; Wu, W. Carboxylate-terminated double-hydrophilic block copolymer as an effective and environmental inhibitor in cooling water systems. Desalination 2012, 304, 33–40. [Google Scholar] [CrossRef]
- Aschauer, U.; Spagnoli, D.; Bowen, P.; Parker, S.C. Growth modification of seeded calcite using carboxylic acids: Atomistic simulations. J. Colloid Interface Sci. 2010, 346, 226–231. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Zhou, Y.; Huang, J. Carboxylate-Terminated Double-Hydrophilic Block Copoly- mer as an Effective and Environmentally Friendly Inhibitor for Carbonate and Sulfate Scales in Cooling Water Systems. Water Air Soil Pollut. 2012, 223, 3601–3609. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Cui, J.; Li, Y.; Liang, Y.; Cao, G. Molecular dynamics simulation and DFT calculation of “green” scale and corrosion inhibitor. Comput. Mater. Sci. 2021, 188, 110229. [Google Scholar] [CrossRef]
- Elkholy, A.E.; El-Taib Heakal, F.; Rashad, A.M.; Zakaria, K. Monte Carlo simulation for guar and xanthan gums as green scale inhibitors. J. Pet. Sci. Eng. 2018, 166, 263–273. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Z.; Wang, B.; He, J. Scale inhibition performance of sodium carboxymethyl cellulose on heat transfer surface at various temperatures: Experiments and molecular dynamics simulation. Int. J. Heat Mass Transf. 2019, 141, 457–463. [Google Scholar] [CrossRef]
- Chen, J.; Xu, H.; Han, J.; Wang, C.; Wu, Q.; Li, C. A Green Multifunctional Antiscaling Inhibitor for Crystallization Control of Ca-Scale Crystals. Chem. Eng. Technol. 2019, 42, 444–453. [Google Scholar] [CrossRef]
- Ji, Y.; Chen, Y.; Le, J.; Qian, M.; Huan, Y.; Yang, W.; Yin, X.; Liu, Y.; Wang, X.; Chen, Y. Highly effective scale inhibition performance of amino trimethylenephosphonic acid on calcium carbonate. Desalination 2017, 422, 165–173. [Google Scholar] [CrossRef]
- Zuo, Y.; Yang, W.; Zhang, K.; Chen, Y.; Yin, X.; Liu, Y. Experimental and theoretical studies of carboxylic polymers with low molecular weight as inhibitors for calcium carbonate scale. Crystals 2020, 10, 406. [Google Scholar] [CrossRef]
- Pu, S.Z.; Wang, Y.N.; He, Q.; Liao, X.P.; Zhang, W.H.; Shi, B. Molecular level understanding of the role of aldehyde in vegetable-aldehyde-collagen cross-linking reaction. Int. J. Quantum Chem. 2012, 112, 2832–2839. [Google Scholar] [CrossRef]
- Zhang, S.; Qu, H.; Yang, Z.; Fu, C.E.; Tian, Z.; Yang, W. Scale inhibition performance and mechanism of sulfamic/amino acids modified polyaspartic acid against calcium sulfate. Desalination 2017, 419, 152–159. [Google Scholar] [CrossRef]
- Chen, C.; Lei, W.; Xia, M.; Wang, F.; Gong, X. Molecular modeling of several phosphonates onto the stepped calcite (011) surface. Desalination 2013, 309, 208–212. [Google Scholar] [CrossRef]
- Pradip, R.B.; Rao, T.K.; Krishnamurthy, S.; Vetrivel, R.; Mielczarski, J.; Cases, J.M. Molecular modeling of interactions of diphosphonic acid based surfactants with calcium minerals. Langmuir 2002, 18, 932–940. [Google Scholar] [CrossRef]
- Pradip, R.B.; Rao, T.K.; Krishnamurthy, S.; Vetrivel, R.; Mielczarski, J.; Cases, J.M. Molecular modeling of interactions of alkyl hydroxamates with calcium minerals. J. Colloid Interface Sci. 2002, 256, 106–113. [Google Scholar] [CrossRef]
- Shi, W.; Xu, W.; Cang, H.; Yan, X.; Shao, R.; Zhang, Y.; Xia, M. Design and synthesis of biodegradable antiscalant based on MD simulation of antiscale mechanism: A case of itaconic acid-epoxysuccinate copolymer. Comput. Mater. Sci. 2017, 136, 118–125. [Google Scholar] [CrossRef]
- Zhou, M.; Gu, Y.H.; Yi, R.J.; Han, H.C. Synthesis and property study of ter-copolymer P(MA-AMPS-HPA) scale inhibitor. J. Polym. Res. 2020, 27, 1–12. [Google Scholar] [CrossRef]
- Cui, C.; Zhang, S. Preparation, Characterization and Performance Evaluation of a Novel Scale Inhibiting and Dispersing Copolymer Containing Natural Tannin. J. Polym. Environ. 2020, 28, 1869–1879. [Google Scholar] [CrossRef]
- Guo, X.; Qiu, F.; Dong, K.; Rong, X.; He, K.; Xu, J.; Yang, D. Preparation and application of copolymer modified with the palygorskite as inhibitor for calcium carbonate scale. Appl. Clay Sci. 2014, 99, 187–193. [Google Scholar] [CrossRef]
- Talib, Y.; Idris, A.; Aslina, S. A tannin-based agent for coagulation and fl occulation of municipal wastewater: Chemical composition, performance assessment compared to Polyaluminum chloride, and application in a pilot plant. J. Environ. Manag. 2016, 184, 494–503. [Google Scholar] [CrossRef]
- Cui, C.C.; Zhang, S.G. Synthesis, characterization and performance evaluation of an environmentally benign scale inhibitor IA/AMPS co-polymer. New J. Chem. 2019, 43, 9472–9482. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; El Basiony, N.M.; Sadeek, S.A.; Migahed, M.A. Scale and corrosion inhibition performance of the newly synthesized anionic surfactant in desalination plants: Experimental, and theoretical investigations. Desalination 2018, 437, 45–58. [Google Scholar] [CrossRef]
- Lin, J.; Yang, Q.; Wen, X.; Cai, Z.Q.; Pi, P.; Cheng, J.; Yang, Z. Synthesis, characterization, and thermal stability studies of bisphenol-A type novolac epoxy-polyurethane coating systems for in-mould decoration ink applications. J. Polym. Res. 2011, 18, 1667–1677. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Li, M.M.; Chen, T.H.; Zhou, Y.F.; Yue, Z.B. Competitive adsorption of heavy metal by extracellular polymeric substances (EPS) extracted from sulfate reducing bacteria. Bioresour. Technol. 2014, 163, 374–376. [Google Scholar] [CrossRef]
- Mady, M.F.; Malmin, H.; Kelland, M.A. Sulfonated nonpolymeric aminophosphonate scale inhibitors-improving the compatibility and biodegradability. Energy Fuels 2019, 33, 6197–6204. [Google Scholar] [CrossRef]
- Wang, Y.; Li, A.; Yang, H. Effects of substitution degree and molecular weight of carboxymethyl starch on its scale inhibition. Desalination 2017, 408, 60–69. [Google Scholar] [CrossRef]
- Yang, L.; Yang, W.Z.; Xu, B.; Yin, X.S.; Chen, Y.; Liu, Y.; Ji, Y.; Huan, Y. Synthesis and scale inhibition performance of a novel environmental friendly and hydrophilic terpolymer inhibitor. Desalination 2017, 416, 166–174. [Google Scholar] [CrossRef]
- Guo, X.Y.; Zhao, X.W.; Xu, Y.H.; Zhang, P.P.; Cheng, Y.M.; Xu, Y. The synthesis of polyaspartic acid derivative PASP-Im and investigation of its scale inhibition performance and mechanism in industrial circulating water. RSC Adv. 2020, 10, 33595–33601. [Google Scholar] [CrossRef]
- Guo, X.; Qiu, F.; Dong, K.; He, K.; Rong, X.; Yang, D.; Guo, X.; Qiu, F.; Dong, K.; He, K.; et al. Scale Inhibitor Copolymer Modified with Oxidized Starch: Synthesis and Performance on Scale Inhibition Scale Inhibitor Copolymer Modified with Oxidized Starch: Synthesis and Performance on Scale Inhibition. Polym. Plast. Technol. Eng. 2013, 52, 37–41. [Google Scholar] [CrossRef]
- Dietzsch, M.; Barz, M.; Schüler, T.; Klassen, S.; Schreiber, M.; Susewind, M.; Loges, N.; Lang, M.; Hellmann, N.; Fritz, M. PAA-PAMPS copolymers as an efficient tool to control CaCO3 scale formation. Langmuir 2013, 29, 3080–3088. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Li, J.; Xu, K.; Ding, L.; Ren, H. The effect of synthesized hydrolyzed polymaleic anhydride (HPMA) on the crystal of calcium carbonate. Desalination 2012, 284, 238–244. [Google Scholar] [CrossRef]
- Olajire, A.A. Accepted Manuscript management technology for oil and gas production. J. Pet. Sci. Eng. 2015, 135, 723–737. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Lu, M.L.; Liu, J.; Chen, H.L.; Chen, Q.L.; Wang, B. Fluorescent-tagged hyper-branched polyester for inhibition of CaSO4 scale and the scale inhibition mechanism. Mater. Today Commun. 2020, 25, 101359. [Google Scholar] [CrossRef]
- Macedo, R.; Marques, N.D.; Paulucci, L.C.S.; Cunha, J.V.M.; Villetti, M.A.; Castro, B.B.; Balaban, R.D. Water-soluble carboxymethylchitosan as green scale inhibitor in oil wells. Carbohydr. Polym. 2019, 215, 137–142. [Google Scholar] [CrossRef]
- Hadfi, A.; Karmal, I.; Ibrahimi, B.E.; Ben-aazza, S.; Errami, M.; Mohareb, S.; Driouiche, A. Experimental investigation and molecular dynamic simulation of Tannic acid as an eco-friendly inhibitor for calcium carbonate scale. J. Mol. Liq. 2021, 340, 117225. [Google Scholar] [CrossRef]
- Li, S.L.; Qu, Q.; Li, L.; Xia, K.; Li, Y.; Zhu, T.T. Bacillus cereus s-EPS as a dual bio-functional corrosion and scale inhibitor in artificial seawater. Water Res. 2019, 166, 155094. [Google Scholar] [CrossRef]
- Yu, W.; Wang, Y.; Li, A.; Yang, H. Evaluation of the structural morphology of starch-graft-poly(acrylic acid) on its scale_inhibition efficiency. Water Res. 2018, 141, 86–95. [Google Scholar] [CrossRef]
- Chaussemier, M.; Pourmohtasham, E.; Gelus, D.; Pécoul, N.; Perrot, H.; Lédion, J.; Cheap-Charpentier, H.; Horner, O. State of art of natural inhibitors of calcium carbonate scaling. A review article. Desalination 2015, 356, 47–55. [Google Scholar] [CrossRef]
- Huang, H.H.; Yao, Q.; Jiao, Q.; Liu, B.L.; Chen, H.L. Polyepoxysuccinic acid with hyper-branched structure as an environmentally friendly scale inhibitor and its scale inhibition mechanism. J. Saudi Chem. Soc. 2019, 23, 61–74. [Google Scholar] [CrossRef]
- Zhang, W.W.; Li, H.J.; Chen, L.W.; Sun, J.N.; Ma, X.Y.; Li, Y.F.; Liu, C.; Han, X.; Pang, B.Y.; Wu, Y.C. Performance and mechanism of a composite scaling-corrosion inhibitor used in seawater: 10-Methylacridinium iodide and sodium citrate. Desalination 2020, 486, 114482. [Google Scholar] [CrossRef]
- Liu, G.Q.; Xue, M.W.; Yang, H. Polyether copolymer as an environmentally friendly scale and corrosion inhibitor in seawater. Desalination 2017, 419, 133–140. [Google Scholar] [CrossRef]
- Du, Q.; Wang, Y.; Li, A.; Yang, H. Scale-inhibition and flocculation dual-functionality of poly(acrylic acid) grafted starch. J. Environ. Manag. 2018, 210, 273–279. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Yao, Q.; Ma, S.; Wu, W.; Sun, W. Synthesis of fluorescent-tagged scale inhibitor and evaluation of its calcium carbonate precipitation performance. Desalination 2014, 340, 1–10. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.S.; Liang, Y.N. Synthesis of polyaspartic acid/graphene oxide grafted copolymer and evaluation of scale inhibition and dispersion performance. Diam. Relat. Mater. 2020, 108, 107949. [Google Scholar] [CrossRef]
- Koutsoukos, P.G.; Amjad, Z. Evaluation of maleic acid based polymers as scale inhibitors and dispersants for industrial water applications. Desalination 2014, 335, 55–63. [Google Scholar]
- Li, H.; Hsieh, M.; Chien, S.; Monnell, J.D.; Dzombak, D.A.; Vidic, R.D. Control of mineral scale deposition in cooling systems using secondary-treated municipal wastewater. Water Res. 2010, 45, 748–760. [Google Scholar] [CrossRef]
- Javad, M.; Moein, A. Experimental analysis of calcium carbonate scale formation and inhibition in waterflooding of carbonate reservoirs. J. Pet. Sci. Eng. 2016, 147, 843–850. [Google Scholar] [CrossRef]
- Canabady-rochelle, L.; Sanchez, C.; Mellema, M.; Banon, S. Calcium carbonate—Hydrolyzed soy protein complexation in the presence of citric acid. J. Colloid Interface Sci. 2010, 345, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Szcześ, A.; Czemierska, M.; Jarosz-Wilkołazka, A. Calcium carbonate formation on mica supported extracellular polymeric substance produced by Rhodococcus opacus. J. Solid State Chem. 2016, 242, 212–221. [Google Scholar] [CrossRef]
- Gao, R.X.; Li, Y.; Zhu, T.T.; Dai, Y.X.; Li, X.H.; Wang, L.; Li, L.; Qu, Q. ZIF-8@s-EPS as a novel hydrophilic multifunctional biomaterial for efficient scale inhibition, antibacterial and antifouling in water treatment. Sci. Total Environ. 2021, 773, 145706. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Zhang, Y.; Yang, M.; Hu, H.; Huang, Z.; Feng, Z.; Chen, D.; Chen, C.; Liang, J. Synthesis, Characterization, and Application of a Multifunctional Cellulose Derivative as an Environmentally Friendly Corrosion and Scale Inhibitor in Simulated Cooling Water Systems. Ind. Eng. Chem. Res. 2018, 57, 10786–10797. [Google Scholar] [CrossRef]
- Rashad, M.M.; Mahmoud, M.H.H.; Ibrahim, I.A.; Abdel-Aal, E.A. Crystallization of calcium sulfate dihydrate under simulated conditions of phosphoric acid production in the presence of aluminum and magnesium ions. J. Cryst. Growth 2004, 267, 372–379. [Google Scholar] [CrossRef]
- Abd-El-Khalek, D.E.; Abd-El-Nabey, B.A.; Abdel-kawi, M.A.; Ramadan, S.R. Investigation of a novel environmentally friendly inhibitor for calcium carbonate scaling in cooling water. Desalin. Water Treat. 2016, 57, 2870–2876. [Google Scholar] [CrossRef]
- Xie, S.; Liu, Z.; Han, G.; Li, W.; Liu, J.; Chen, Z. 3, 5-dibromo salicylaldehyde schiff’s base. Comput. Theor. Chem. 2015, 1063, 50–62. [Google Scholar] [CrossRef]
- Xu, X.; Xiao, J.; Huang, H.; Li, J.; Xiao, H. Molecular dynamic simulations on the structures and properties of. J. Hazard. Mater. 2010, 175, 423–428. [Google Scholar] [CrossRef]
- Desiraju, G.R. Hydrogen bridges in crystal engineering: Interactions without borders. Acc. Chem. Res. 2002, 35, 565–573. [Google Scholar] [CrossRef]
- Perry, I.V.; Thomas, D.; Cygan, R.T.; Mitchell, R. Molecular models of a hydrated calcite mineral surface. Geochim. Cosmochim. Acta 2007, 71, 5876–5887. [Google Scholar] [CrossRef]
- Kang, S.W.; Kang, K.; Kim, M.A.; Jeon, N.R.; Kim, S.M.; Jeon, J.S.; Nho, C.W.; Um, B.H. Phytoestrogenic activity of Aceriphyllum rossii and rapid identification of phytoestrogens by LC-NMR/MS and bioassay-guided isolation. Eur. Food Res. Technol. 2014, 239, 237–246. [Google Scholar] [CrossRef]







| Surface | Antiscalant | Etot | Esur+water | Epoly+water | Ewater | Einter | Ebin |
|---|---|---|---|---|---|---|---|
| (104) | VTE | −46,637.28 | −46,575.47 | −787.18 | −759.86 | −34.49 | 34.49 |
| MVTE | −47,010.13 | −46,460.63 | −1146.16 | −714.71 | −118.04 | 118.04 |
| Surface | Antiscalant | ΔEnon-bond | ΔEelectrostatic | ΔEvdw | Edef |
|---|---|---|---|---|---|
| (104) | VTE | −47,240.41 | −51,737.61 | 4502.92 | 15.03 |
| MVTE | −47,685.23 | −52,318,069 | 4640.26 | 58.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Zhang, L.; Wang, L.; Zhang, Q.; Luan, L. Anti-Scale Performance and Mechanism of Valonia Tannin Extract for Calcium Carbonate in Circulating Cooling Water System. Sustainability 2023, 15, 8811. https://doi.org/10.3390/su15118811
He Z, Zhang L, Wang L, Zhang Q, Luan L. Anti-Scale Performance and Mechanism of Valonia Tannin Extract for Calcium Carbonate in Circulating Cooling Water System. Sustainability. 2023; 15(11):8811. https://doi.org/10.3390/su15118811
Chicago/Turabian StyleHe, Zhenbo, Li Zhang, Lihong Wang, Qiang Zhang, and Lingyu Luan. 2023. "Anti-Scale Performance and Mechanism of Valonia Tannin Extract for Calcium Carbonate in Circulating Cooling Water System" Sustainability 15, no. 11: 8811. https://doi.org/10.3390/su15118811
APA StyleHe, Z., Zhang, L., Wang, L., Zhang, Q., & Luan, L. (2023). Anti-Scale Performance and Mechanism of Valonia Tannin Extract for Calcium Carbonate in Circulating Cooling Water System. Sustainability, 15(11), 8811. https://doi.org/10.3390/su15118811






