Application of Luminescent Bacteria Bioassay in the Detection of Pollutants in Soil
Abstract
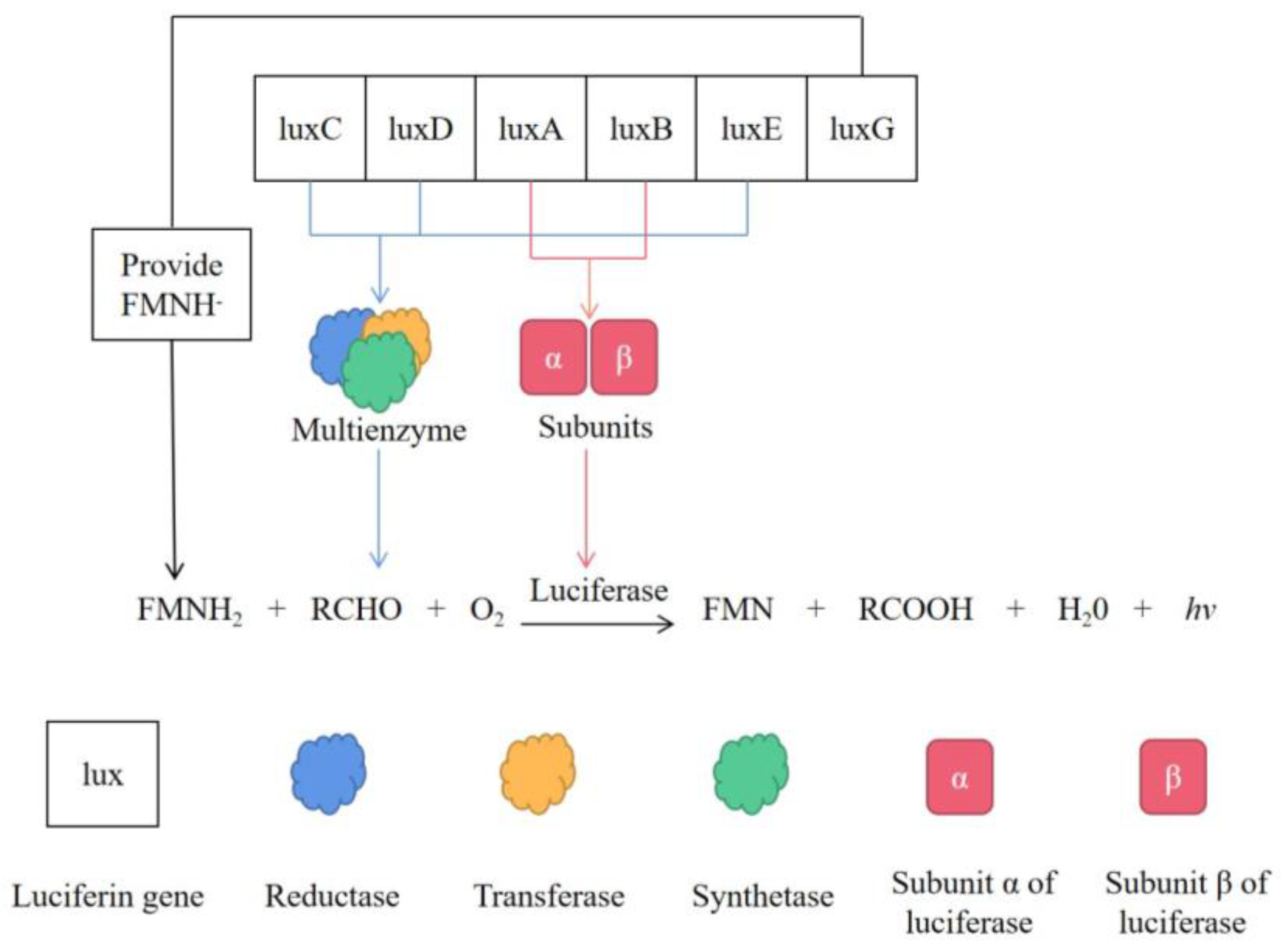
:1. Introduction
2. Data Source Search Methods
3. The Application of a Traditional Luminescent Bacteria Bioassay in the Detection of Pollutants in Soil
3.1. Vibrio fischeri in the Detection of Pollutants in Soil
3.2. Photobacterium phosphoreum in the Detection of Pollutants in Soil
3.3. Vibrio qinghaiensis in the Detection of Pollutants in Soil
4. Application of an Improved Luminescent Bacteria Bioassay in the Detection of Pollutants in Soil
4.1. Recombinant Luminescent Bacteria Bioassay in the Detection of Pollutants in Soil
4.2. Application of MNP-Based Biosensors in Soil Detection
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, X.; Cui, Y.; Li, Y. Research status of soil remediation in China. Technol. Innov. Appl. 2016, 152, 147. (In Chinese) [Google Scholar]
- Liu, Z.; Gao, Y.; Wang, J.; Zhou, W. Present situation and prevention measures of soil environmental pollution in China. Heilongjiang Environ. J. 2022, 35, 97–99. (In Chinese) [Google Scholar]
- Li, Y.; He, X.; Zhu, W.; Li, H.; Wang, W. Bacterial bioluminescence assay for bioanalysis and bioimaging. Anal. Bioanal. Chem. 2021, 414, 75–83. [Google Scholar] [CrossRef]
- Wang, C. Determination of eight elements in farmland sediment by X-ray fluorescence spectrometry. Chem. Anal. Meterage 2022, 31, 40–44. (In Chinese) [Google Scholar]
- Ning, W.; Yang, P.; Wang, H.; Han, L.; Cao, M.; Luo, J. Evaluating a Sampling Regime for Estimating the Levels of Contamination and the Sources of Elements in Soils Collected from a Rapidly Industrialized Town in Guangdong Province, China. Arch. Environ. Contam. Toxicol. 2022, 82, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Gao, Z.; Liu, L.; Zhu, Q.; Hu, G.; Zhou, X. Acute toxicity assessment of drinking water source with luminescent bacteria: Impact of environmental conditions and a case study in Luoma Lake, East China. Front. Environ. Sci. Eng. 2020, 14, e20970-171. [Google Scholar] [CrossRef]
- Rogowska, J.; Cieszynska-Semenowicz, M.; Ratajczyk, W.; Wolska, L. Micropollutants in treated wastewater. Ambio 2020, 49, 487–503. [Google Scholar] [CrossRef]
- Yang, J.; Hu, S.; Liao, A.; Weng, Y.; Liang, S.; Lin, Y. Preparation of freeze-dried bioluminescent bacteria and their application in the detection of acute toxicity of bisphenol A and heavy metals. Food Sci. Nutr. 2022, 10, 1841–1853. [Google Scholar] [CrossRef]
- Li, R.; Yuan, Y.; Li, C.; Sun, W.; Yang, M.; Wang, X. Environmental Health and Ecological Risk Assessment of Soil Heavy Metal Pollution in the Coastal Cities of Estuarine Bay-A Case Study of Hangzhou Bay, China. Toxics 2020, 8, 75. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Wang, C.; Zhu, Y.; Cui, Y. Probabilistic-fuzzy risk assessment and source analysis of heavy metals in soil considering uncertainty: A case study of Jinling Reservoir in China. Ecotoxicol. Environ. Saf. 2021, 222, 112537. [Google Scholar] [CrossRef]
- Sun, R. Study on the Joint Toxicity of Pollutants with Different Curve Types to Vibrio fischeri. Master’s Thesis, Xi’an University of Science and Technology, Xi’an, China, 2021. [Google Scholar]
- Zeng, Q. Advances in evaluating the toxicity of environmental pollutants in water using recombinant luminescent bacteria. China Resour. Compr. Util. 2019, 37, 97–101, 108. (In Chinese) [Google Scholar]
- Fan, Y.; Liu, S.; Qu, R.; Li, K.; Liu, H. Polymyxin B sulfate inducing time-dependent antagonism of the mixtures of pesticide, ionic liquids, and antibiotics to Vibrio qinghaiensis sp.-Q67. RSC Adv. 2017, 7, 6080–6088. [Google Scholar] [CrossRef]
- Li, J. Research progress on evaluation of water quality by biological toxicity test. China High New Technol. 2018, 36, 31–33. (In Chinese) [Google Scholar]
- Xu, C.; An, X.; Wang, L.; Wang, Z.; Sun, L.; Li, X.; Liu, L. Application of Luminescent Bacteria in the Detection of Environmental Biological Toxicity. Liaoning Chem. Ind. 2018, 47, 256–258. (In Chinese) [Google Scholar]
- Strotmann, U.; Flores, D.P.; Konrad, O.; Gendig, C. Bacterial Toxicity Testing: Modification and Evaluation of the Luminescent Bacteria Test and the Respiration Inhibition Test. Processes 2020, 8, 1349. [Google Scholar] [CrossRef]
- Kolosova, E.M.; Sutormin, O.S.; Stepanova, L.V.; Shpedt, A.A.; Rimatskaya, N.V.; Sukovataya, I.E.; Kratasyuk, V.A. Bioluminescent enzyme inhibition-based assay for the prediction of toxicity of pollutants in urban soils. Environ. Technol. Innov. 2021, 24, 101842. [Google Scholar] [CrossRef]
- Zhu, B.; Wu, B.; Xie, S. Test principle of biological toxicity meter and application of environmental toxicity detection. Environ. Dev. 2019, 31, 156–158. (In Chinese) [Google Scholar]
- Wan, S.; Li, F.; Wang, J.; Jiang, G.; Wu, P.; Wu, J. Detection and Evaluation of Biotoxicity of Cd Contaminated Soil by Luminescent Bacteria. J. Anhui Agric. Sci. 2022, 50, 57–60. (In Chinese) [Google Scholar]
- Heisterkamp, I.; Gartiser, S.; Kalbe, U.; Bandow, N.; Glossmann, A. Assessment of leachates from reactive fire-retardant coatings by chemical analysis and ecotoxicity testing. Chemosphere 2019, 226, 85–93. [Google Scholar] [CrossRef]
- He, D.; Chen, R.; Zhu, E.; Chen, N.; Yang, B.; Shi, H.; Huang, M. Toxicity bioassays for water from black-odor rivers in Wenzhou, China. Environ. Sci. Pollut. Res. Int. 2015, 22, 1731–1741. [Google Scholar]
- Gartiser, S.; Heisterkamp, I.; Schoknecht, U.; Burkhardt, M.; Ratte, M.; Ilvonen, O.; Brauer, F.; Bruckmann, J.; Dabrunz, A.; Egeler, P.; et al. Results from a round robin test for the ecotoxicological evaluation of construction products using two leaching tests and an aquatic test battery. Chemosphere 2017, 175, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Xie, Y.; Mao, W.; Lu, Y.; Wang, Y.; Li, H.; Zhang, C. Efficient biodegradation of tris-(2-chloroisopropyl) phosphate by a novel strain Amycolatopsis sp. FT-1: Process optimization, mechanism studies and toxicity changes. J. Hazard. Mater. 2023, 443, 130149. [Google Scholar] [CrossRef] [PubMed]
- Strotmann, U.; Thouand, G.; Pagga, U.; Gartiser, S.; Heipieper, H.J. Toward the future of OECD/ISO biodegradability testing-new approaches and developments. Appl. Microbiol. Biotechnol. 2023, 107, 2073–2095. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Martin, T.J.; Price, O.R.; Snape, J.R.; van Egmond, R.A.; Finnegan, C.J.; Schafer, H.; Davenport, R.J.; Bending, G.D. Refinement of biodegradation tests methodologies and the proposed utility of new microbial ecology techniques. Ecotoxicol. Environ. Saf. 2015, 111, 9–22. [Google Scholar] [CrossRef]
- Sharifian, S.; Homaei, A.; Hemmati, R.; Khajeh, K. Light emission miracle in the sea and preeminent applications of bioluminescence in recent new biotechnology. J. Photochem. Photobiol. B Biol. 2017, 172, 115–128. [Google Scholar] [CrossRef]
- Delatour, E.; Pagnout, C.; Zaffino, M.L.; Duval, J.F.L. Comparative Analysis of Cell Metabolic Activity Sensing by Escherichia coli rrnB P1-lux and Cd Responsive-Lux Biosensors: Time-Resolved Experiments and Mechanistic Modelling. Biosensors 2022, 12, 763. [Google Scholar] [CrossRef]
- Sharon, Y.-K.; Shimshon, B. Molecular manipulations for enhancing luminescent bioreporters performance in the detection of toxic chemicals. Adv. Biochem. Eng. Biotechnol. 2014, 145, 137–149. [Google Scholar]
- Yadav, N.; Garg, V.K.; Chhillar, A.K.; Rana, J.S. Detection and remediation of pollutants to maintain ecosustainability employing nanotechnology: A review. Chemosphere 2021, 280, 130792. [Google Scholar] [CrossRef]
- Wang, X.; Qu, R.; Wei, Z.; Yang, X.; Wang, Z. Effect of water quality on mercury toxicity to Photobacterium phosphoreum: Model development and its application in natural waters. Ecotoxicol. Environ. Saf. 2014, 104, 231–238. [Google Scholar] [CrossRef]
- Zhang, S.; Kong, X.; Jiang, Y.; Lv, J.; Wu, N.; Zhang, J.; Ma, Y.; Zhou, Y. Review of Application and Research of Biological Monitoring Technologies in Aquatic Environment. Environ. Prot. Sci. 2015, 41, 103–107. (In Chinese) [Google Scholar]
- GB/T 15441-1995; Determination of Acute Toxicity of Water Quality by Luminescent Bacteria Bioassay. Standards Press of China: Beijing, China, 1995.
- Wang, D.; Wang, S.; Bai, L.; Nasir, M.S.; Li, S.; Yan, W. Mathematical Modeling Approaches for Assessing the Joint Toxicity of Chemical Mixtures Based on Luminescent Bacteria: A Systematic Review. Front. Microbiol. 2020, 11, 1651. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.Y.; Wang, X.C.; Ngo, H.H.; Guo, W.; Wu, M.N.; Wang, N. Bioassay based luminescent bacteria: Interferences, improvements, and applications. Sci. Total Environ. 2014, 468–469, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kusumahastuti, D.K.A.; Sihtmae, M.; Kapitanov, I.V.; Karpichev, Y.; Gathergood, N.; Kahru, A. Toxicity profiling of 24 l-phenylalanine derived ionic liquids based on pyridinium, imidazolium and cholinium cations and varying alkyl chains using rapid screening Vibrio fischeri bioassay. Ecotoxicol. Environ. Saf. 2019, 172, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, K.A.; Leusch, F.D.L.; van de Merwe, J.P. Review of ecologically relevant in vitro bioassays to supplement current in vivo tests for whole effluent toxicity testing—Part 1: Apical endpoints. Sci. Total Environ. 2022, 851, 157817. [Google Scholar] [CrossRef]
- UNE-EN ISO 11348-3-2009; Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio fischeri (Luminescent Bacteria Test)—Part 3: Method Using Freeze-Dried Bacteria. European Standards: Brussels, Belgium, 2009.
- Wang, Z.; Chen, F.; Xu, Y.; Huang, P.; Liu, S. Protein Model and Function Analysis in Quorum-Sensing Pathway of Vibrio qinghaiensis sp.-Q67. Biology 2021, 10, 638. [Google Scholar] [CrossRef]
- Cui, B. Rapid Detection of Acute Toxicity in Drinking Water Based on Luminescent Bacteria. Master’s Thesis, Shandong Normal University, Jinan, China, 2017. [Google Scholar]
- Zhuo, P.; Wang, Y.; Xue, L.; Mi, Q.; Li, S. Research progress on the application of Vibrio qinghaiensis in environmental pollutant monitoring. J. Tianshui Norm. Univ. 2014, 34, 22–26. (In Chinese) [Google Scholar]
- Adnan, N.A.; Halmi, M.I.E.; Abd Gani, S.S.; Zaidan, U.H.; Abd Shukor, M.Y. Comparison of Joint Effect of Acute and Chronic Toxicity for Combined Assessment of Heavy Metals on Photobacterium sp.NAA-MIE. Int. J. Environ. Res. Public Health 2021, 18, 6644. [Google Scholar] [CrossRef]
- Wei, S.; Dong, L.; Zhang, Y.; Pei, Y.; Xu, S. Application Research Progress of Luminescent Bacteria Method in Pesticides Pollution Toxicity Detection. Mod. Agric. Sci. Technol. 2019, 739, 121–124. (In Chinese) [Google Scholar]
- Zinaida, M.K.; Aleksandra, S.T.; Ilia, V.Y. 1001 lights: Luciferins, luciferases, their mechanisms of action and applications in chemical analysis, biology and medicine. Chem. Soc. Rev. 2016, 45, 6048–6077. [Google Scholar]
- Wang, D.; Bai, L.; Li, S.; Yan, W. Similarities and Differences in Quorum Sensing-Controlled Bioluminescence between Photobacterium phosphoreum T3 and Vibrio qinghaiensis sp.-Q67. Appl. Sci. 2022, 12, 2066. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, D.; Li, S.; Yan, W. Advances in the application of Photobacterium phosphoreum on joint toxicity detection. Environ. Sci. Technol. 2022, 45, 117–131. (In Chinese) [Google Scholar]
- Hu, X.; He, Z.; Li, H. Research progress of the detection mechanism of luminescent bacterial toxicity and its application. Food Mach. 2018, 34, 179–184. (In Chinese) [Google Scholar]
- Li, R.; Ru, N.; Li, M.; Song, W.; Sun, S.; Jia, R. Experimental study of the emergency monitoring on different types of pollutants by Vibrio fischeri comprehensive toxicity method. Saf. Environ. Eng. 2015, 22, 104–109. (In Chinese) [Google Scholar]
- Yang, H. Toxicities of Heavy Metals to Vibrio fischeri. Environ. Sci. Manag. 2015, 40, 140–142. (In Chinese) [Google Scholar]
- Yu, X.; Jiang, P.; Zhang, H.; Chen, W.; Xue, Y.; Zhang, W. Assessment of acute soil toxicity and ecological risk in microwave remediation of petroleum hydrocarbon contaminated sites. Environ. Chem. 2021, 40, 3413–3420. (In Chinese) [Google Scholar]
- Shi, H.; Yuan, M.; Meng, Y.; Xu, H.; Cui, R. Study on joint toxic effects of copper, chromium and dichlorvos on Vibrio fischeri. Environ. Dev. 2019, 31. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, H.; Shi, J.; Su, Y.; Li, W.; Wilkinson, K.J.; Xie, B. Acute toxicity evaluation of nanoparticles mixtures using luminescent bacteria. Environ. Monit. Assess. 2020, 192, 484. [Google Scholar] [CrossRef]
- Abbas, M.; Adil, M.; Ehtisham-ul-Haque, S.; Munir, B.; Yameen, M.; Ghaffar, A.; Shar, G.A.; Tahir, M.A.; Iqbal, M. Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: A review. Sci. Total Environ. 2018, 626, 1295–1309. [Google Scholar] [CrossRef]
- Jiang, W.; Xiao, X.; Wang, K.; Wu, J.; Zhang, L.; Lu, F. Combined toxicity model an analysis of soil detection with Vibrio fischeri. J. Huaqiao Univ. Nat. Sci. 2021, 42, 809–816. (In Chinses) [Google Scholar]
- Mariani, L.; Grenni, P.; Caracciolo, A.B.; Donati, E.; Rauseo, J.; Rolando, L.; Patrolecco, L. Toxic response of the bacterium Vibrio fischeri to sodium lauryl ether sulphate residues in excavated soils. Ecotoxicology 2020, 29, 815–824. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, Y.; Zheng, Y.; Bao, C.; Huang, X.; Ding, Y.; Cai, Q. Joint toxicity of binary complexes of cartap, spirotetramat, copper, and cadmium to Vibrio fischeri. J. Agro-Environ. Sci. 2019, 38, 2080–2085. (In Chinese) [Google Scholar]
- Qiu, A.; Wang, Y.; Zhang, S.; Peng, Q.; Chen, Z. Joint toxic effects of carbofuran, Cd and Cu to Vibrio fischeri. J. Agro-Environ. Sci. 2017, 36, 869–875. (In Chinese) [Google Scholar]
- Cunha, D.; Muylaert, S.; Nascimento, M.; Felix, L.; de Andrade, J.J.D.; Silva, R.; Bila, D.; da Fonseca, E.M. Concentration and toxicity assessment of contaminants in sediments of the Itaipu–Piratininga lagoonal system, Southeastern Brazil. Reg. Stud. Mar. Sci. 2021, 46, 101873. [Google Scholar] [CrossRef]
- Giovanella, P.; Duarte, L.D.; Kita, D.M.; De Oliveira, V.M.; Sette, L.D. Effect of biostimulation and bioaugmentation on hydrocarbon degradation and detoxification of diesel-contaminated soil: A microcosm study. J. Microbiol. 2021, 59, 634–643. [Google Scholar] [CrossRef]
- Tao, M. Study on the Evaluation of Combined Toxicity and Mechanism of Three Typical Disinfectants on Vibrio-qinghaiensis sp.-Q67. Master’s Thesis, Anhui Jianzhu University, Hefei, China, 2021. [Google Scholar]
- Zhang, J.; Chen, P.; Tian, H.; Ma, Y.; Song, Y.; Jia, J.; Wang, J. Test for assessment of toxicity of three typical toxic contaminants to luminescent bacteria in soil. Environ. Sci. Technol. 2014, 37, 15–18. (In Chinese) [Google Scholar]
- Chen, W.; Zhao, Y.; Zheng, G.; Ma, Y.; Lei, C.; Cai, Q. Evaluation of tannery wastewater toxicity and reduction effect based on zebrafish and luminescent bacteria. Asian J. Ecotoxicol. 2014, 9, 358–366. (In Chinese) [Google Scholar]
- Xu, H.; Meng, Y.; Li, A.; Tang, W.; Huang, W.; Huang, M. Determination and evaluation of biotoxicity of heavy metal contaminated soil by luminescent bacteria. Environ. Prot. Chem. Ind. 2019, 39, 538–544. (In Chinese) [Google Scholar]
- Zeng, J.; Chen, F.; Li, M.; Wu, L.; Zhang, H.; Zou, X. The mixture toxicity of heavy metals on Photobacterium phosphoreum and its modeling by ion characteristics-based QSAR. PLoS ONE 2019, 14, e0226541. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, X.; Si, X.; Yang, R.; Zhou, J.; Quan, X. Environmentally persistent free radical generation on contaminated soil and their potential biotoxicity to luminous bacteria. Sci. Total Environ. 2019, 687, 348–354. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, X.; Zhao, J.; Lin, F.; Bao, Z.; He, Y.; Wang, L.; Shi, Z. Toxicity in different molecular-weight fractions of sludge treating synthetic wastewater containing 4-chlorophenol. Int. Biodeterior. Biodegrad. 2015, 104, 251–257. [Google Scholar] [CrossRef]
- Shen, W.; Zhu, N.; Cui, J.; Wang, H.; Dang, Z.; Wu, P.; Luo, Y.D.; Shi, C. Ecotoxicity monitoring and bioindicator screening of oil-contaminated soil during bioremediation. Ecotoxicol. Environ. Saf. 2016, 124, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hu, S.; Wu, M.; Liao, A.; Liang, S.; Lin, Y. Construction of luminescent Escherichia coli via expressing lux operons and their application on toxicity test. Appl. Microbiol. Biotechnol. 2022, 106, 6317–6333. [Google Scholar] [CrossRef] [PubMed]
- Jian, Q.; Gong, L.; Li, T.; Wang, Y.; Wu, Y.; Chen, F.; Qu, H.; Duan, X.; Jiang, Y. Rapid Assessment of the Toxicity of Fungal Compounds Using Luminescent Vibrio qinghaiensis sp. Q67. Toxins 2017, 9, 335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, S.; Deng, X. Research progress in application of the freshwater luminecent bacteria Vibrio qinghaiensis Q-67 in toxicity of environment hormonse. J. Green Sci. Technol. 2016, 12, 95–97. (In Chinese) [Google Scholar]
- Xu, W.; Jiang, Z.; Zhao, Q.; Zhang, Z.; Su, H.; Gao, X.; Ye, Z. Acute toxicity assessment of explosive-contaminated soil extracting solution by luminescent bacteria assays. Environ. Sci. Pollut. Res. Int. 2016, 23, 22803–22809. [Google Scholar] [CrossRef]
- Du, X.; Wang, C. Prediction of the toxicity of phenol derivatives to Vibrio-qinghaiensis by neural network method. Asian J. Ecotoxicol. 2016, 11, 90–94. (In Chinese) [Google Scholar]
- Chen, Y.; Liu, H.; Song, L. Application of Biotoxicity Test on Industrial Contaminated Site Investigation. Adm. Tech. Environ. Monit. 2017, 29, 59–63. (In Chinese) [Google Scholar]
- Xiu, L.; Li, Z.; Wang, X.; An, P. Research on the toxic effects of pollutants on luminescent bacteria. Sci. Technol. Inf. 2014, 12, 98–100. (In Chinese) [Google Scholar]
- Chen, S.; Zhang, Y.; Shang, J.; Xu, G. Biological Toxicity Effects of Soil Pollution Caused by Galvanized Wastewater Based on Vibrio Qinghaiensis sp.-Q67. J. Forensic Med. 2020, 36, 445–452. (In Chinese) [Google Scholar]
- Liu, M.; Guo, C.; Zhu, C.; Lv, J.; Yang, W.; Wu, L.; Xu, J. Vertical profile and assessment of soil pollution from a typical coking plant by suspect screening and non-target screening using GC/QTOF-MS. Sci. Total Environ. 2021, 810, 151278. [Google Scholar] [CrossRef]
- Han, D. Study on the Characteristics of Heavy Metal Pollution and Biotoxicity in the Environment around Smelters. Master’s Thesis, Hebei University, Baoding, China, 2020. [Google Scholar]
- Huang, Z.; Tao, M.; Zhang, J.; Dong, X.; Luo, Z.; Zhou, N. Quantitative evaluation on the antagonism between heavy mental and pesticide pollutants to Vibrio qinghaiensis sp.-Q67. Environ. Chem. 2020, 39, 2441–2449. (In Chinese) [Google Scholar]
- Xu, X.; Xue, Y.; Liu, F.; Jin, S.; Jiang, Y.; Jiang, S.; Shi, X.; Xie, X. Screening of acute toxicity and genetic toxicity of soil leachates from abandoned pesticide factory contaminated site. Asian J. Ecotoxicol. 2017, 12, 223–232. (In Chinese) [Google Scholar]
- Mehinto, A.C.; Jia, A.; Snyder, S.A.; Jayasinghe, B.S.; Denslow, N.D.; Crago, J.; Schlenk, D.; Menzie, C.; Westerheide, S.D.; Leusch, F.D.L.; et al. Interlaboratory comparison of in vitro bioassays for screening of endocrine active chemicals in recycled water. Water Res. 2015, 83, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Panitlertumpai, N.; Nakbanpote, W.; Sangdee, A.; Boonapatcharoen, N.; Prasad, M.N.V. Potentially toxic elements to maize in agricultural soils-microbial approach of rhizospheric and bulk soils and phytoaccumulation. Environ. Sci. Pollut. Res. Int. 2018, 25, 23954–23972. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Pinas, F.; Rodea-Palomares, I.; Leganes, F.; Gonzalez-Pleiter, M.; Munoz-Martin, M.A. Evaluation of the ecotoxicity of pollutants with bioluminescent microorganisms. Adv. Biochem. Eng. Biotechnol. 2014, 145, 65–135. [Google Scholar]
- Song, Y.; Jiang, B.; Tian, S.; Tang, H.; Liu, Z.; Li, C.; Jia, J.; Huang, W.; Zhang, X.; Li, G. A whole-cell bioreporter approach for the genotoxicity assessment of bioavailability of toxic compounds in contaminated soil in China. Environ. Pollut. 2014, 195, 178–184. [Google Scholar] [CrossRef]
- Zhang, L. Application of Luminescent Bacteria in Water Quality Monitoring. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2020. [Google Scholar]
- Durand, M.J.; Hua, A.; Jouanneau, S.; Cregut, M.; Thouand, G. Detection of Metal and Organometallic Compounds with Bioluminescent Bacterial Bioassays. Adv. Biochem. Eng. Biotechnol. 2016, 3, 77–99. [Google Scholar]
- Zeng, N.; Wu, Y.; Chen, W.; Huang, Q.; Cai, P. Whole-Cell Microbial Bioreporter for Soil Contaminants Detection. Front. Bioeng. Biotechnol. 2021, 9, 622994. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Schillereff, D.N.; Chiverrell, R.C.; Tefsen, B.; Wells, M. Whole-cell biosensors for determination of bioavailable pollutants in soils and sediments: Theory and practice. Sci. Total Environ. 2022, 811, 152178. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Y.; Liu, Y.; Xiang, Y.; Liu, G.; Zhang, Q.; Yin, Y.; Cai, Y.; Jiang, G. Advances in bacterial whole-cell biosensors for the detection of bioavailable mercury: A review. Sci. Total Environ. 2023, 868, 161709. [Google Scholar] [CrossRef]
- Sekhon, S.S.; Ahn, J.Y.; Ahn, J.M.; Park, J.M.; Min, J.; Kim, Y. Stress specific Escherichia coli biosensors based on gene promoters for toxicity monitoring. Mol. Cell. Toxicol. 2014, 10, 369–377. [Google Scholar] [CrossRef]
- Zhu, Y.; Elcin, E.; Jiang, M.; Li, B.; Wang, H.; Zhang, X.; Wang, Z. Use of whole-cell bioreporters to assess bioavailability of contaminants in aquatic systems. Front. Chem. 2022, 10, 1018124. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.W.; Seo, H.B.; Belkin, S.; Gu, M.B. An optical detection module-based biosensor using fortified bacterial beads for soil toxicity assessment. Anal. Bioanal. Chem. 2020, 412, 3373–3381. [Google Scholar] [CrossRef] [PubMed]
- Brutesco, C.; Preveral, S.; Escoffier, C.; Descamps, E.C.T.; Prudent, E.; Cayron, J.; Dumas, L.; Ricquebourg, M.; Adry-anczyk-Perrier, G.; de Groot, A.; et al. Bacterial host and reporter gene optimization for genetically encoded whole cell biosensors. Environ. Sci. Pollut. Res. Int. 2017, 24, 52–65. [Google Scholar] [CrossRef]
- Evrim, E.; Avni, Ö.H. Inorganic Cadmium Detection Using a Fluorescent Whole-Cell Bacterial Bioreporter. Anal. Lett. 2020, 53, 2715–2733. [Google Scholar]
- Fang, G.; Hu, L.; Huang, G.; Zhao, J.; Ying, G. The Application of Gene Recombinant Luminescent Bacteria to Environmental Sample Toxicity Test. J. S. China Norm. Univ. 2020, 52, 60–67. (In Chinese) [Google Scholar]
- Wang, Y.; Li, D.; He, M. Application of internal standard method in recombinant luminescent bacteria test. J. Environ. Sci. 2015, 35, 128–134. [Google Scholar] [CrossRef]
- He, W.; Hu, Z.; Yuan, S.; Zhong, W.; Mei, Y.; Dai, C. Bacterial Bioreporter-Based Mercury and Phenanthrene Assessment in Yangtze River Delta Soils of China. J. Environ. Qual. 2018, 47, 562–570. [Google Scholar] [CrossRef]
- Patel, R.; Zaveri, P.; Mukherjee, A.; Agarwal, P.; More, P.; Munshi, N.S. Development of fluorescent protein-based biosensing strains: A new tool for the detection of aromatic hydrocarbon pollutants in the environment. Ecotoxicol. Environ. Saf. 2019, 182, 109450. [Google Scholar] [CrossRef]
- Jiang, B.; Lian, L.; Xing, Y.; Zhang, N.; Chen, Y.; Lu, P.; Zhang, D. Advances of magnetic nanoparticles in environmental application: Environmental remediation and (bio)sensors as case studies. Environ. Sci. Pollut. Res. Int. 2018, 25, 30863–30879. [Google Scholar] [CrossRef]
- Verma, M.L.; Rani, V. Biosensors for toxic metals, polychlorinated biphenyls, biological oxygen demand, endocrine disruptors, hormones, dioxin, phenolic and organophosphorus compounds: A review. Environ. Chem. Lett. 2021, 19, 1657–1666. [Google Scholar] [CrossRef]
- Hanoglu, S.B.; Harmanci, D.; Ucar, N.; Evran, S.; Timur, S. Recent Approaches in Magnetic Nanoparticle-Based Biosensors of miRNA Detection. Magnetochemistry 2023, 9, 23. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.; Aifantis, K.E.; Fan, Y.; Feng, Q.; Cui, F.; Watari, F. Current investigations into magnetic nanoparticles for biomedical applications. J. Biomed. Mater. Res. Part A 2016, 104, 1285–1296. [Google Scholar] [CrossRef]
- Jiang, B.; Huang, W.E.; Li, G. Construction of a bioreporter by heterogeneously expressing a Vibrio natriegens recA::luxCDABE fusion in Escherichia coli, and genotoxicity assessments of petrochemical-contaminated groundwater in northern China. Environ. Sci. Process. Impacts 2016, 18, 751–759. [Google Scholar] [CrossRef]
- Chen, B.; Xie, H.; Zhang, A.; Liu, N.; Li, Q.; Guo, J.; Su, B. Synthesis of PEI-Functionalized Magnetic Nanoparticles for Capturing Bacteria. J. Wuhan Univ. Technol. -Mater. Sci. Ed. 2019, 34, 236–242. [Google Scholar] [CrossRef]
- Jiang, N.; Ying, G.; Liu, S.; Shen, L.; Hu, J.; Dai, L.J.; Yang, X.; Tian, G.; Su, B. Amino acid-based biohybrids for nano-shellization of individual desulfurizing bacteria. Chem. Commun. 2014, 50, 15407–15410. [Google Scholar] [CrossRef] [PubMed]
- Malekzad, H.; Zangabad, P.S.; Mirshekari, H.; Karimi, M.; Hamblin, M.R. Noble metal nanoparticles in biosensors: Recent studies and applications. Nanotechnol. Rev. 2017, 6, 301–329. [Google Scholar] [CrossRef]
- Bohara, R.A.; Thorat, N.D.; Pawar, S.H. Role of functionalization: Strategies to explore potential nano-bio applications of magnetic nanoparticles. RSC Adv. 2016, 6, 43989–44012. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, B.; Yu, Q. Genetic Engineering-Facilitated Coassembly of Synthetic Bacterial Cells and Magnetic Nanoparticles for Efficient Heavy Metal Removal. ACS Appl. Mater. Interfaces 2020, 12, 22948–22957. [Google Scholar] [CrossRef]
- Saed, D.; Nassar, H.N.; El-Gendy, N.S.; Zaki, T.; Moustafa, Y.M.; Badr, I.H.A. The Enhancement of Pyrene Biodegradation by Assembling MFe3O4 Nano-sorbents on the Surface of Microbial Cells. Energy Sources Part A Recovery Util. Environ. Eff. 2014, 36, 1931–1937. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, S.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A novel strategy to immobilize bacteria on polymer particles for efficient adsorption and biodegradation of soluble organics. Nanoscale 2017, 9, 11530–11536. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Li, H.; Zong, S.; Jiang, B.; Li, G.; Ejenavi, O.; Zhu, J.; Zhang, D. Magnet bioreporter device for ecological toxicity assessment on heavy metal contamination of coal cinder sites. Sens. Actuators B. Chem. 2016, 222, 290–299. [Google Scholar] [CrossRef]
- Zhang, K.; Bao, K.; Xiong, Z.; Li, T. Didactic experimental design for characterization of biotoxicity in contaminated soils with MNP–P.phosphoreum biosensor. Exp. Technol. Manag. 2022, 39, 209–213. (In Chinese) [Google Scholar]


| Luminescent Bacteria | Source | Salinity Effect | Temperature | pH | Resuscitation Fluid | Toxicity Control | References |
|---|---|---|---|---|---|---|---|
| Vibrio fischeri | Ocean | Yes | 18–30 °C | 6.5–7.5 | 3% NaCl solution | HgCl2 | [39] |
| Photobacterium phosphoreum | Ocean | Yes | 20–25 °C | 6.5–7.5 | 3% NaCl solution | HgCl2 | [39,40] |
| Vibrio qinghaiensis | Fresh water | No | 18–30 °C | 6.0–9.0 | 0.85% NaCl solution | Phenol | [39,40,41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Liu, M.; Song, X.; Wang, D. Application of Luminescent Bacteria Bioassay in the Detection of Pollutants in Soil. Sustainability 2023, 15, 7351. https://doi.org/10.3390/su15097351
Zhang K, Liu M, Song X, Wang D. Application of Luminescent Bacteria Bioassay in the Detection of Pollutants in Soil. Sustainability. 2023; 15(9):7351. https://doi.org/10.3390/su15097351
Chicago/Turabian StyleZhang, Kai, Meng Liu, Xinlong Song, and Dongyu Wang. 2023. "Application of Luminescent Bacteria Bioassay in the Detection of Pollutants in Soil" Sustainability 15, no. 9: 7351. https://doi.org/10.3390/su15097351




